Introduction. Bacterial infection is a frequent complication in patients with decompensated liver cirrhosis and is related to high mortality rates during follow-up of these individuals. We sought to evaluate the diagnostic value of C-reactive protein (CRP) and procalcitonin (PCT) in diagnosing infection and to investigate the relationship between these biomarkers and mortality after hospital admission.
Material and methods. Prospective study that included cirrhotic patients admitted to the hospital due to complications of the disease. The diagnostic accuracy of CRP and PCT for the diagnosis of infection was evaluated by estimating the sensitivity and specificity and by measuring the area under the receiver operating characteristics curve (AUROC).
Results. A total of 64 patients and 81 hospitalizations were analyzed during the study. The mean age was 54.31 ± 11.87 years with male predominance (68.8%). Significantly higher median CRP and PCT levels were observed among infected patients (P < 0.001). The AUROC of CRP and PCT for the diagnosis of infection were 0.835 ± 0.052 and 0.860 ± 0.047, respectively (P = 0.273). CRP levels > 29.5 exhibited sensitivity of 82% and specificity of 81% for the diagnosis of bacterial infection. Similarly, PCT levels > 1.10 showed sensitivity of 67% and specificity of 90%. Significantly higher levels of CRP (P = 0.026) and PCT (P = 0.001) were observed among those who died within three months after admission.
Conclusion. CRP and PCT were reliable markers of bacterial infection in subjects admitted due to complications of liver cirrhosis and higher levels of these tests are related to short-term mortality in those patients.
Bacterial infections are common in patients with liver cirrhosis, especially in the decompensated phase.1 This complication quadruplicates the mortality risk, with 30% of patients who acquire bacterial infection dying within a month.2 The higher prevalence of infection among patients with advanced degree of liver dysfunction is probably related to abnormalities in the immune system associated with increased bacterial translocation, including impairment of macrophage Fcγ-receptor-mediated clearance of antibody coated bacteria, deficiencies in the complement system, down-regulation of monocyte HLA-DR expression and depressed neutrophil phagocytic and intracellular killing.3–5
Recognizing bacterial infection in patients with cirrhosis may be difficult as the value of SIRS criteria for the detection of sepsis in cirrhosis is decreased.6 Hyperdynamic circulation leads to tachycardia in the absence of infection, patients receiving beta-blockers have a reduced heart rate, hepatic encephalopathy may cause tachypnea, and hypersplenism decreases white blood cell count.7 Additionally, the bacterial cultures require 24-48 h for providing any results, thus delaying diagnosis and treatment.8 Therefore, the identification of other clinical and laboratory parameters for the early diagnosis of bacterial infection would be of special interest in those patients.
Over the last few years, inflammatory biomarkers, such as C-reactive protein (CRP) and procalcitonin (PCT) have been investigated as tools for early diagnosis of bacterial infection in a variety of clinical settings.9–11 It is known that CRP and PCT are mostly produced by the hepatocytes, and its serum levels increases rapidly in response to non-specific inflammation.12,13 Thus, it is possible that the performance of these biomarkers could be impaired in patients with hepatic dysfunction.14,15 Nevertheless, some studies have shown that CRP and PCT are reliable markers for the diagnosis of bacterial infection in patients with chronic liver disease.16–18
The high admission rates for bacterial infection in patients with decompensated liver cirrhosis justify the search for biomarkers that can provide an early diagnosis, and allow appropriate treatment. In addition, given the poor outcome of infectious complications in those subjects, these tests could also be related to higher mortality rates, becoming important tools in prognostic evaluation of patients with cirrhosis admitted due to complications of chronic liver disease. In this context, the purpose of the present study was to evaluate the performance of CRP and PCT for the diagnosis of bacterial infection in patients with decompensated liver cirrhosis, and to investigate the association between theses biomarkers and short-term mortality.
Material and MethodsPatientsThis is a prospective study that included all patients admitted to the emergency room of a Brazilian tertiary hospital due to acute complications of liver cirrhosis (ascites, hepatic encephalopathy and upper gastrointestinal bleeding secondary to portal hypertension). Patients in the following situations were excluded: hospitalization for elective procedures, admissions not related to complications of liver cirrhosis, doubtful diagnosis of liver cirrhosis, use of antibiotics for a period longer than 12 h before sampling for laboratory tests and refusal or inability of the patient or responsible to understand the terms of informed consent.
The diagnosis of cirrhosis was established either histologically (when available) or by the combination of clinical, imaging, and laboratory findings in patients with evidence of portal hypertension.
The study protocol complies with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee on Human Research of the Federal University of Santa Catarina.
MethodsPatients were evaluated within 12 h of admission by one of the researchers involved in the study, and the following clinical variables were collected: age, gender, race, current alcohol intake (defined as any alcohol intake in the month prior to hospitalization), illicit drug use, associated diseases, prophylactic antibiotics, cirrhosis etiology, current and previous decompensation. All subjects underwent laboratory evaluation at admission, and the following tests were performed for this study: total leukocytes, neutrophils, sodium, creatinine, albumin, total bilirubin, international normalized ratio (INR) and CRP. An aliquot of the serum sampled at admission was stored at -20 °C for PCT measurement. Evaluation of mortality on the seventh day and at the third month after admission was performed by phone call, in case of hospital discharge.
Individuals with suspected infection at hospital admission were submitted to clinical examination to confirm this diagnosis and to establish the primary source of infection. A retrospective analysis of medical records was also performed to assess the presence of infection.
The diagnosis of infection was made according the criteria of the Center for Diseases Control.19 Spontaneous bacterial peritonitis (SBP) was diagnosed when the neutrophil count of the ascitic fluid was ≥ 250 neutrophils/mm3 in the absence of intraabdominal source of infection, regardless of negative culture.20 The diagnosis of systemic inflammatory response syndrome (SIRS) and sepsis was established based on the definition of the American College of Chest Physicians/Society Of Critical Care Medicine (ACCP/SCCM) and on diagnostic criteria of the committee of the Consensus Conference in 1992.21 Hepatic encephalopathy was graded according to the West-Haven criteria.22 Severity of liver disease was estimated by the Child-Pugh classification system23 and MELD (Model for End-Stage Liver Disease)24 calculated based on laboratory tests performed on admission.
Determination of serum CRP and PCTCRP and PCT measurements were performed on samples collected on hospital admission. CRP serum concentrations were analyzed by immunonephelometry with the reagent CardioPhase® hsCRP and a Siemens BNII device (analytical sensitivity of 0.175 mg/L). Quantitative analysis of PCT was performed by imunochemiluminescence using Siemens Advia-Centaur XP device and the reagent ADVIA Centaur® BRAHMS PCT (quantitation limits between 0.02 and 75 ng/mL).
Statistical analysisThe normality of the variable distribution was determined by the Kolmogorov-Smirnov test. Continuous variables were compared using Student’s t test in the case of normal distribution or Mann-Whitney test in the remaining cases. Categorical variables were evaluated by chi-square test. The diagnostic accuracy of CRP and PCT was analyzed by ROC curves (receiver operating characteristics). Based on the ROC, the best cutoffs points to predict the presence of infection were chosen. The sensitivity, specificity, positive and negative predictive values were calculated for the selected cutoffs. Comparisons of the ROC curves were performed by MedCalc software version 9.3 (MedCalc Software, Mariakerke, Belgium) using the technique described by Hanley & McNeil.25 A p value of < 0.05 was considered statistically significant. All tests were two-tailed and were performed by the SPSS software, version 17.0 (SPSS, Chicago, IL, USA).
ResultsSample characteristicsWe evaluated all hospital admissions due to decompensated liver cirrhosis during the period between December 2010 and November 2011. Twenty admissions were excluded because of antibiotics use for a period longer than 12 h before sampling for laboratory tests. Thus, the final sample was composed of 64 patients and a total of 81 admissions.
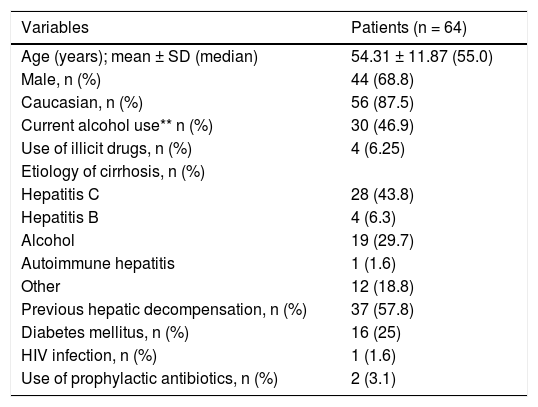
Table 1 exhibits the characteristics of the included patients. The mean age was 54.31 ± 11.87 years, 87.5% were Caucasians, and a male predominance was observed (68.8%). Current alcohol consumption and a history of illicit drug abuse were reported by 46.9 and 6.5% of the patients, respectively. The main causes of liver cirrhosis were hepatitis C (43.8%) and alcohol abuse (29.7%). Other causes of cirrhosis included: cryptogenic (n = 5), hepatitis B (n = 4), non-alcoholic steatohepatitis (n = 3), primary biliary cirrhosis (n = 3), hemochromatosis and autoimmune hepatitis (n = 1). Previous history of cirrhosis decompensation was observed in 37 (59.7%) patients, while two subjects (3.1%) were on antibiotic prophylaxis.
Clinical and epidemiological characteristics of patients hospitalized with decompensated cirrhosis.*
| Variables | Patients (n = 64) |
|---|---|
| Age (years); mean ± SD (median) | 54.31 ± 11.87 (55.0) |
| Male, n (%) | 44 (68.8) |
| Caucasian, n (%) | 56 (87.5) |
| Current alcohol use** n (%) | 30 (46.9) |
| Use of illicit drugs, n (%) | 4 (6.25) |
| Etiology of cirrhosis, n (%) | |
| Hepatitis C | 28 (43.8) |
| Hepatitis B | 4 (6.3) |
| Alcohol | 19 (29.7) |
| Autoimmune hepatitis | 1 (1.6) |
| Other | 12 (18.8) |
| Previous hepatic decompensation, n (%) | 37 (57.8) |
| Diabetes mellitus, n (%) | 16 (25) |
| HIV infection, n (%) | 1 (1.6) |
| Use of prophylactic antibiotics, n (%) | 2 (3.1) |
SD: standard deviation. HIV: human immunodeficiency virus.
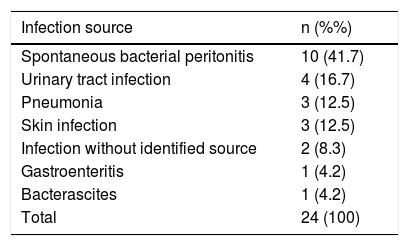
Infections were diagnosed in 24 admissions (29.6%) and sepsis was observed in 13 (54.2%) of those. The most frequent infections were SBP (41.7%) and urinary tract infection (16.7%) (Table 2). Infection was confirmed by culture in 14 (58.3%) admissions, and Escherichia coli and Enterococcus sp. were found in 72% of the positive cultures. Other isolated bacteria included: Klebsiela pneumoniae, Staphylococcus sp., Streptococcus sp., and Morganella morganii.
Source of infection in patients with decompensated liver cirrhosis admitted to the emergency unit.
| Infection source | n (%%) |
|---|---|
| Spontaneous bacterial peritonitis | 10 (41.7) |
| Urinary tract infection | 4 (16.7) |
| Pneumonia | 3 (12.5) |
| Skin infection | 3 (12.5) |
| Infection without identified source | 2 (8.3) |
| Gastroenteritis | 1 (4.2) |
| Bacterascites | 1 (4.2) |
| Total | 24 (100) |
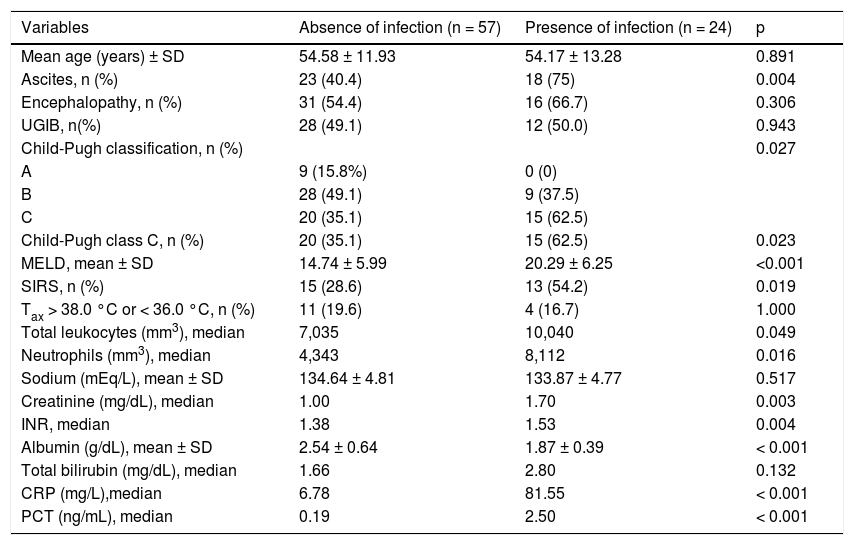
The infected patients were compared to the uninfected ones (Table 3). The median age was similar between the groups. On the other hand, infection at admission was associated with significantly higher MELD scores and a higher rate of individuals classified as Child-Pugh C. No differences between the two groups were noted regarding the presence of hepatic encephalopathy (p = 0.306) and upper gastrointestinal bleeding (p = 0.943), while ascites and SIRS were more frequent in infected patients (p = 0.004 and p = 0.019, respectively).
Clinical and laboratory variables associated with infection on admission.
| Variables | Absence of infection (n = 57) | Presence of infection (n = 24) | p |
|---|---|---|---|
| Mean age (years) ± SD | 54.58 ± 11.93 | 54.17 ± 13.28 | 0.891 |
| Ascites, n (%) | 23 (40.4) | 18 (75) | 0.004 |
| Encephalopathy, n (%) | 31 (54.4) | 16 (66.7) | 0.306 |
| UGIB, n(%) | 28 (49.1) | 12 (50.0) | 0.943 |
| Child-Pugh classification, n (%) | 0.027 | ||
| A | 9 (15.8%) | 0 (0) | |
| B | 28 (49.1) | 9 (37.5) | |
| C | 20 (35.1) | 15 (62.5) | |
| Child-Pugh class C, n (%) | 20 (35.1) | 15 (62.5) | 0.023 |
| MELD, mean ± SD | 14.74 ± 5.99 | 20.29 ± 6.25 | <0.001 |
| SIRS, n (%) | 15 (28.6) | 13 (54.2) | 0.019 |
| Tax > 38.0 °C or < 36.0 °C, n (%) | 11 (19.6) | 4 (16.7) | 1.000 |
| Total leukocytes (mm3), median | 7,035 | 10,040 | 0.049 |
| Neutrophils (mm3), median | 4,343 | 8,112 | 0.016 |
| Sodium (mEq/L), mean ± SD | 134.64 ± 4.81 | 133.87 ± 4.77 | 0.517 |
| Creatinine (mg/dL), median | 1.00 | 1.70 | 0.003 |
| INR, median | 1.38 | 1.53 | 0.004 |
| Albumin (g/dL), mean ± SD | 2.54 ± 0.64 | 1.87 ± 0.39 | < 0.001 |
| Total bilirubin (mg/dL), median | 1.66 | 2.80 | 0.132 |
| CRP (mg/L),median | 6.78 | 81.55 | < 0.001 |
| PCT (ng/mL), median | 0.19 | 2.50 | < 0.001 |
SD: standard deviation. UGIB: upper gastrointestinal bleeding. MELD: model for end-stage liver disease. SIRS: systemic inflammatory response syndrome. INR: international normalized ratio. CRP: C-reactive protein. PCT: procalcitonin. Tax: axillary temperature.
Infection at admission was associated with higher median values of total leukocytes (p = 0.049), neutrophils (p = 0.016), creatinine (p = 0.003) and INR (p = 0.004). The mean serum albumin levels were lower in infected patients (p < 0.001), but no differences in serum sodium (p = 0.517) and total bilirubin (p = 0.132) was found between the groups.
The median serum CRP and PCT levels were 12.3 mg/L (mean 47.02 ± 69.95 mg/L) and 0.35 ng/mL (mean 2.93 ± 7.16), respectively. Infection at admission was associated with significantly higher median levels of CRP (81.55 vs. 6.78 mg/L, p < 0.001) and PCT (2.50 vs. 0.19 ng/mL, p < 0.001) as compared to the uninfected group. No statistical differences were observed when patients with infection confirmed by culture were compared to infected subjects with negative cultures regarding CRP and PCT levels (P > 0.05).
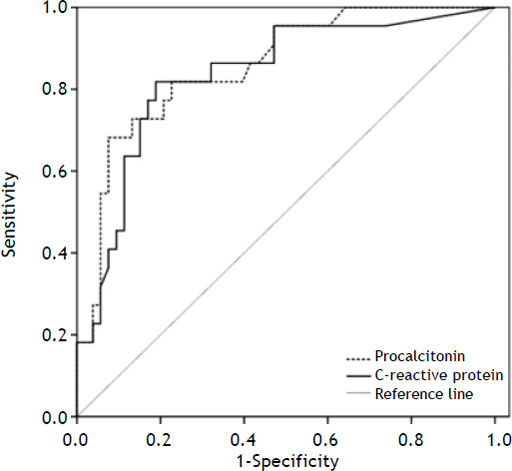
Performance of CRP and PCT for the diagnosis of infection at hospital admissionThe performance of serum CRP and PCT for the diagnosis of infection was evaluated by ROC curve (Figure 1). All hospitalizations were included in the analysis of the PCT accuracy; however, CRP levels were available in 75 admissions. The areas under the ROC curve for CRP and PCT for diagnosing infection were 0.835 ± 0.052 and 0.856 ± 0.047, respectively (p = 0.273).
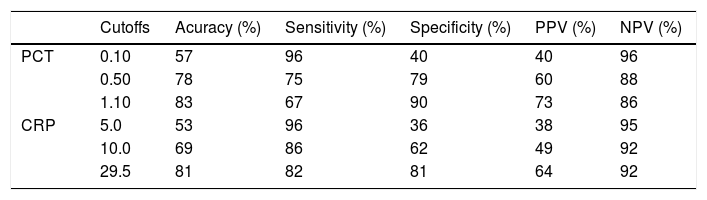
Based on the ROC curve, three cutoffs were chosen to predict the absence or presence of infection at admission (Table 4). The best overall performance for CRP was observed at a cutoff of 29.5 mg/L. This value exhibited accuracy, sensitivity and specificity of 81, 82, and 81%, respectively. Similarly, the best results for PCT were observed at a cutoff point of 1.10 ng/mL. This value exhibited accuracy, sensitivity and specificity of 83, 67, and 90%, respectively.
Diagnostic accuracy of C-reactive protein and procalcitonin in diagnosing infection at hospital admission.
| Cutoffs | Acuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| PCT | 0.10 | 57 | 96 | 40 | 40 | 96 |
| 0.50 | 78 | 75 | 79 | 60 | 88 | |
| 1.10 | 83 | 67 | 90 | 73 | 86 | |
| CRP | 5.0 | 53 | 96 | 36 | 38 | 95 |
| 10.0 | 69 | 86 | 62 | 49 | 92 | |
| 29.5 | 81 | 82 | 81 | 64 | 92 |
PCT: procalcitonin (ng/mL). CRP: C-reactive protein (mg/L). PPV: positive predictive value. NPV: negative predictive value.
The performance of CRP and PCT for identifying patients with infection decreased in those with more advanced liver disease. The AUROCs for CRP and PCT were 0.925 ± 0.040 and 0.949 ± 0.035 in Child-Pugh A/B group and 0.729 ± 0.094 and 0.709 ± 0.098 for Child-Pugh C subjects, respectively. CRP levels at a cutoff of 29.5 mg/L showed an accuracy of 88%, sensitivity of 89%, and specificity of 88% in the Child-Pugh A/B group and an accuracy of 72%, sensitivity of 77%, and specificity of 68% in Child-Pugh C patients. Similar findings were noted regarding PCT levels. In Child-Pugh A/B group, the cutoff of 1.10 ng/mL exhibited accuracy, sensitivity and specificity of 91, 78, and 95%, respectively. However, in Child-Pugh C subjects, the same value showed an inferior performance, with an accuracy of 71%, sensitivity of 60%, and specificity of 80%.
Association between CRP and PCT levels and the severity of liver diseaseWith respect to CRP concentrations and Child-Pugh classification, the study showed a trend towards higher median CRP levels in those patients classified as C when compared to Child-Pugh A/B patients (29.5 vs. 8.6 mg/L, p = 0.084). Likewise, the median of PCT concentrations was significantly higher in the Child C group than in the Child A/B group (0.57 vs. 0.19 ng/mL, p = 0.002). These results are similar even when evaluating only non-infected patients, with higher levels of CRP (19.7 vs. 6.3 mg/L, p = 0.047) and PCT (0.38 vs. 0.16 ng/mL, p = 0.002) in Child C group as compared to Child-Pugh A/B patients. Median values of CRP and PCT were also evaluated according to the MELD score. Patients with MELD score < 15 showed lower concentrations of CRP (6.6 vs. 41.1 mg/L, p = 0.002) and PCT (0.17 vs. 0.89 ng/mL, p < 0.001) as compared to those with MELD score > 15. The results are comparable also when only non-infected patients were evaluated, with higher levels of CRP (23.4 vs. 5.9 mg/L, p = 0.019) and PCT (0.37 vs. 0.16 ng/mL, p = 0.004) in those with MELD score > 15.
Association between inflammatory markers levels and short-term mortalityFor the assessment of mortality, patients admitted more than once were analyzed only regarding the last hospitalization. Ten patients (15.6%) died before the seventh day and 22 (34.9%) died before the ninetieth day after admission. One patient loss to follow-up and was excluded from the analysis. No association between infection at admission and death before the seventh day of hospitalization was observed (p = 1.000). The rate of infections among those who died before the day 90 after hospital admission was numerically higher as compared to those who survived; however, no statistical difference was observed (36.4 vs. 19.0%, p = 0.129).
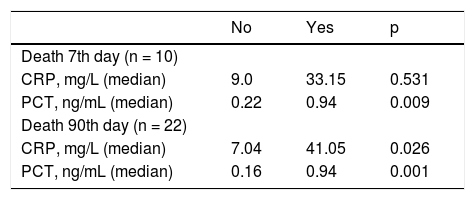
The correlation between CRP and PCT levels and death on the seventh and ninetieth days after admission was also evaluated (Table 5). No association was observed between CRP levels and death before the seventh day (p = 0.531). However, subjects who died before the seventh day showed significantly higher PCT levels as compared to survivors (0.22 vs. 0.94 ng/mL, p = 0.009). Similarly, significantly higher CRP (7.04 vs. 41.05 mg/L, p = 0.026) and PCT levels (0.16 ng/mL vs. 0.94 ng/mL, p = 0.001) were observed among those who died before the ninetieth day after admission as compared to the survivors. Additional variables related to death before the ninetieth day in univariate analysis were: MELD score (P < 0.001), creatinine (P < 0.001), INR (P < 0.001), albumin (P = 0.002), total bilirubin (P < 0.001), SIRS (P = 0.007), Child-Pugh C (P = 0.007), current alcohol use (P = 0.004) and hepatic encephalopathy (P = 0.014). Age, gender, previous decompensation, ascites, upper gastrointestinal bleeding, infection confirmed by positive culture, SBP, urinary tract infection, pneumonia and serum sodium were not related to mortality (P > 0.05).
Relationship between inflammatory markers levels and death before the seventh and ninetieth day after admission.
| No | Yes | p | |
|---|---|---|---|
| Death 7th day (n = 10) | |||
| CRP, mg/L (median) | 9.0 | 33.15 | 0.531 |
| PCT, ng/mL (median) | 0.22 | 0.94 | 0.009 |
| Death 90th day (n = 22) | |||
| CRP, mg/L (median) | 7.04 | 41.05 | 0.026 |
| PCT, ng/mL (median) | 0.16 | 0.94 | 0.001 |
CRP: C-reactive protein. PCT: procalcitonin. *Lost contact with one patient during 3-month follow-up.
Patients with liver cirrhosis are at high risk of several serious complications during hospitalization, including sepsis, acute respiratory failure, and death.26 Consequently, prompt diagnosis and treatment of infectious complications are essential to improve the prognosis of these individuals.27 Although there are previous data related to inflammatory markers levels for the diagnosis of bacterial infection in patients with cirrhosis, information about the usefulness of these tests in patients admitted for complications of the liver disease is still lacking.
Previous studies involving a variable number of patients found conflicting results regarding the analysis of CRP as a marker of infection in patients with liver cirrhosis, with areas under de ROC curve ranging from 0.641 to 0.930.16–18,28,29 An interesting fact observed in these studies was the difference in the cutoffs used to identify infection, with values raging between 9.2 and 80 mg/L. This significant variation can be explained by methodological differences across the studies, such as distinct inclusion criteria, differences in the severity of infection episodes, and the relatively small number of individuals studied. In the present study, the best cutoff for CRP evaluated by the ROC curve was 29.5 mg/L. Similarly, a cross-sectional study that evaluated 98 patients with liver cirrhosis admitted to an emergency unit suggested a CRP cutoff of 24.7 mg/dL as the best value for diagnosing bacteremia and sepsis in this population.16 This cutoff showed a sensitivity and specificity of 80% for the diagnosis of sepsis and sensitivity of 73% and specificity of 68% for predicting bacteremia.16 On the other hand, Papp, et al. prospectively evaluated 368 patients with liver cirrhosis admitted to a hospital unit and found that 10 mg/L was the best cutoff for CRP in diagnosing infection, with sensitivity of 84% and specificity of 91%.17 One must consider that 25% of patients in the Hungarian study had mild infections, mainly in the urinary tract.17 In our study, we noticed a considerable decrease in accuracy and specificity when using a CRP cutoff of 10 mg/L. This discrepancy can be explained by the high proportion of patients with severe infections in the present study, with more than half of infected subjects exhibiting signs of sepsis. It is important to highlight that the comparison of cut-off points across different studies may be hampered by methodological differences, including the clinical scenario and the diagnostic criteria for bacterial infection.
PCT has a favorable kinetic property, exhibiting a rapid peak of plasma levels (at around 6 h) after endotoxemia.30 and thus, potentially allowing an early diagnosis of bacterial infection and helping the diagnostic approach. However, as PCT (and also of CRP) is mainly synthesized by the liver, there were doubts about the usefulness of this biomarker in patients with cirrhosis. Bota, et al. prospectively evaluated 864 patients hospitalized in an intensive care unit, 79 of those with the diagnosis of liver cirrhosis.29 No differences were found between the concentrations of CRP and PCT in patients with or without liver cirrhosis, suggesting that although the liver is considered the main producer of these inflammatory biomarkers, its serum concentrations are not suppressed in patients with chronic liver failure.29 This idea is reinforced by our findings and by other studies that showed higher CRP and PCT levels in Child-Pugh C regardless the presence of bacterial infection.17
In the present study, the area under the ROC curve for PCT in the diagnosis of infection was 0.856. There are few studies evaluating the diagnostic accuracy of PCT in patients with chronic liver disease, but the results reported were in general favorable.16,17,28,29,31,32 In these studies, the area under the ROC curve ranged between 0.680 and 0.980, which is in agreement with our findings. In this study, the cutoff that showed the best overall performance was 1.10 ng/mL, with an accuracy of 83%, sensitivity of 67%, and 90% of specificity. Connert and colleagues prospectively evaluated 127 patients with liver cirrhosis with or without decompensation and reported the cutoff of 0.58 ng/mL as the most accurate for the diagnosis of infection in decompensated patients.31 In the study by Elefsiniotis, et al., PCT was measured in 106 patients hospitalized with acute or chronic liver disease, and the cutoff of 0.5 ng/mL showed the best accuracy in the diagnosis of bacterial infection.32 However, 46% of non-infected patients with alcoholic hepatitis and superimposed cirrhosis, as well as 30% of non-infected patients with acute viral hepatitis, especially those with significant fibrosis or cirrhosis, showed slightly higher PCT. The authors concluded that a cutoff value > 0.5 ng/mL is required for the differential diagnosis of infection in these particular groups of patients with liver disease.32 Hence, as discussed above for the CRP, it is likely that higher cutoffs show better performance in patients admitted to emergency units, who often present with more serious infections and other medical conditions, which can lead to increased serum levels of acute phase markers.
Despite the numerical difference, no statistically significant differences in mortality rates on the seventh and ninetieth days after hospital admission were found between infected and non-infected cirrhotic patients. These findings are probably justified by the relatively small sample size, given that previous studies have shown that infection causes a considerable deterioration in the prognosis of patients with chronic liver disease.33
In the present study, higher levels of CRP and PCT on admission were associated with mortality before the ninetieth day. These findings are similar to those reported by Cervoni, et al., who demonstrated that in patients with advanced liver cirrhosis, CRP levels ≥ 29 mg/L were independent predictors of six-month mortality.34 Moreover, in a study involving 368 patients with liver cirrhosis, higher CRP levels were also good predictors of infection in the next three months.17 Nevertheless, a retrospective study including 202 cirrhotic patients fail to demonstrate a relationship between CRP and mortality in 30 days.35 However, the decrease in CRP levels at four or five days after admission was related to lower mortality rates.35 Contradicting results were also found in studies evaluating the relationship between PCT levels and mortality. Connert, et al. demonstrated an association between PCT concentrations > 0.58 ng/mL and mortality after two months of follow-up.31 However, this correlation was not observed by Cervoni, et al., in a study that followed 148 cirrhotic patients with Child-Pugh score ≥ B8 for a longer period (up to six months).34 It is likely that the disparate results observed in the mentioned studies reflect the different clinical scenarios evaluated and specific characteristics of the study designs. Moreover, in the present study, the absence of multivariate analysis evaluating the factors independently related to mortality may limit the interpretation of the data. However, given the relatively small number of events (22 deaths), we cannot ensure a minimum number of events per variable to perform a logistic regression analysis including all variables of interest.
We acknowledge some limitations to our analysis. The relatively small number of patients and the inclusion of individuals who were readmitted to the hospital due to hepatic decompensation may allow the occurrence of selection bias, limiting the interpretation of the results. However, the number of patients included in most studies evaluating inflammatory markers for the diagnosis of infection in patients with hepatic cirrhosis was similar to our sample. Even so, the results presented herein require external validation by prospective studies with a greater number of patients, especially with regard to the chosen cutoffs of the acute-phase markers. Another limitation is the fact that our study was conducted in an emergency room without standardized infection diagnosis at the time of admission, which might have provided a misleading categorization of the study subjects. However, the prospective nature of this study, with follow-up of all patients included at hospital admission and the analysis of medical records, makes such a bias unlikely.
In conclusion, CRP and PCT showed good accuracy in diagnosing bacterial infection in cirrhotic patients admitted due to complications of the disease. Cutoffs higher than those previously suggested in the literature may be required, especially in the context of patients admitted to emergency rooms in whom more severe infections and other medical complications might be associated with significantly increased levels of acute-phase markers. The association between higher CRP and PCT levels and short-term mortality suggest that these biomarkers have a potential as prognostic predictors in patients with liver cirrhosis.
Potential Conflict of InterestNothing to report.
AcknowledgmentsThe authors would like to thank the Laboratório Médico Santa Luzia for the support in the determination of the serum procalcitonin levels.