Curcumin, a polyphenol, is a natural compound that has been widely studied as a hepatoprotector; however, only a few studies have examined its ability to reduce fibrosis in previously established cirrhosis. The objective of this study was to investigate whether curcumin could reduce carbon tetrachloride (CCl4)-induced fibrosis and if so, to determine the action mechanisms involved in the reduction process.
Materials and MethodsCCl4 was administered to male Wistar rats (400 mg/kg, three times a week, i. p.) for 12 weeks; curcumin (100 mg/kg body weight twice per day, p. o.) was administered from week 9–12 of CCl4 treatment. Biochemical markers of hepatic injury and oxidative stress were evaluated. Hematoxylin and eosin, Masson’s trichrome stains, transmission electron microscopy; immunohistochemistry, and zymography assays were carried out. Moreover, Smad3 and α-SMA mRNA and protein levels were studied. Western blotting by TGF-β, CTGF, Col-I, MMP-13, NF-κB, IL-1, IL-10, Smad7, pSmad3, and pJNK proteins was developed.
Results and ConclusionsCurcumin reduced liver damage, oxidative stress, fibrosis, and restored normal activity of MMP-9 and MMP-2. Besides, curcumin restored NF-κB, IL-1, IL-10, TGF-β, CTGF, Col-I, MMP-13, and Smad7 protein levels. On the other hand, curcumin decreased JNK and Smad3 phosphorylation. Furthermore, curcumin treatment decreased α-SMA and Smad3 protein and mRNA levels. Curcumin normalized GSH, and NF-κB, JNK-Smad3, and TGF-β-Smad3 pathways, leading to a decrement in activated hepatic stellate cells, thereby producing its antifibrotic effects.
Despite the progress in the study and management of liver diseases, millions of people worldwide still suffer from chronic hepatic illness. The incidence and prevalence of fibrosis, conducing to cirrhosis, is important to understanding the burden of hepatic disease [1]. However, no effective antifibrotic therapy has been approved to date. Activated hepatic stellate cells (HSCs) are the major drivers of liver fibrogenesis, producing exacerbated amounts of extracellular matrix (ECM) proteins. Therefore, pharmacological treatment to decrease activated HSCs may constitute an effective antifibrotic therapy [2,3].
Transforming growth factor-β (TGF-β) is widely recognized for its profibrogenic effects, inducing several profibrogenic factors, and most importantly inducing HSC activation. Several studies have reported that TGF-β mediates its effects through Smad3 phosphorylation in its MH2 domain (carboxyl-terminal); however, in recent years, it has been reported that Smad3 actively participates in the profibrogenic process independent of its activation via TGF-β [4]. The MAPK family can phosphorylate Smad3 in its linker domain, resulting in the induction of PAI-1 expression, which is involved in HSC proliferation and migration processes, favoring fibrosis [5,6].
Curcumin, a polyphenol found in the root of the Curcuma longa plant, has been widely reported to be a hepatoprotective plant in various liver diseases [7–10]. However, the mechanism of action of curcumin in reducing fibrosis has not been completely elucidated. Furthermore, the effect of curcumin in the noncanonical Smad profibrotic pathway has not been investigated. Consequently, the objective of this study was to investigate whether curcumin could reduce hepatic fibrosis in a carbon tetrachloride (CCl4)-induced model of cirrhosis and if so, to determine the canonical/noncanonical Smad3 pathways, and/or HSCs activation/deactivation pathways that may be involved in this protective effect.
2Materials and methods2.1Animal treatmentMale Wistar rats (initially weighing 100–120 g.) were provided by the Laboratory Animals Production and Experimentation Unit (UPEAL). Rats were maintained on a standard diet of Rat Chow® with free access to drinking water under controlled conditions. After the acclimation period (1 week), the rats (n = 32) were administered the following treatments: The control group (n = 8) was given 0.3% carboxymethylcellulose at a dose of 1 mL daily through an intragastric tube. The CCl4 group (n = 8) was administered 400 mg CCl4/kg body weight i.p. three times per week, dissolved in mineral oil for 12 weeks. The CCl4 + curcumin group (n = 8) was administered CCl4, as in the CCl4 group for 12 weeks, plus 100 mg of curcumin/kg twice a day at the beginning of the week 9 of CCl4 treatment for 4 weeks, by gavage. The curcumin only group (n = 8) was administered 100 mg of curcumin/kg twice a day for 4 weeks by gavage. The curcumin dose in this study has been previously evaluated and used with successful results [9,10]. Rats were euthanized under ketamine (100 mg/kg) and xylazine (8 mg/kg body weight) anesthesia. All rats were sacrificed following 12 weeks of the experiment. Blood was collected by cardiac puncture and centrifuged at 1300 rpm for 15 min; serum obtained was used for biochemical determinations. The liver was quickly removed, sectioned, and appropriately stored before each analysis was conducted. The rest of the liver was stored at −72 °C.
All animal care and experimental procedures complied with the Institution’s guidelines, as well as the Mexican official regulation (NOM- 062-ZOO-1999). This research protocol (protocol number 207−16) was approved by the institutional animal care and use committee from CINVESTAV-IPN.
2.2Biochemical analysesLiver damage was assessed by measuring the activities of alanine aminotransferase (ALT) [11], alkaline phosphatase (AP) [12], and γ-glutamyl transpeptidase (γ-GTP) [13] in serum samples of blood that were centrifuged at 1300 rpm for 15 min. Protein concentration was determined via the Bradford method using bovine serum albumin as a standard [14]. The content of reduced glutathione (GSH) in the liver was evaluated in fresh tissue samples [15]. Small pieces of liver were separated for glycogen determination using anthrone reagent [16]. Collagen concentrations were determined by measuring the hydroxyproline content in the liver samples as described previously [17].
2.3Hematoxylin, eosin, Masson’s trichrome stains, and immunohistochemistryLiver samples were fixed with 10% formaldehyde (in 1X PBS) for 24 h, then, hematoxylin and eosin (H & E) and Masson’s trichrome stains were performed; next, samples were mounted with Permount polymer. Sections were prepared for immunohistochemistry (IHC) as described previously [18]. The antibodies used for IHC are shown in Table 1. All stained slides were visualized using a light microscope (80i, Eclipse; Nikon, Tokyo, Japan).
Antibodies used in Western blot and immunohistochemistry techniques.
| Protein | Company | Origin | Catalog number | WB dilution | IHQ dilution |
|---|---|---|---|---|---|
| TGF-β | Millipore | CA, USA | 05−1423 | 1:500 | 1:25 |
| α-SMA | Sigma-Aldrich | Missouri, USA | A5691 | 1:500 | 1:25 |
| CTGF | Santa Cruz Biotechnology | CA, USA | SC-365,970 | 1:500 | ______ |
| COL-1 | Sigma-Aldrich | Missouri, USA | C2456 | 1:500 | ______ |
| MMP-13 | Millipore | MA, USA | MAB13426 | 1:500 | ______ |
| NF-κB (P65) | Millipore | MA, USA | MAB3026 | 1:500 | 1:50 |
| IL-1β | Millipore | Cambridge, UK | AB1832P | 1:500 | 1:25 |
| IL-10 | Thermo Fisher Scientific | CA, USA | ARC9102 | 1:500 | ______ |
| Smad3 | Abcam | MA, USA | ab65847 | 1:500 | ______ |
| Psmad3L | Abcam | Cambridge, UK | ab63403 | 1:250 | ______ |
| Smad7 | Abcam | Cambridge, UK | ab90086 | 1:500 | ______ |
| JNK | Cell Signaling Technology | MA, USA | 9252 | 1:500 | ______ |
| pJNK | Abcam | Cambridge, UK | ab131499 | 1:500 | ______ |
| β-actin | Thermo Fisher Scientific | CA, USA | AM4302 | 1:500 | ______ |
Proteolytic activity was assayed with gelatin-substrate acrylamide gels as described previously [19]. Gels were digitized and then analyzed densitometrically with an image analysis software (ImageJ, 1.46 r, NIH, Bethesda, Maryland, USA).
2.5Western blot assaysProtein analysis by western blot was performed as previously described [20]. Antibodies used for western blot are shown in Table 1. Protein concentration was measured using the bicinchoninic acid method [21]. The images were digitized, and the intensity of each band was quantified using densitometric scanning with ImageJ software (NIH) [22].
2.6RNA extraction, RT-PCR, and qRT-PCRTissue RNA was extracted and assayed as previously described [18,20] and β-Actin was used as the housekeeping gene to normalize mRNA levels. Gels were digitized. qRT-PCR was performed using 0.5 μL of cDNA added to 4.5 μL of master mix (Kapa SYBR Fast Universal qPCR Kit REF KK4601, Merck Mexico). Reaction conditions recommended by the manufacturer were followed. qRT-PCR was performed in an Eco thermocycler (Illumina). The relative mRNA levels of α-SMA (Forward 5′-GAATGAACGCTTCCGCTGCC-3′/Reverse 5′-TCCTGTCAGCAATGCCTGGG-3ʹ) and Smad3 (Forward 5′-TGATCCCTCCAATTCAGAGC-3′/Reverse 5′-AAAGACCTCCCCTCCAATGT-3′) were calculated by the comparative threshold cycle (Ct) method using β-actin (Forward 5ʹ TGGCACCACACCTTCTACA-3ʹ/Reverse 5ʹ-TCACGCACGATTTCCC-3ʹ) as an internal control for normalization. The fold change in the expression of each target gene was calculated by the 2−ΔΔCt method [23].
2.7Transmission electron microscopyRepresentative liver tissues were processed for transmission electron microscopy (TEM) as previously described [24].
2.8Statistical analysisResults are expressed as mean values ± SE. Comparisons were carried out via one-way variance analysis followed by Tukey’s test, utilizing the Graph Pad Prism software, version 6.01 (GraphPad Software Inc., San Diego, CA, USA). Differences were considered statistically significant when P was <0.05. The group sizes referred to independent values.
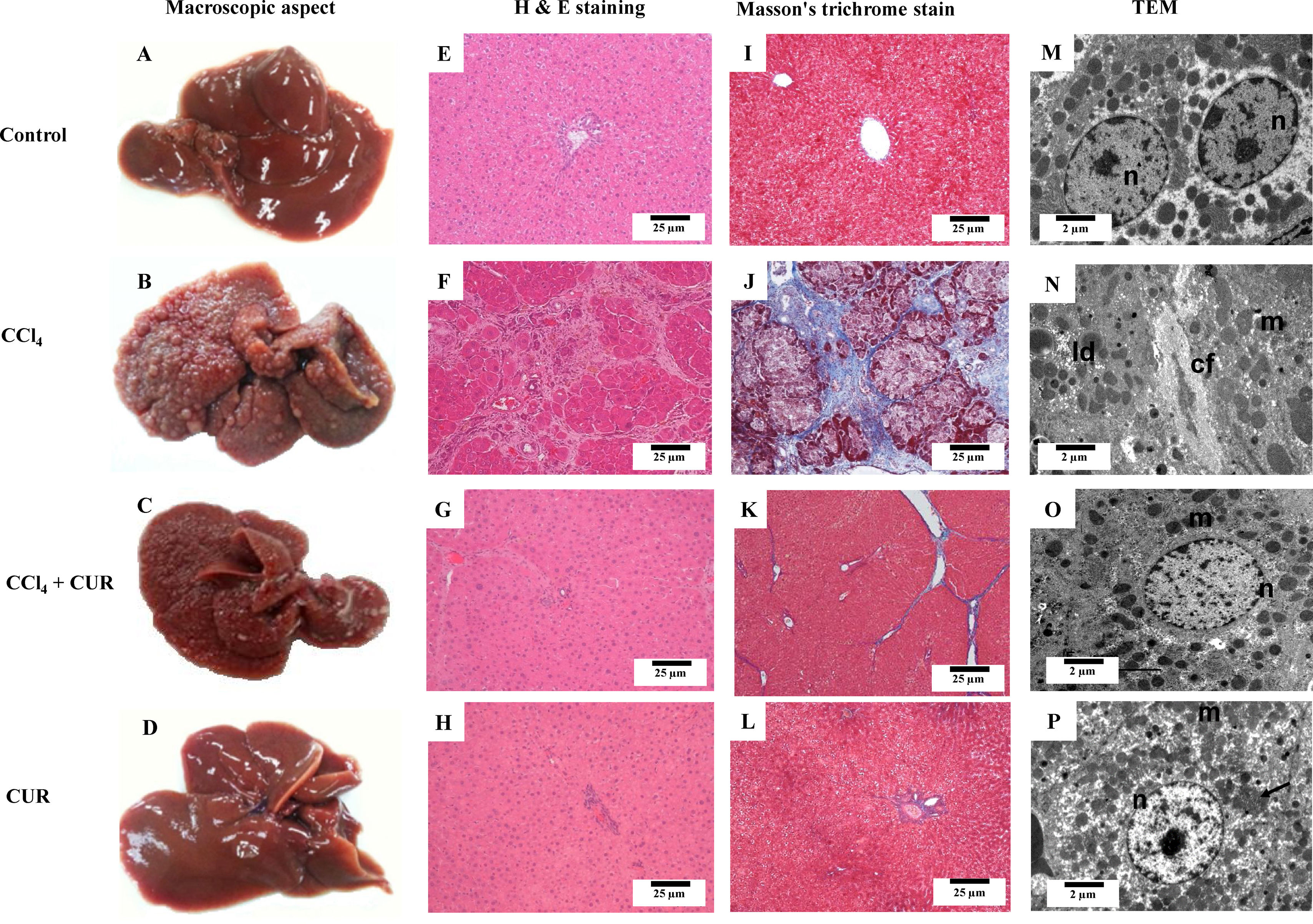
3Results3.1Curcumin attenuated macroscopic, microscopic, and ultrastructural alterations produced by CCl4The control rat liver had an intense brown color, smooth, and soft surface (Fig. 1A). Treatment with CCl4 produced liver macronodular fibrosis (Fig. 1B) and curcumin attenuated the macroscopic effects produced by CCl4 administration (Fig. 1C). Hepatic tissue from CCl4-treated rats (Fig. 1F) showed a distortion of the parenchyma, bile duct proliferation, and steatosis but curcumin decreased these alterations. Large areas of normal hepatocytes were found, but some hepatocytes maintained discrete deposits of hemosiderin and nuclear atypia (Fig. 1G). Fig. 1J shows a cirrhotic liver section stained with Masson’s trichrome, where large amounts of collagen can be observed forming regeneration nodules. In the CCl4 + curcumin group, normal areas were observed, as well as a considerable decrease in collagen fibers (Fig. 1K). The control group showed a normal liver ultrastructure by TEM images, where several mitochondria and typical nuclei can be observed, and the liver appeared healthy (Fig. 1M). On the other hand, cirrhotic livers showed abundant lipid droplets and disorganized mitochondria with loss of cristae and internal structure as well as large areas of collagen deposition (Fig. 1N). Importantly, hepatic parenchyma from animals intoxicated with CCl4 plus curcumin (Fig. 1O) had fewer collagen fibers, and alterations were attenuated. Curcumin-only-treated rats showed no alterations when compared to the control group.
Macroscopic, microscopic, and ultrastructural effects of curcumin in rats treated with CCl4. Macroscopic appearance (A-D) and representative liver sections stained using H&E (E-H), Masson's trichrome (I-L), and transmission electron microscopy (TEM) (M-P) of livers of the control rats, rats treated with CCl4 for 12 weeks (CCl4), rats treated with CCl4 + curcumin (CUR) (CCl4 + CUR) and rats treated with CUR alone. n: nucleus; m: mitochondria; ld: lipid droplets; cf: collagen fibers.
After CCl4 intoxication, ALT, FA, and γ-GTP serum activities were significantly elevated; curcumin treatment partial or completely ameliorated these alterations (Figs. 2A-C). GSH and glycogen contents were elevated during liver cirrhosis, and curcumin restored these parameters (Figs. 2D and E). Healthy rats treated with curcumin did not exhibit any modified parameters.
Curcumin attenuated serum indicators of hepatic injury restored oxidative stress markers, and glycogen content in rats treated with CCl4. Activities of alkaline phosphatase (AP) (A), γ-glutamyl transpeptidase (γ-GTP) (B), and alanine aminotransferase (ALT) (C), liver reduced glutathione (GSH) (D) and glycogen levels (E) were determined in control rats, rats treated with CCl4 for 12 weeks (CCl4), rats treated with CCl4 + curcumin (CUR) (CCl4+ CUR), and rats treated with CUR alone. Each bar represents the average value of eight rats (n = 8) ± standard error. a means P < 0.05 with respect to the control group and b means P < 0.05 with respect to the CCl4 group.
Treatment with CCl4 for 12 weeks increased immunoreactivity to NF-κB (p65) and IL-1β in hepatic tissue; interestingly, intense positivity colocalized with collagen fibers accumulation (arrows) (Figs. 3B and G). In the group treated with CCl4 and curcumin, immunoreactivity to NF-κB (Fig. 3C) and IL-1β (Fig. 3H) decreased significantly. The percentage of immunoreactivity to NF-κB and IL-1β is shown in Figs. 3E and J, respectively. Besides, protein levels of NF-κB, IL-1β, and IL-10 increased significantly by CCl4 intoxication compared to the control group, and curcumin administration restored basal levels of NF-κB, IL-1β, and IL-10 (Figs. 3K-M). Rats treated with only curcumin showed no changes in cytokines levels.
Administration of curcumin attenuated inflammatory environment in rats with experimental cirrhosis. Representative NF-κB (AD), and IL-1β (FI) immunohistochemistry. The percentage of immunoreactivity to NF-κB (E), and IL-1β (J). Representative NF-κB (p65) (K), IL-1β (L), and IL-10 (M) western blot (WB) of the control rats livers, rats treated with CCl4, rats treated with CCl4 + curcumin (CUR), and rats treated with CUR alone. Immunohistochemistry positivity is indicated by arrows; results are expressed as % of the area with immunoreactivity. Each bar represents the average value of three rats (n = 3) (each rat with three fields averaged) ± standard error. In WB, densitometric analyses were performed using β-actin as internal control. Each bar represents the average value of four rats (n = 4) ± standard error. a means P < 0.05 with respect to the control group and b means P < 0.05 with respect to the CCl4 group.
Collagen, quantitatively assessed as the hepatic hydroxyproline content, significantly increased in the CCl4 group when compared to the control group; notably, after curcumin administration for 4 weeks, collagen values decreased significantly (Fig. 4A). Protein levels of CTGF, MMP-13, and activity of MMP-2 and MMP-9 were elevated compared to the control group, and curcumin administration restored these factors to normal levels (Figs. 4B-E). Treatment of healthy rats with curcumin did not modify proteins or activities.
Curcumin reduced collagen accumulation and extracellular matrix remodeling proteins in cirrhotic rats with experimental liver cirrhosis. Total collagen levels (A), representative western blot (WB) of CTGF (B), and MMP-13 (C) as well as representative MMP-2 (D) and MMP-9 (E) zymography (F) of livers derived from control rats, rats treated with CCl4 for 12 weeks (CCl4), rats treated with CCl4+ curcumin (CUR) (CCl4+ CUR), and rats treated with CUR alone. In the total collagen graph, each bar represents the average value of eight rats (n = 8) ± standard error. In WB, densitometric analyses were performed using β-actin as the internal control and each bar represents the average value of four rats (n = 4) ± standard error. In the zymography assay, densitometric analyses were performed and each bar represents the average value of three rats (n = 3) ± standard error. a means P < 0.05 with respect to the control group and b means P < 0.05 with respect to the CCl4 group.
Hepatic parenchyma from CCl4-damaged rats exhibited large areas of positive reaction to TGF-β and α-SMA and this reaction colocalized with fibrotic zones, rich in collagen and HSCs (arrows) (Figs. 5B and G), and curcumin decreased TGF-β and α-SMA positivity (Figs. 5C and H); the percentages of immunoreactivity to TGF-β and α-SMA are shown in Figs. 5E and J, respectively. Western blotting results showed that TGF-β and α-SMA protein levels in the livers of CCl4-treated rats were significantly elevated compared to those in the control group and that curcumin significantly reversed this alteration (Figs. 5K and L). At the mRNA level, hepatotoxicity significantly increased α-Sma levels and curcumin reversed this increase (Fig. 5M). Rats treated with CCl4 showed decreased levels of Smad7, and curcumin administration significantly attenuated this effect (Fig. 5N). Curcumin administered to healthy rats did not modify the studied parameters.
Effect of curcumin on hepatic stellate cells transdifferentiation and TGF-β pathway. Representative TGF-β (AD), and α-SMA (FI) immunohistochemistry. The percentage of immunoreactivity to TGF-β (E), and α-SMA (J). Representative western blot (WB) of TGF-β (K) and α-SMA (L). Representative α-SMA qRT-PCR graph and RT-PCR gel (M), and representative Smad7 WB (N) of control rats, rats treated with CCl4, rats treated with CCl4 + curcumin (CUR), and rats treated with CUR alone. Immunohistochemistry positivity is indicated by arrows; results are expressed as % of the area with immunoreactivity. Each bar represents the average value of three rats (n = 3) (each rat with three fields averaged) ± standard error. In WB, densitometric analyses were performed using β-actin as internal control. Each bar represents the average value of four rats (n = 4) ± standard error. In qRT-PCR, β-actin was used as a reference gene to normalize α-SMA mRNA levels. The relative change of expression was calculated using the 2ΔΔCt method. Each bar represents the average value of three rats (n = 3) ± standard error. a means P < 0.05 with respect to the control group and b means P < 0.05 with respect to the CCl4 group.
Fig. 6 shows that CCl4 increased the pJNK/JNK ratio (A), and levels of pSmad3L (B), Smad3 (C) proteins, and Smad3 mRNA (D). Curcumin significantly reversed these effects. Curcumin administered to healthy rats did not modify the studied parameters.
Administration of curcumin inhibits the JNK-Smad3 pathway. Representative western blot (WB) of pJNK (A), pSmad3L (B), and Smad3 (C), as well as Smad3 qRT-PCR graph and RT-PCR gel (D) of control rats, rats treated with CCl4 for 12 weeks (CCl4), rats treated with CCl4 + curcumin (CCl4 + CUR), and rats treated with CUR alone. In WB, densitometric analyses were performed using JNK or β-actin as internal controls. Each bar represents the average value of four rats (n = 4) ± standard error. In qRT-PCR β-actin was used as a reference gene to normalize Smad3 mRNA levels. The relative change of expression was calculated using the 2−ΔΔCt method. Each bar represents the average value of three rats (n = 3) ± standard error. a means P < 0.05 with respect to the control group and b means P < 0.05 with respect to the CCl4 group.
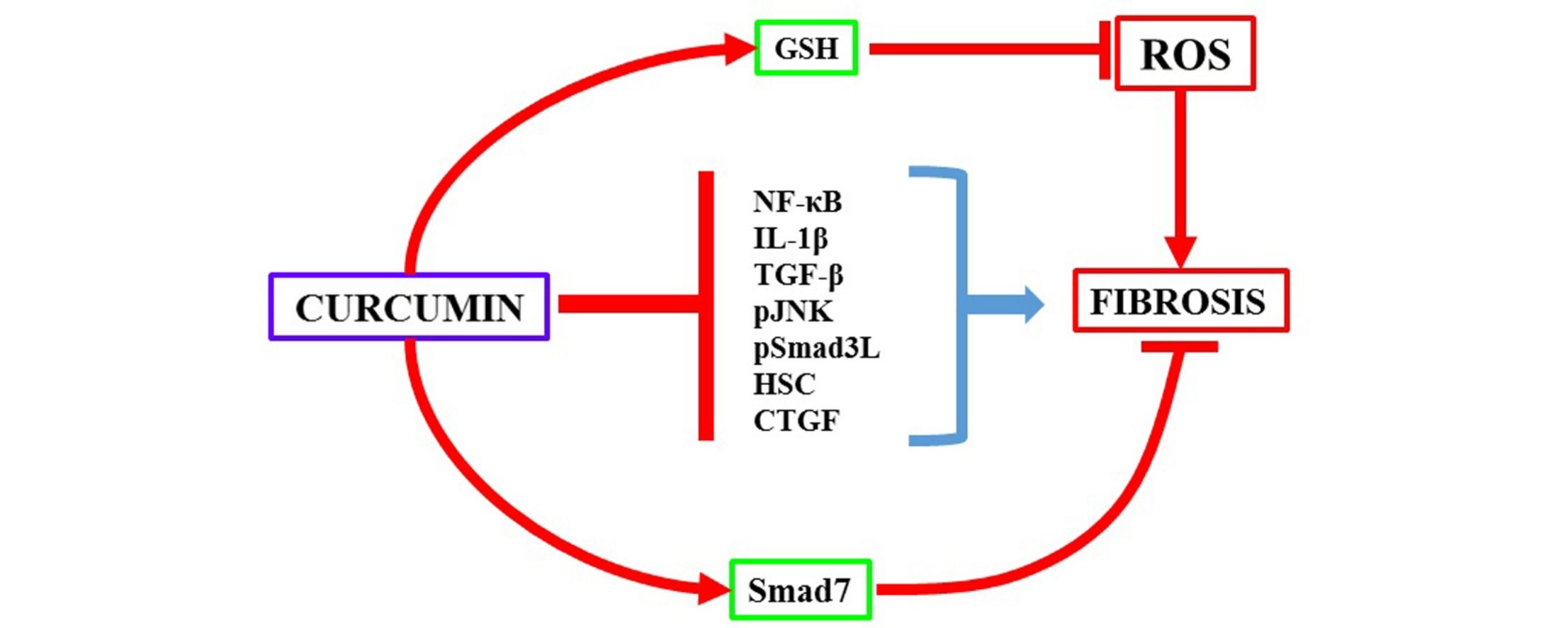
This study provides evidence that curcumin administration attenuates CCl4-induced liver cirrhosis. Our results suggest that an antifibrotic action of curcumin may be associated with its ability to deactivate and/or eliminate transdifferentiated HSCs, the most active producers of fibrotic tissue in the liver parenchyma. Curcumin downregulated the TGF-β signaling pathway, the main HSCs transdifferentiation inductor, as well as the JNK-Smad3 pathway, involved in the proliferation and migration of HSCs. Moreover, curcumin restored Smad7 levels, an important antifibrotic protein. This polyphenol helped to attenuate the proinflammatory cytokine cascade by blocking the NF-κB factor.
4.1Curcumin downregulates the profibrogenic JNK-Smad3 pathwayPreviously, it has been reported that curcumin reduces acute and chronic liver damage [7–10]. In the present study, it was observed, for the first time, that curcumin re-established normal JNK and Smad3, mRNA, and protein levels that were upregulated by CCl4-induced chronic liver damage, thereby leading to downregulation of the JNK-Smad3 pathway; as a result, profibrogenic and mitogenic pSmad3L [5,6] was decreased and progression of fibrosis was attenuated. It seems that more Smad protein is synthesized in the CCl4 group (please note that Smad3 mRNA was increased) and more Smad3 is phosphorylated. Our results suggest that phosphorylation is increased too because pJNK was upregulated in the CCl4 group. In summary, the present results suggest that CCl4 increased the synthesis and phosphorylation of Smad3, and curcumin attenuated both effects.
4.2Curcumin alleviates fibrosis by increasing the antifibrotic Smad7 proteinCurcumin re-established normal Smad7 levels, which is a negative regulator of the liver fibrogenic process. Smad7 intracellularly binds to TβRI, preventing Smad3 phosphorylation and Smads3-Smad4 heterocomplex formation, thus blocking the profibrogenic activity in the canonical TGF-β pathway. Additionally, in the nucleus, Smad7 binds to DNA and impedes binding of the Smad3-Smad4 heterocomplex to DNA [25–28].
During chronic liver damage, Smad7 levels remain low, leading to an uncontrolled fibrotic response; low levels of Smad7 in activated HSCs may exacerbate TGF-β profibrogenic effects during the progression of liver fibrosis [29,30]. Therefore, the upregulation of Smad7 by curcumin seems to be another antifibrotic mechanism of this molecule by blocking the TGF-β pathway, which may help in triggering the resolution of fibrosis.
4.3Curcumin reduces the number of activated HSCIt is worth noting that curcumin treatment resulted in a reduction in the number of activated HSCs. This effect could be mediated by an increase in Smad7 levels and downregulation of TGF-β canonical and non-canonical pathways or other curcumin-independent effects such as deactivation, senescence, or apoptosis of HSCs [31–34]. Taken together, the results of the present study and evidence from previous studies, suggest that one of the main antifibrotic mechanisms of curcumin is the decrease in activated profibrogenic HSCs.
4.4Curcumin sustains beneficial effects by regulating MMPsMMPs are key regulators of the synthesis and degradation of ECM [35], which is the main reservoir of signaling molecules by binding growth factors such as CTGF, TGF-β, and PDGF [36]. MMP13 is responsible for the cleavage of CTGF [37]. Furthermore, the high proteolytic activities of MMP2 and 9 promote the dissociation of TNF-α and IL-1β from ECM, conducing to an exacerbation of the inflammatory process [38]. The results of the present study suggest that curcumin may support antifibrotic activity by modulating MMPs, thus regulating the release from the ECM of pro-inflammatory and pro-fibrogenic factors.
4.5Curcumin attenuates the proinflammatory NF-κB pathwayIt has been reported that NF-κB inhibition promotes apoptosis of HSCs, preventing liver fibrosis consequently [39,40]. Curcumin reduces NF-κB mRNA, as well as Toll-like receptor 2, Toll-like receptor 4, and MyD88 adapter in activated HSCs, leading to their apoptosis [41–43]. Therefore, it is likely that NF-κB inhibition by curcumin may explain, in part, its antifibrotic actions by both blocking inflammation and inducing HSC apoptosis.
5ConclusionIt can be concluded that curcumin attenuated CCl4-induced cirrhosis by downregulating canonical and non-canonical Smad pathways, restoring Smad 7 levels, blocking NF-κB proinflammatory cytokine production, and decreasing the number of activated HSCs in the hepatic parenchyma. These results may be of clinical importance in the treatment of cirrhotic patients; however, more basic and controlled prospective studies in humans are required before suggesting curcumin as a safe anti-cirrhotic remedy.
Limitations and perspectivesActivated HSCs are the main responsible for fibrogenesis. Therefore, it is generally recognized that the most suitable approach to induce remission of liver fibrosis consists of “eliminating” activated HSCs. Herein, we found that curcumin reduced activated HSCs. However, the mechanism remains to be dilucidated. The possible mechanisms include apoptosis, necrosis, and senescence of these cells. The deactivation of profibrogenic HSCs to a quiescent state seems to be a suitable approach. Therefore, research on the molecular pathways leading to the “elimination” of activated HSCs will provide specific targets to produce regression of the fibrotic state. Moreover, the specific molecular targets of curcumin (or any putative antifibrotic drug) on activated HSCs must be investigated to illuminate the antifibrotic pathways involved and to improve therapy.
FundingThis work was supported by the National Council of Science and Technology (Conacyt) of Mexico, No. 253037 to Muriel P., No. 237523 to Shibayama M., and No. 239516 to Segovia J.; fellowship No. 358378 to Hernández-Aquino E. from Conacyt.
Conflict of interestThe authors have no conflict of interest to report.
The authors thank Laura D. Buendia-Montaño, Rafael Leyva, Benjamín E. Chavez, and Ricardo Gaxiola for their excellent technical assistance. The authors also acknowledge the Animal Lab Facility, UPEAL-Cinvestav.