Introduction and aim. In chronic hepatitis B (CHB) patients with equivocal indication for antiviral therapy, therapeutic decision currently depends on histopathology of the liver. We aimed to evaluate if acoustic radiation force impulse (ARFI) in conjunction with aspartate transaminase to platelet ratio index (APRI) and fibrosis-4 (FIB-4) score could replace liver biopsy to indicate treatment for CHB.
Material and methods. We prospectively enrolled 101 clinically non-cirrhotic patients whose serum alanine aminotransferase was mildly elevated (1-2 folds above the upper normal limit) despite a high viral load (HBV DNA > 2,000 IU/mL). All participants underwent liver biopsy, and measurement of ARFI, APRI and FIB-4. The ability of the markers to distinguish fibrosis ≥ METAVIR F2 was evaluated.
Results. According to histopathology, liver fibrosis was METAVIR F0 in 2 (2.0%), F1 in 43 (42.6%), F2 in 34 (33.7%), F3 in 16 (15.8%), and F4 in 6 (5.9%) patients, and was correlated with ARFI (p = 0.0001), APRI (p = 0.012), and FIB-4 (p = 0.004). The six patients with cirrhosis were included for analysis, and received antiviral therapy. The C statistics of ARFI, APRI, and FIB-4 for fibrosis ≥ F2 were 0.70 (95% confidence interval [CI], 0.59-0.80), 0.62 (95% CI, 0.51-0.73), and 0.64 (0.53-0.75), respectively. The cut-off values for 95% sensitivity and 95% specificity to identify significant fibrosis were 0.97 m/sec and 1.36 m/sec for ARFI, 0.36 and 1.0 for APRI, 0.63 and 2.22 for FIB-4, respectively. Using a combination of these 3 indices, 44 patients (43.6%) could be spared a liver biopsy procedure.
Conclusions. A combination of ARFI, APRI, and FIB-4 may spare some CHB patients with equivocal indication for antiviral treatment a liver biopsy.
Chronic hepatitis B (CHB) is a leading cause of end-stage liver disease and hepatocellular carcinoma around the world. Significant advances in antiviral therapy for CHB over the past two decades have resulted in the use of interferon-based therapy and nucleos(t)ide analogues (NAs), which have been shown to inhibit virus replication and may effectively improve clinical outcomes. Serum levels of viral DNA, and elevated levels of alanine aminotransferase (ALT), in the absence of hepatic decompensation, liver cirrhosis, or immune suppression are currently used as therapeutic indications for antiviral treatment.
Serum viral load and ALT elevation are not always consistent with each other, and when there is a discrepancy, major international guidelines suggest reliance on histopathology obtained by biopsy to guide antiviral treatment.1–3 Initiation of medication is recommended in patients with substantial liver fibrosis. The desire to avoid invasive procedures has resulted in a tremendous enthusiasm for developing non-invasive methods that can replace liver biopsies.4,5 Previous studies have shown that tissue elastography can be used to discriminate the severity of liver fibrosis through measurement of shear wave velocity by acoustic radiation force impulse (ARFI) image,6 and blood-based indices such as Aspartate transaminase to platelet ratio index (APRI)7,8 and fibrosis-4 (FIB-4) score.9–11 However, the performance of these indices in guiding indications for antiviral therapy in CHB has not been elucidated.
In this study, we prospectively recruited CHB patients with discrepant serum gradients of viral DNA and ALT. None of the patients had liver cirrhosis, hepatic decompensation, immunosuppression, or other clear indications for antiviral treatment. We performed liver biopsies and measured ARFI, APRI, as well as FIB-4 levels in all participants in order to evaluate the performance of these non-invasive methods in distinguishing patients with or without significant liver fibrosis.
Materials And MethodsStudy design and patientsThis cross-sectional observational study recruited a total of 101 CHB patients at a teaching hospital in southern Taiwan (E-Da Hospital, Kaohsiung, Taiwan) between February 7, 2012 and July 24, 2014. Inclusion criteria were: age > 20 years, history of CHB for more than 6 months, serum HBV DNA > 2,000 IU/mL, highest serum ALT > 1 fold of upper limit of normal (ULN), but < 2 X ULN on at least two occasions (≥ 3 months apart) in the preceding one year. Exclusion criteria were: co-infection with human immunodeficiency virus, hepatitis C virus (HCV), or hepatitis D virus (HDV), previous exposure to antiviral treatment for CHB, prior diagnosis of liver cirrhosis by conventional ultrasound or clinical evidence of portal hypertension, hepatic decompensation, malignant diseases, organ transplantation, or bleeding tendency. The study protocol was approved by the institutional review board of E-Da Hospital (EMRP36100N).
Methods of measurementParticipants were interviewed and physically examined. Blood tests included hemogram, biochemistry, determination of serology markers of hepatitis B virus (HBV), and determination of serum viral load. Percutaneous liver biopsy was done under real-time sonographic guidance. Histopathological specimens were evaluated independently by an experienced pathologist (I-Wai Chang) who was blinded to the patients’ clinical information. The blood-based indices for liver fibrosis, APRI and FIB 4, were calculated from their original formulae as previously described.12,13 APRI was derived as follows: (aspartate aminotransferase [AST]/ ULN)/platelets (PLT) x 100. FIB 4 score was derived as follows: age [yr] X AST [U/L])/((PLT [109/L]) X (ALT [U/L])1/2).
ARFI was measured by the same examiner (YCH) on the day of liver biopsy. ARFI is a unique mode for tissue elasticity available in the commercialized ultrasound machine (Acuson S2000; Siemens AG, Germany). ARFI measurement was focused in the right lobe of liver through intercostal spaces when patients were lying flat. The probe was placed approximately 2 cm below the liver capsule, over segments 8 or 5, and away from ductular or vascular structures. Shear wave velocity of the targeted area was captured at a motionless image when the patient held breathing. The measurement was repeated 10 times in all patients, and was represented by the median value.
Outcome measuresThe primary outcome was liver fibrosis stage as evaluated by METAVIR scoring system. Based on current practice guidelines which recommend antiviral therapy in patients with a METAVIR fibrosis score of 2 points or more (≥ F2), our participants were categorized into two groups according to their fibrosis status (the F0 or F1 group and the ≥ F2 group).
Data analysis and statistical methodsQuantitative data were summarized as mean ± standard deviation (SD) and categorical variables as percentages. Fisher’s exact test was used to compare proportions of categorical variables. Unpaired and paired student’s t-tests were used to compare means of continuous variables between groups and within groups respectively. The diagnostic accuracy of blood tests and ARFI for liver fibrosis was evaluated by plotting the receiver operating characteristic (ROC) curve, and calculating the area under the curve (AUC). We reported the sensitivity, specificity, positive predictive value, and negative predictive value, of the non-invasive strategy for determination of antiviral indication. All tests were two-tailed. A P value less than 0.05 was considered to be statistically significant.
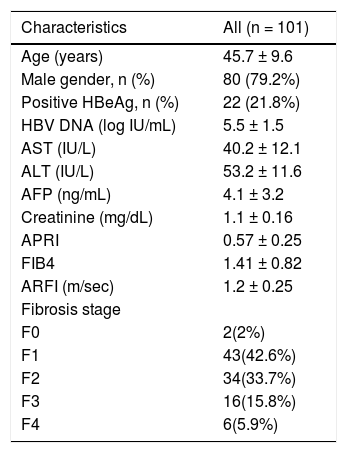
ResultsDemographic characteristics of the study subjectsThis study screened 154 patients without a clear indication for antiviral therapy, and a total of 101 participants met the inclusion and exclusion criteria and were enrolled in this study. Baseline characteristics of these participants are shown in table 1. The mean serum viral load was greater than 105 IU/mL. Twenty two (21.8%) patients remained hepatitis B e-antigen positive. According to liver histopathology, a majority of the patients were staged at either METAVIR F1 (n = 43, 42.6%) or F2 (n = 34, 33.7%). Of note, although cirrhosis was documented on the liver tissue samples of 6 patients, it was not disclosed by clinical assessment prior to biopsy.
Characteristics of participants.
| Characteristics | All (n = 101) |
|---|---|
| Age (years) | 45.7 ± 9.6 |
| Male gender, n (%) | 80 (79.2%) |
| Positive HBeAg, n (%) | 22 (21.8%) |
| HBV DNA (log IU/mL) | 5.5 ± 1.5 |
| AST (IU/L) | 40.2 ± 12.1 |
| ALT (IU/L) | 53.2 ± 11.6 |
| AFP (ng/mL) | 4.1 ± 3.2 |
| Creatinine (mg/dL) | 1.1 ± 0.16 |
| APRI | 0.57 ± 0.25 |
| FIB4 | 1.41 ± 0.82 |
| ARFI (m/sec) | 1.2 ± 0.25 |
| Fibrosis stage | |
| F0 | 2(2%) |
| F1 | 43(42.6%) |
| F2 | 34(33.7%) |
| F3 | 16(15.8%) |
| F4 | 6(5.9%) |
HBV: Hepatitis B virus. AST: aspartate aminotransferase. ALT: alanine transaminase. AFP: alpha-fetoprotein. APRI: aspartate transaminase to platelet ratio index. FIB-4: fibrosis-4. ARFI: acoustic radiation force impulse.
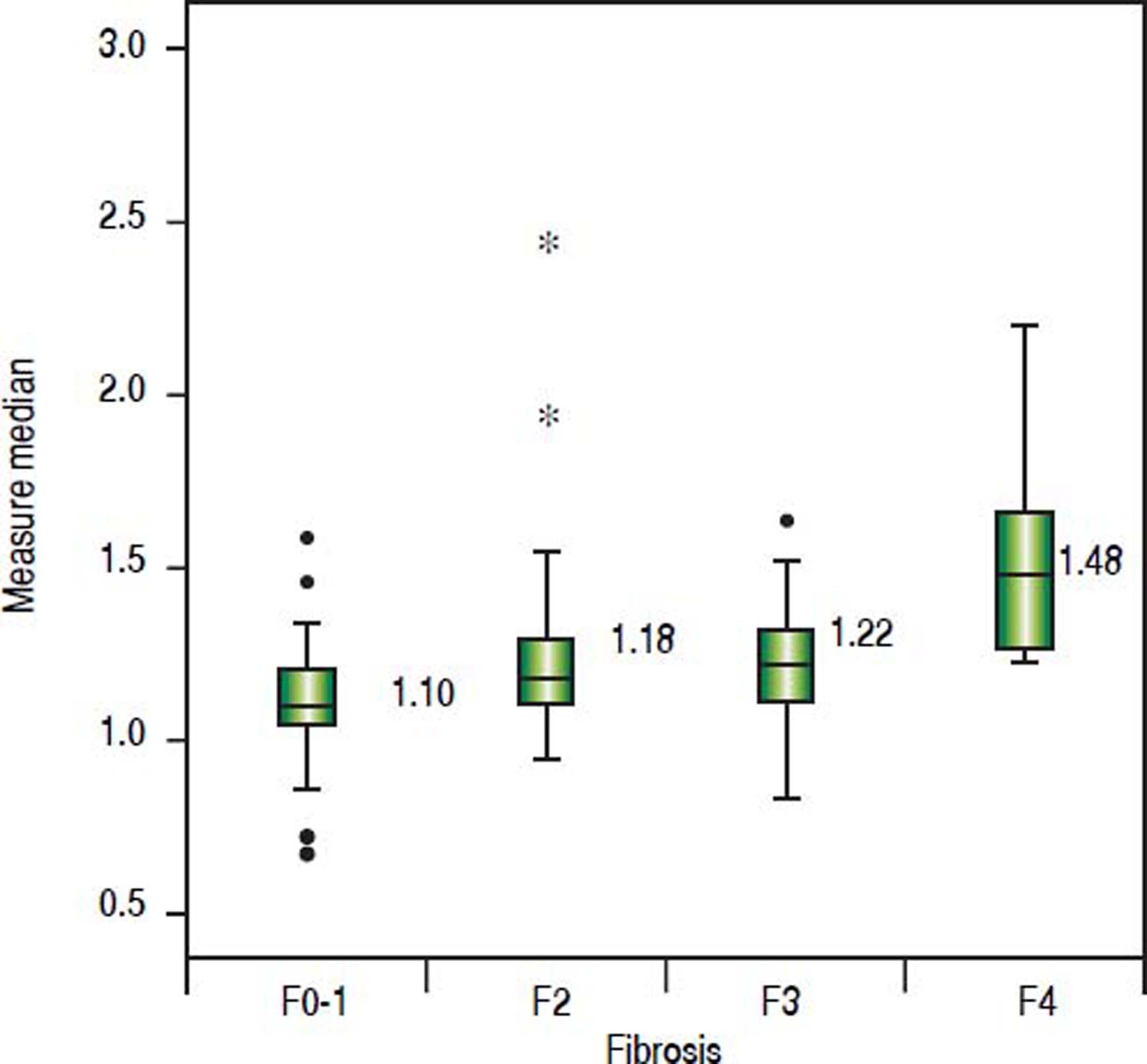
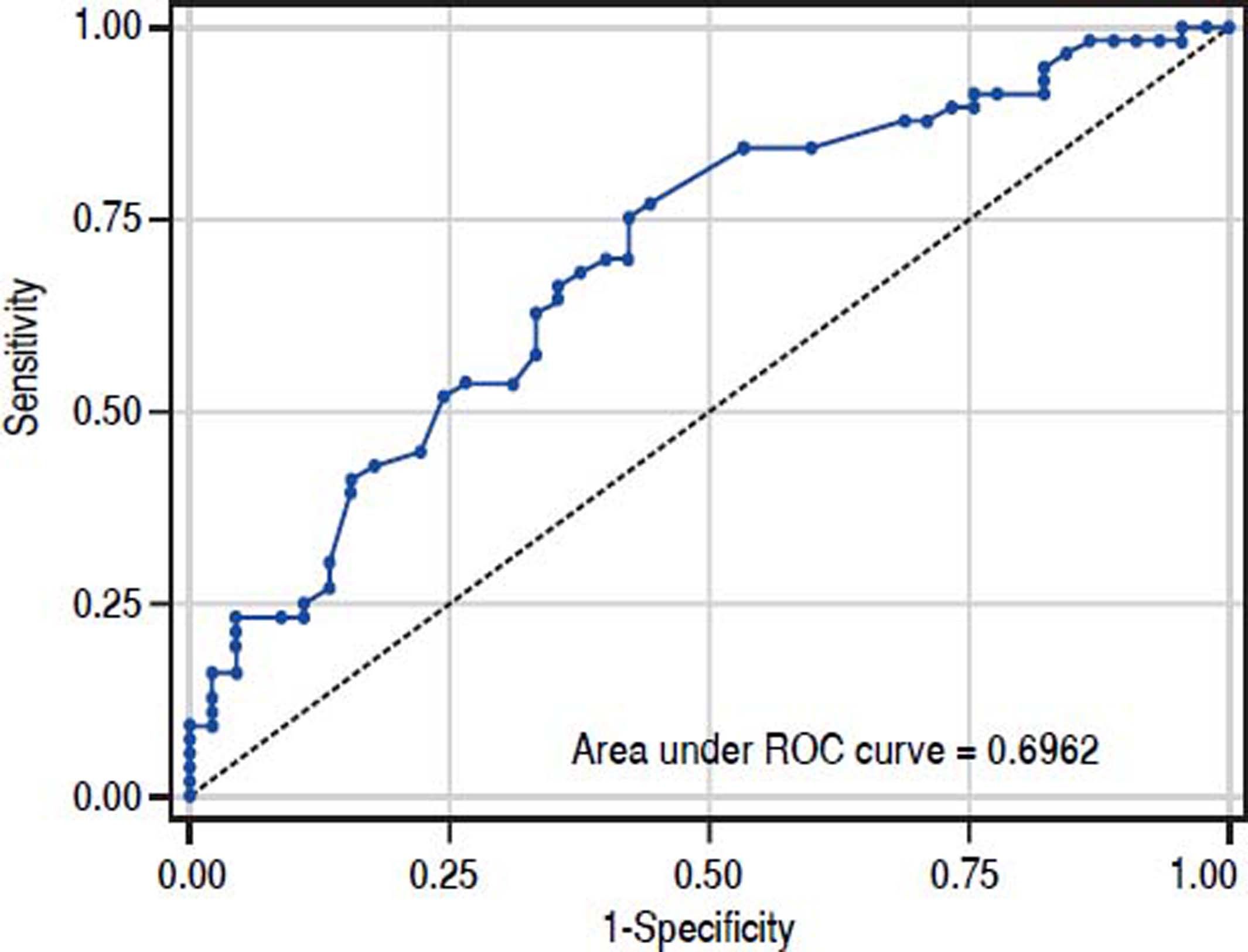
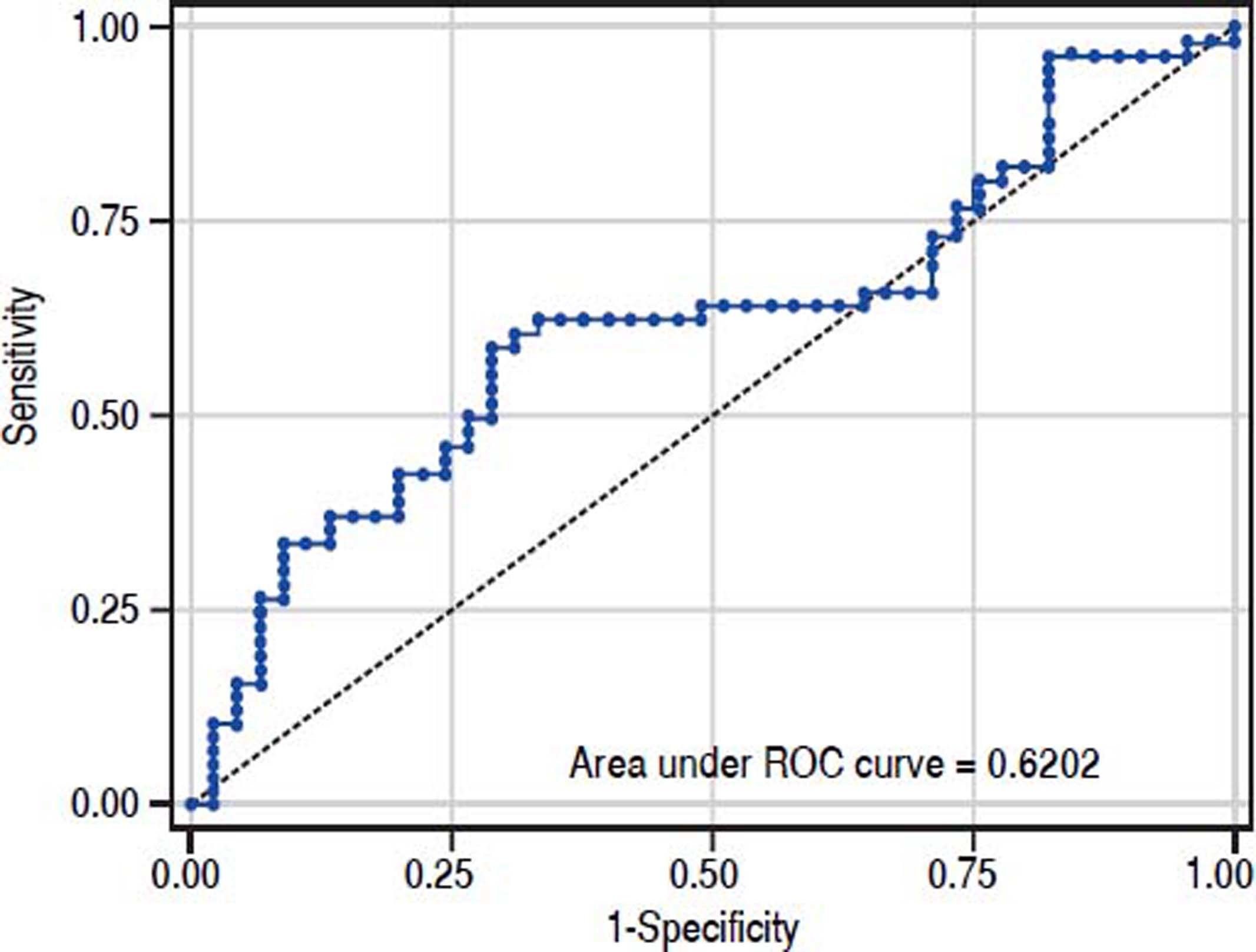
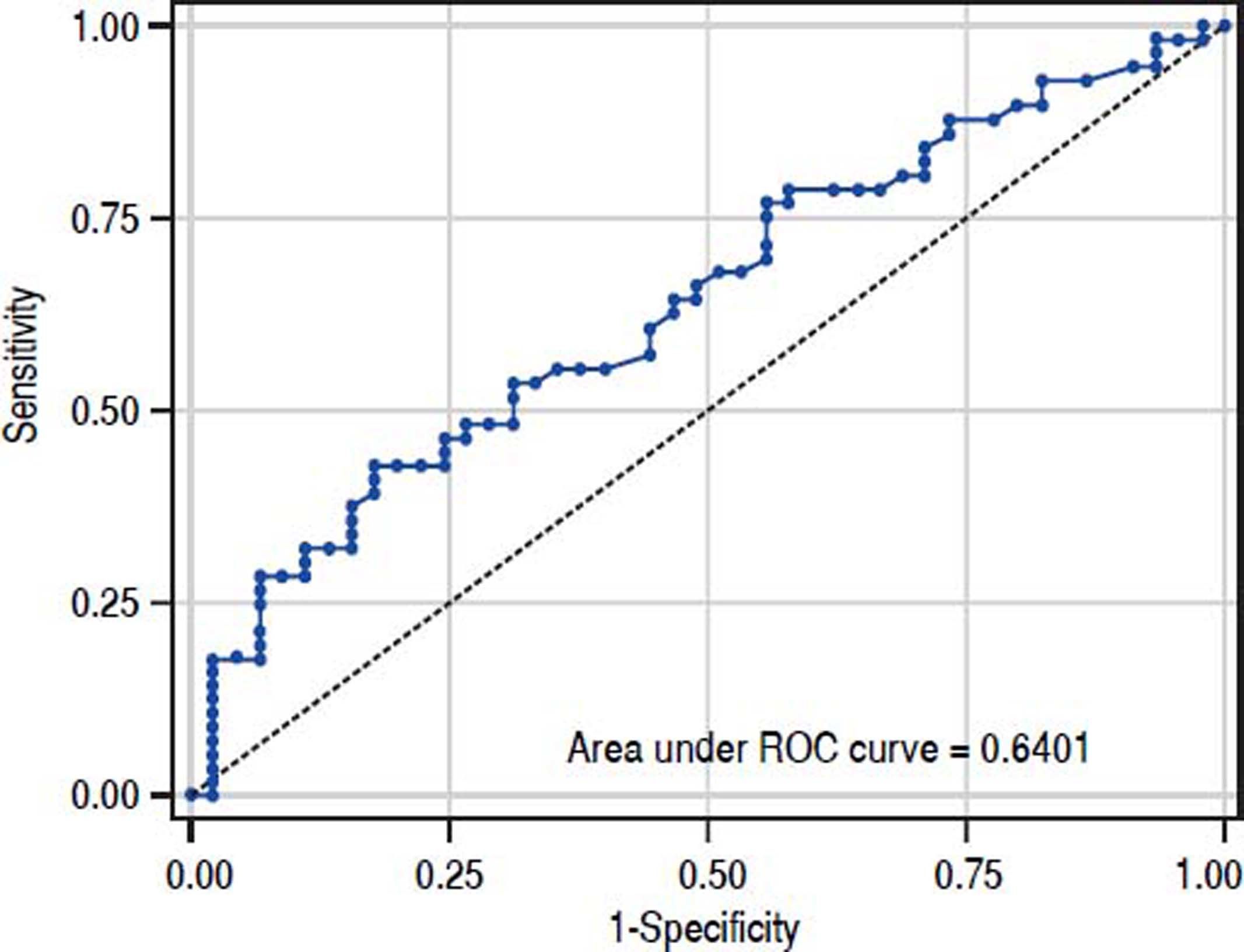
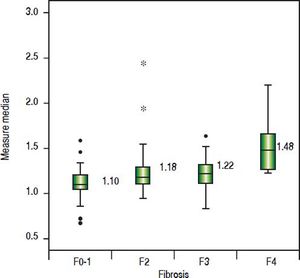
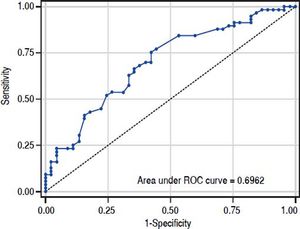
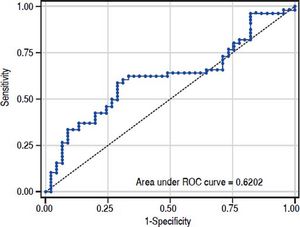
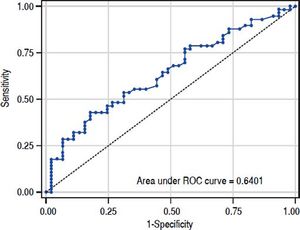
The value of ARFI measurement increased with the severity of liver fibrosis (Figure 1). There was a significant correlation between all 3 non-invasive assessments and fibrosis stage, with the Spearman’s ρ of 0.38 for ARFI (p = 0.0001), 0.25 for APRI (p = 0.012), and 0.28 for FIB-4 (p = 0.004). The C statistics of ARFI (Figure 2), APRI (Figure 3), and FIB-4 (Figure 4) for fibrosis stage ≥ 2 were 0.70 (95% confidence interval [CI], 0.59-0.80), 0.62 (95% CI, 0.51-0.73), and 0.64 (95% CI, 0.53-0.75), respectively.
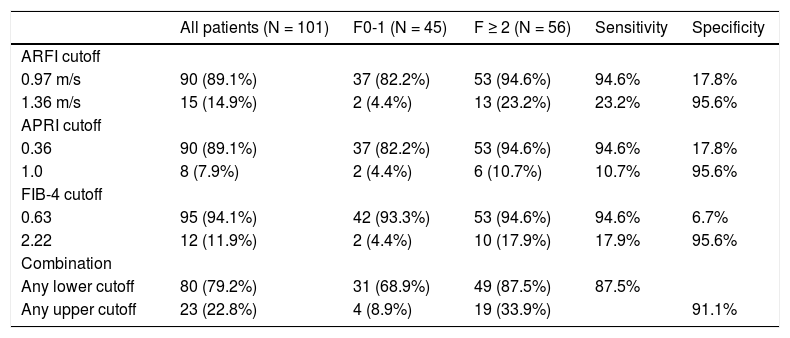
Cutoff values of ARFI, APRI, and FIB-4 to spare liver biopsyThe measured value with 95% sensitivity and that with 95% specificity for fibrosis ≥ F2 were chosen as the lower and upper cutoffs to identify patients who may be spared liver biopsy, since significant liver fibrosis was very unlikely when the measurement fell below the lower cutoff but almost certain when it was above the upper one. The upper and lower cutoff points to distinguish significant liver fibrosis were 0.97 m/sec and 1.36 m/sec for ARFI, 0.36 and 1.0 for APRI, 0.63 and 2.22 for FIB-4, respectively (Table 2). A combination of these 3 non-invasive methods would spare liver biopsy in a total of 44 (43.6%) patients who had measurements below the lower cutoff, or above the upper cutoff, with an overall sensitivity of 87.5% and specificity of 91.1%.
Sensitivity and specificity of ARFI, APRI, FIB-4, and the combination to distinguish significant liver fibrosis (METAVIR F ≥ 2).
| All patients (N = 101) | F0-1 (N = 45) | F ≥ 2 (N = 56) | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| ARFI cutoff | |||||
| 0.97 m/s | 90 (89.1%) | 37 (82.2%) | 53 (94.6%) | 94.6% | 17.8% |
| 1.36 m/s | 15 (14.9%) | 2 (4.4%) | 13 (23.2%) | 23.2% | 95.6% |
| APRI cutoff | |||||
| 0.36 | 90 (89.1%) | 37 (82.2%) | 53 (94.6%) | 94.6% | 17.8% |
| 1.0 | 8 (7.9%) | 2 (4.4%) | 6 (10.7%) | 10.7% | 95.6% |
| FIB-4 cutoff | |||||
| 0.63 | 95 (94.1%) | 42 (93.3%) | 53 (94.6%) | 94.6% | 6.7% |
| 2.22 | 12 (11.9%) | 2 (4.4%) | 10 (17.9%) | 17.9% | 95.6% |
| Combination | |||||
| Any lower cutoff | 80 (79.2%) | 31 (68.9%) | 49 (87.5%) | 87.5% | |
| Any upper cutoff | 23 (22.8%) | 4 (8.9%) | 19 (33.9%) | 91.1% |
This prospective study showed ARFI, APRI, and FIB correlated with degree of liver fibrosis among non-cirrhotic CHB patients with high viral load and slightly elevated ALT. After combination of these three methods, 44(43.6%) patient could spare liver biopsy. To our knowledge, it is the first article that focuses on this population, in which the degree of liver fibrosis is crucial to determine antiviral therapy.
APRI and FIB-4 are biomarkers with high applicability and goof reproducibility for fibrosis evaluation. However, the value might be affected by some factors such as inflammation, hemolysis, and Gilbert syndrome. Liver stiffness measurement is another kind of modality.4 ARFI is an ultrasound-based elastography method that is integrated into a conventional ultrasound machine. Comparing to transient elastography (TE), ARFI is more technic-dependent, however, it can be easily applied in a routine abdominal ultrasound practice, and it overcomes the limitations of TE, such as obesity and ascites.4 Besides, ARFI has been shown to have a higher rate of reliable measurements compared to TE.5 However, it had some disadvantage included narrow range of value, inadequate accuracy to discriminate intermediated stage fibrosis and, not well validated as TE, APRI, and FIB-4.5 Besides, it could be affected by body mass index.14
In the present study, APRI tended to present less accurate result than ARFI and FIB-4, which was correlated with other reports.15,16 However, the predictive accuracy of the three modalities were all lower than previous studies. The area under RCO for significant fibrosis of ARFI, FIB-4, and APRI were 0.7, 0.64, and 0.62 respectively. Reviewing previous studies, the area under ROC for significant fibrosis ranged from 0.70-0.91,0.74-0.76, and 0.72-0.79 for ARFI, FIB-4, and APRI respectively.14–19 In fact, previously reported results were inconsistent as well. Liu, et al.15 reported that ARFI and APRI had acceptable predictive value for fibrosis stage (area under ROC: 0.91 and 0.79). However, Tai, et at.20 reported the cirrhosis predictive accuracy of ARFI was worse than conventional ultrasound in CHB patients, and ARFI was influenced by liver inflammation. A meta-analysis17 showed APRI had limited value in CHB patients. The different targeted population might mainly contribute to the discrepancy between our results and others. Comparing to previous reports, our patient population was very homogeneous. Most of them were clinically non-cirrhotic CHB patients with high viral load and slightly elevated ALT (1–2 folds of upper normal limit). Besides, most of the patients were classified as F1(42.6%) or F2 (33.7%). In contrast, the background conditions were very heterogeneous in previous studied with wide range ALT level and fibrosis distribution. Furthermore, the ARFI measurement was performed on the same day of liver biopsy in our study. On the other hand, the period between ARFI measurement and liver biopsy might be up to 4 weeks in one previous study.18
The strength of this prospective study was its strict inclusion criteria among a homogeneous and unique population. However, this study also had some important limitations. First, we recognize that the sample size is relatively small, which precludes the development of a multivariate-adjusted model. Second, external validation by an independent cohort is necessary before the reported cutoff points can be applied in daily practice. Third, as it remains debatable whether percutaneous needle biopsy can serve as the gold standard to stage hepatic fibrosis, we cannot exclude possible variability in the sampling of liver tissue and interpretation of data.21–23
In conclusion, our study demonstrated that measurements of ARFI, APRI, and FIB-4 were modestly correlated with severity of liver fibrosis in a unique CHB population composed of non-cirrhotic patients with mildly elevated ALT despite high viral load. Combination of these non-invasive methods may spare “some” but not all patients for a liver biopsy.
Abbreviation- •
ALT: alanine aminotransferase.
- •
APRI: aspartate transaminase to platelet ratio index.
- •
ARFI: acoustic radiation force impulse.
- •
AST: aspartate aminotransferase.
- •
AUC: area under the curve.
- •
CHB: chronic hepatitis B.
- •
CHC: chronic hepatitis C.
- •
CI: confidence interval.
- •
FIB-4: fibrosis-4.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HDV: hepatitis D virus.
- •
NAs: nucleos(t)ide analogues.
- •
PLT: platelet.
- •
ROC: operating characteristic.
- •
SD: standard deviation.
- •
TE: transient elastography.
- •
ULM: upper limit of normal.
The grand is supported by E-Da Hospital (EDAHP104003), Tomorrow Medical Foundation (105–2), and the Ministry of Science and Technology (MOST-105-2314-B-650-001-MY2)
AcknowledgementThe authors are grateful to “Liver Disease Prevention & Treatment Research Foundation, Taiwan” for the support of the study and Ms. Ying-Ju Lee for her efficient assistance