Biliary tract complications are an important cause of morbidity and mortality after liver transplantation (LT) occurring in 5% to 25% of patients. The most common biliary complication in LT recipients are strictures representing approximately half of these biliary adverse events. Bile duct strictures can be divided into anastomotic biliary strictures (ABS) and non-anastomotic biliary strictures (NABS) depending on their location in the biliary tree, being ABS the most encountered type. Several risk factors identified in previous studies can predispose to the development of ABS and NABS, especially those related to surgical techniques and donor characteristics. Magnetic resonance cholangiopancreatography (MRCP) is the recommended noninvasive imaging test for detecting post-LT biliary strictures, given its high sensitivity and specificity. Once the diagnosis of a biliary stricture after LT has been made, endoscopic retrograde cholangiopancreatography (ERCP) is the preferred initial therapy with good short and long-term results. Biliary sphincterotomy plus balloon dilation (BD) with placement of multiple plastic stents (MPS) has been the classic endoscopic approach for treating ABS, although fully-covered metallic stents (FCSEMS) have emerged as an alternative thanks to shorter total duration of stenting and fewer endoscopic procedures compared to MPS. In this review, we provide a practical update on the management of biliary strictures after LT, focusing our attention on the available evidence in the endoscopic therapy.
Liver transplantation (LT) is the definitive treatment for acute and end-stage liver diseases of all etiologies and is also performed in patients with hepatocellular carcinoma. Survival rates in Europe have improved significantly, achieving rates of 86% at one year and 74% at five years after LT [1]. Despite these major improvements, biliary adverse events are common after orthotopic LT compromising graft survival and increasing patient morbidity and even mortality [2,3].
2Incidence and risk factorsThe overall estimated incidence of biliary adverse events following LT ranges from 5% to 25% in most observational studies [2,4–6], with an approximate incidence of 10% to 15% in recipients of deceased donor livers and a higher range of 15% to 30% in recipients of living donor livers [5–10]. Up to 40–50% of these post-LT biliary events are caused by biliary strictures. They are divided into anastomotic biliary strictures (ABS) and non-anastomotic biliary strictures (NABS), depending on their location; ABS is the most common type [11]. Strictures that occur within the first 8 weeks following LT are typically due to technical problems during surgery. In contrast, strictures that develop beyond this early period are primarily attributed to factors such as vascular insufficiency, ischemia, as well as complications related to healing and fibrosis [11–13].
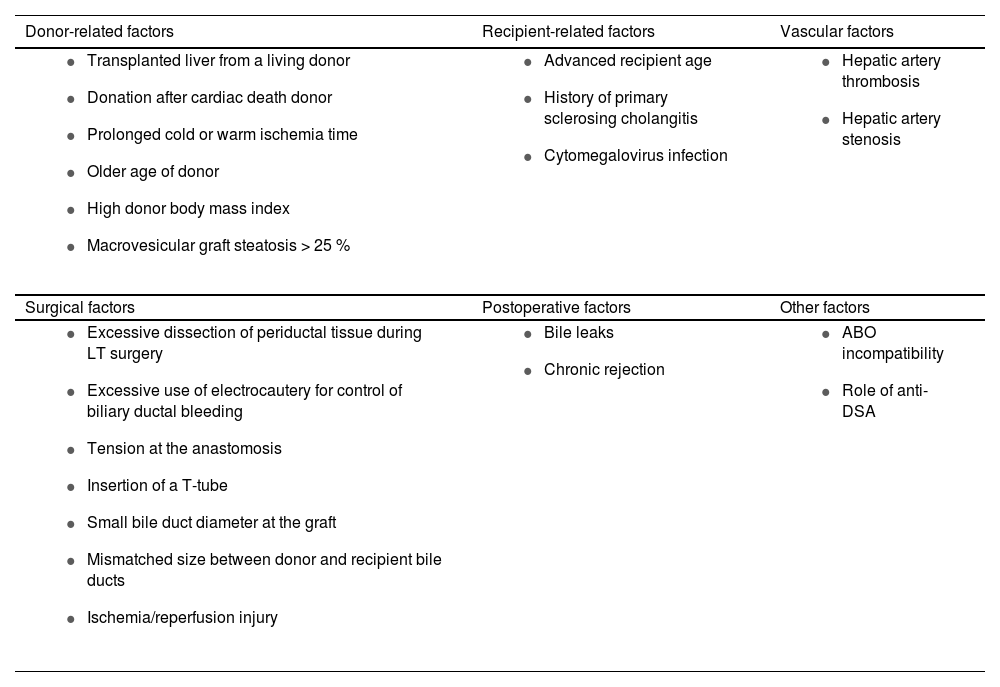
Several observational studies, systematic reviews and meta-analysis have identified multiple risk factors for developing biliary strictures [14–17]. Table 1 summarizes the main factors.
Risk factors for biliary strictures after liver transplantation.
| Donor-related factors | Recipient-related factors | Vascular factors |
|---|---|---|
|
|
|
| Surgical factors | Postoperative factors | Other factors |
|
|
|
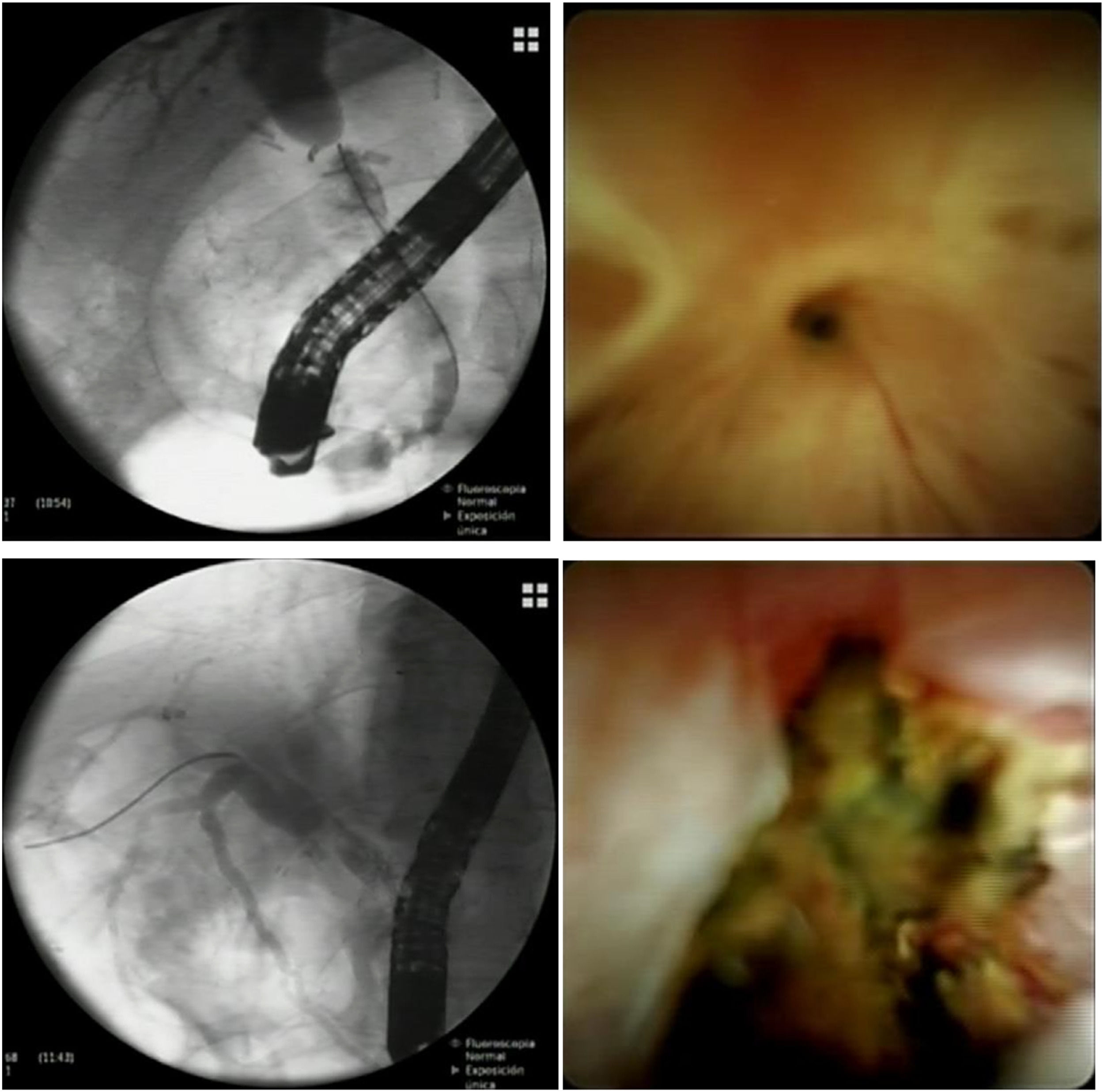
Anastomotic strictures are defined as single, short, isolated strictures within 5 to 10 mm of the biliary anastomosis. On cholangiogram, the stricture typically appears as a thin narrowing of the biliary anastomosis without clear passage of contrast medium (Fig. 1). With per-oral cholangioscopy (POCS) the stricture is either surrounded by scarring or inflammatory signs (erythema, edema, ulceration) (Fig. 1). ABS can develop at any time after LT surgery, but the majority of them occur between the second month and the first year after transplantation [18,19]. Depending on the time of occurrence, they can be separated in early and late. Early ABS generally develop in the first three months after LT and are mostly related to technical/surgical factors, while late ABS develop three months after LT and are associated with local ischemia and fibrosis [4,20,21].
ERCP and POCS images of anastomotic biliary strictures. The pictures above are from the same patient and represent a very tight fibrotic ABS successfully crossed with a guidewire using POCS. The pictures below are from the same patient and show an inflamed and ulcerated ABS in a patient with ischemic cholangiopathy.
In certain cases, there may be a temporary narrowing of the anastomosis observed in patients one to two months after LT. This narrowing typically results from postoperative swelling and inflammation and is not usually considered a true stricture [22], so unless there is reason to suspect a biliary adverse event, there is no need for endoscopic intervention.
2.1.2Non-anastomotic biliary strictures (NABS)NABS are defined as irregularities or duct narrowing in the biliary tree away from the surgical anastomosis. They can be either intrahepatic or extrahepatic, and create biliary sludge accumulation proximal to the stricture, leading to the formation of casts [19,22]. They constitute between 10% to 25% of all stricture-related adverse events following LT, with reported incidences ranging from 0.5% to 10% [5,20,23]. NABS can be present for up to one year following surgery. They are mainly due to hepatic artery thrombosis or other forms of biliary ischemia (prolonged cold and/or warm ischemia) and are more prevalent when donor organs have been retrieved after cardiac death [24].
3Clinical and diagnosis of biliary stricturesClinical presentation of biliary strictures is heterogeneous. There is a wide spectrum of symptoms such as anorexia, pruritus, jaundice, abdominal pain, fever or recurrent cholangitis. The majority of LT recipients have asymptomatic elevations in liver enzymes, primarily in a cholestatic pattern (increased levels of alkaline phosphatase and/or gamma-glutamyl transferase compared with aminotransferases) and this may represent the first sign of presentation of a biliary stricture.
The evaluation of a suspected biliary stricture generally begins with an abdominal ultrasound (US) as first step. US imaging can be used to study the presence of dilated bile ducts, examine the liver parenchyma for the presence of liver abscesses or other fluid collections, and perform a Doppler study to rule out any vascular abnormalities in the hepatic vessels. In case of any vascular abnormality such as hepatic artery stenosis or occlusion in the Doppler study, then a computed tomography scan with angiogram is obtained to confirm the diagnosis.
Sometimes, only a US is needed to confirm the diagnosis of a biliary stricture, especially when it shows features of bile duct obstruction such as proximal biliary ductal dilation with stones. In patients with a high pretest probability for a bile duct stricture and suggestive US findings, proceeding directly to endoscopic retrograde cholangiopancreatography (ERCP) is considered prudent [25,26].
Abdominal US may not be sufficiently sensitive in some cases (sensitivity of US varying from 38% to 77% [13,21,27,28] and a magnetic resonance cholangiopancreatography (MRCP) should be performed. MRCP is a highly reliable noninvasive technique for identifying biliary strictures after LT, and it is recommended by recent society guidelines and meta-analyses [25,26,29]. It provides a complete map of the biliary tract and represents the most accurate noninvasive imaging test for detecting post-LT biliary adverse events. A recent meta-analysis of 20 studies showed that MRCP has a sensitivity of 95% and a specificity of 90% for diagnosing post-LT biliary strictures [26].
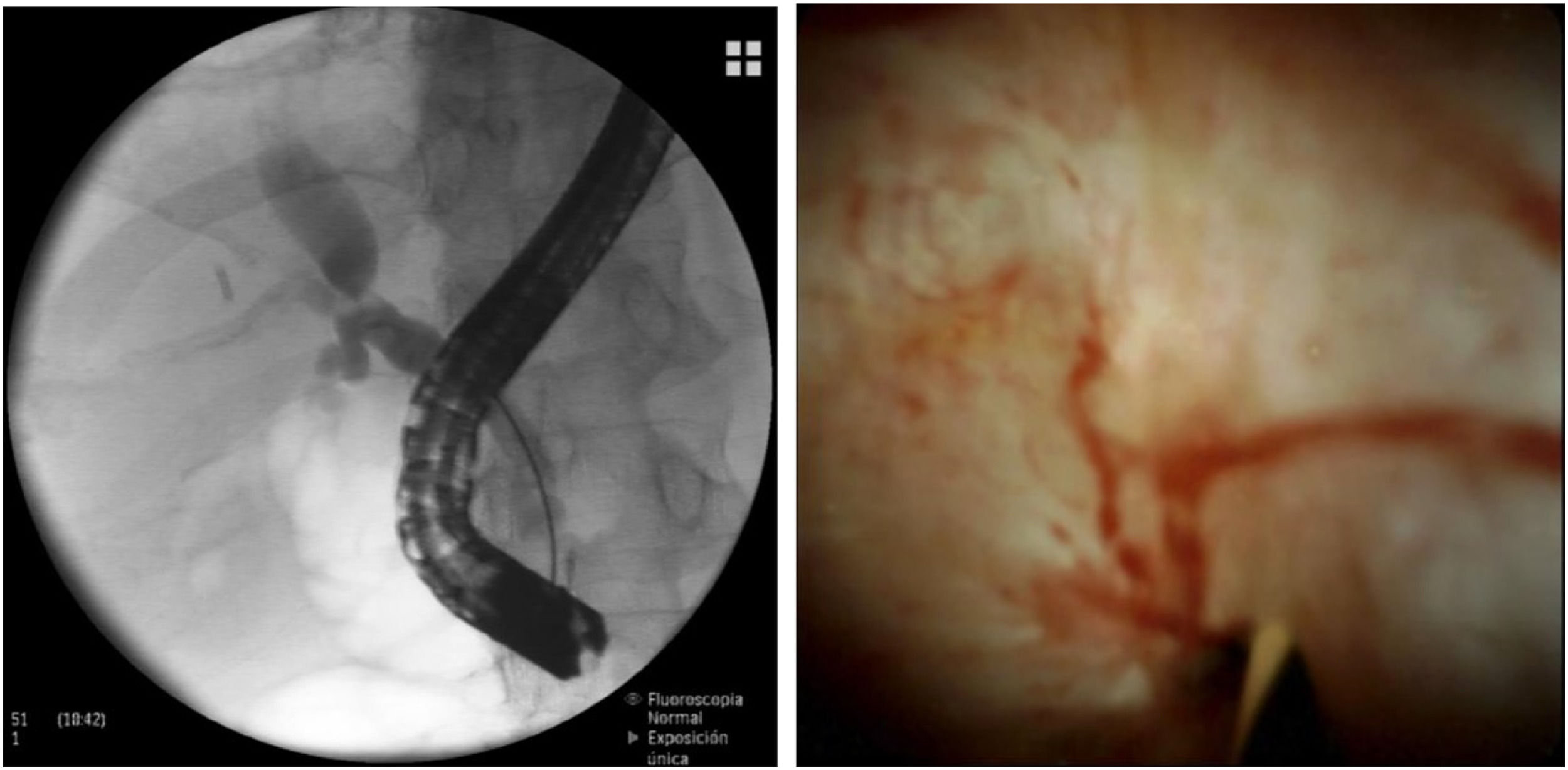
Finally, POCS may represent another valid option in the diagnostic work-up of patients with post-LT bile duct strictures. It is feasible in LT recipients and it enables an excellent visualization of the bile duct, allowing for selective guidewire placement in the proximal biliary tree of the graft and adequate biopsy sampling of strictures [30–34] (Fig. 2).
ERCP and POCS images of a tight ABS that could not be crossed with fluoroscopy despite using multiple guidewires. In this case, POCS enabled an excellent visualization of the anastomotic stricture, which showed scarring and mild erythema. POCS allowed for selective guidewire placement in the biliary tree of the graft and subsequent endoscopic treatment was performed.
Two distinct visual patterns that are easily identified with POCS (scarring and mild erythema vs. erythema/edema with sloughing and/or ulceration) can provide important diagnostic information and predict the outcomes of endoscopic therapy in patients with biliary strictures after LT [35]. The presence of significant ulceration and sloughing in a biliary stricture after LT requires a much longer period of biliary stenting and, therefore, more ERCP sessions [35].
4Management of biliary stricturesThe main goal of treating all biliary strictures is to restore the bile outflow through the narrowed area identified on cholangiography. The aim is to improve liver tests and resolve cholestasis, itching, jaundice, abdominal pain and/or recurrent cholangitis.
4.1Endoscopic treatment of post-LT biliary stricturesEndoscopic retrograde cholangiopancreatography (ERCP) remains as the preferred initial therapy for the management of these complications, as recently published by the latest guidelines [25,26,36]. If ERCP is unsuccessful, due to factors such as difficult stenosis, intrahepatic or hilar stenosis, or anatomic variations (mainly post-surgical), it may be necessary to consider a percutaneous intervention or surgical approach.
4.2Endoscopic treatment of anastomotic biliary stricturesIn patients with post-LT biliary strictures, ERCP usually requires biliary sphincterotomy plus balloon dilation (BD) and multiple plastic stents (MPS) placement over a selective guidewire. The guidewire is passed selectively across the stricture and placed in the proximal biliary tree of the graft, a step that is necessary prior to stent placement. In a few cases, this selective guidewire placement may fail using conventional ERCP techniques, particularly when ABS are very tight or significantly kinked. Some retrospective studies [32,34,37] have shown that POCS is a useful endoscopic tool for selective guidewire placement across complex biliary strictures when classic ERCP methods fail. POCS has the potential to address this issue thanks to its excellent image resolution, with a technical success rate of assisting guidewire placement in complex strictures around 75% [32,34,37]. This, in turn, facilitates subsequent endoscopic therapy and reduces the need for alternative treatments like surgery.
After the index procedure, ERCP is typically repeated every three months to exchange stents and increase their diameter in order to achieve stricture resolution and minimize long-term recurrence. Plastic stents are usually replaced at three-month intervals to mitigate increased risk of stent occlusion and bacterial cholangitis. The majority of patients following the MPS strategy often require multiple ERCP sessions, normally ranging between 3 and 5 endoscopic procedures. With this specific approach, the rate of ABS resolution is around 70–95% [38–43] and the rate of ABS recurrence is around 3–20% [38–43].
Over the past ten years, self-expandable metallic stents (SEMS) have emerged as an alternative for MPS in order to reduce the challenges linked to multiple stent placement, the risk of stent occlusion and the need for several endoscopic sessions. In particular, fully covered SEMS (FCSEMS), given the benign nature of post-LT biliary strictures, offers some advantages such as quicker and easier placement compared to plastic stents. Additionally, they provide greater radial force after the initial ERCP and can remain functional for longer than the 3-month period typically employed in the MPS approach. Table 2 illustrates the endoscopic technique for both plastic stent and SEMS placement.
Endoscopic technique for both plastic stent and SEMS placement in the index ERCP.
| PLASTIC STENT PLACEMENT TECHNIQUE | FCSEMS PLACEMENT TECHNIQUE |
|---|---|
|
|
To date, 4 RCTs [44–47] have investigated this specific comparison between MPS and transpapillary-placed SEMS in patients with post-LT ABS. The stricture resolution rate with SEMS in these four studies was around 83–100% and the stricture recurrence rate was 14–32%, which does not differ significantly from MPS numbers. In a systematic review [48] of these 4 RCTs (including 103 FCSEMS patients and 102 MPS patients), stricture resolution (OR 1.05, 95%CI 0.43–2,56), stricture recurrence (OR 2.37, 95%CI 0.54–10.38) and overall adverse events (OR 0.91, 95%CI 0.32–2.62) were similar between MPS and FCSEMS. Moreover, FCSEMS were associated with shorter total duration of stenting (mean difference [MD] −105 days, 95%CI −202 to −8 days) and fewer endoscopic procedures compared to MPS (MD −1.86, 95%CI −3.12 to −0.6). The existing evidence of achieving clinical success with SEMS with fewer ERCPs has also been corroborated by other RCTs focused on benign biliary strictures of other etiologies [49].
When evaluating the ideal duration for FCSEMS therapy, findings from one meta-analysis [50] and a five-year follow-up observational study [51] have suggested that stricture recurrence rates tend to decrease when FCSEMS are left in place for over 4 months, with an optimal range of stent indwelling time between 4 and 6 months. Beyond this six-month period, the likelihood of tissue overgrowth and stent embedment increases and some experts advise for FCSEMS exchange.
One concern with the use of FCSEMS in ABS may be its migration rate, ranging from 10% to 33% [44–46]. To minimize this, some authors have analyzed the potential anti-migration effect of intraductal fully covered metallic stents (ID-FCSEMS) for treating post-LT ABS. Two large retrospective studies [52,53] have evaluated recently the performance of ID-FCSEMS in post-LT ABS. One study reported the UK experience and included 162 patients [52]. The ID-FCSEMS was placed for a median of 15 weeks and the stricture resolution was 81%. Of these patients with stricture resolution, 10% had stricture recurrence, which occurred after a median of 19 weeks. The migration rate reported of ID-FCSEMS occurred in 2% of cases. The other study retrospectively compared ID-FCSEMS vs. MPS and included 80 patients (44 with ID-FCSEMS and 36 with MPS) [53]. The resolution and recurrence rates of ABS in patients treated with ID-FCSEMS were consistent with previously reported data (93% and 33%, respectively), whereas stent migration occurred in 16% of cases.
Latest guidelines [25,26,36] support the use of FCSEMS slightly in favor to MPS as the preferred stent for patients with biliary strictures after LT, with a low to moderate quality of evidence. Robust data regarding the use of ID-FCSEMS is still lacking and current guidelines do not make specific statements on this matter.
4.3Endoscopic treatment of non-anastomotic biliary stricturesPost-LT NABS are more complex and challenging to manage endoscopically due to the fact that they are often multiple, diffuse, have small caliber and normally are located in the proximal or intrahepatic biliary tree of the graft [17,19]. Endoscopic therapy for extrahepatic NABS typically consists of ERCP and sphincterotomy followed by stricture dilation and placement of plastic stents. Plastic stents are replaced every three months for approximately one year, and usually these strictures require prolonged treatment including BD and removals of casts and debris. The stricture resolution rate reported with NABS is around 35–65% [22,54,55], although the number of studies is limited and the follow-up is usually short.
Patients with NABS strictures have an increased risk of poor graft survival [22] and imply worse prognosis when they develop early after LT [54], generally related to ischemic cholangiopathy provoking severe bile duct injury. Certain patients may require additional interventions to address NABS, including procedures such as percutaneous transhepatic cholangiography or surgical revisions. Even with optimal endoscopic treatment, up to 50% of patients with NABS continue to experience progressive biliary disease, ultimately leading to retransplantation or death [22,54,55].
4.4Monitoring after endoscopic therapyAfter stricture resolution, patients undergo regular monitoring with liver function tests every one to three months. If liver enzyme levels exceed their baseline values, one potential explanation is the recurrence of the stricture and an imaging test (preferably MRCP) is obtained for further evaluation. Patients with post-LT bile duct strictures need ongoing surveillance and clinical follow-up due to the possibility of stricture recurrence. Clinical observations indicate that risk factors for recurrence include initial occurrence of the stricture beyond 6 months after LT surgery, the presence of very tight ABS and NABS.
5ConclusionsBiliary strictures after LT a common adverse event after the surgery. The most frequent are ABS which are single, short, and isolated. Most patients have asymptomatic elevations in liver enzymes, with cholestasis. The evaluation of a suspected biliary stricture generally starts with an abdominal ultrasound followed by an MRCP. Therapy is usually performed with ERCP including biliary dilatation of the anastomosis along with plastic or FCSEMS placement with a stricture resolution rate > 80%. After stricture resolution, patients must regularly follow-up with liver function tests every one to three months.
FundingAndrés Cárdenas is funded by the Instituto de Salud Carlos III and Plan Estatal de Investigación Ciéntifica y Técnica y de Innovación (Grant No PI19/00752) and has received funding for this work by “Fundación Marta Balust”.
Author contributionsAlex Bofill (AB) conducted extensive literature search and wrote the review. Andres Cardenas (AC) defined the scope of the project, provided guidance during revision and ensured the paper's quality.