Background and objective. Steatohepatitis is a common cause of liver disease due to alcohol (ALD) or non-alcoholic fatty liver disease (NAFLD). We performed this study to compare natural history of ALD and NAFLD.
Material and methods. Retrospective analysis of ALD or NAFLD patients managed at our center (2007-2011). ALD diagnosed by excluding other liver diseases (except HCV) and alcohol abuse of > 40 g/d in women and > 60 g/d in men for > 5 years. NAFLD diagnosed by excluding other liver diseases and a history of alcohol use of < 10 g/d. Cirrhosis was diagnosed using biopsy for uncertain clinical diagnosis.
Results. Compared to patients with NAFLD (n = 365; mean age 50 yrs; 43% males; 53% diabetic), ALD patients (n = 206; mean age 51 yrs; 68% males; 24% diabetic) presented more often with cirrhosis or complications(46 vs. 12%; P< 0.0001) with a higher MELD score (13 ± 7 vs. 8 ± 8; P<0.0001). On logistic regression, ALD diagnosis was associated with presence of cirrhosis by over 4-fold (4.1 [1.8-9.1]) even after excluding 23 patients with concomitant HCV. Over median follow up of about 3 and 4 yrs among ALD and NAFLD patients respectively, ALD patients more frequently developed cirrhosis or its complications including HCC with worse transplant free survival (90 vs. 95%; P = 0.038).
Conclusions. Compared to NAFLD, ALD patients present at an advanced stage of liver disease with a faster progression on follow-up. Prospective multicenter studies are needed to identify potential barriers to early referral of ALD patients as basis for development of strategies to improve outcome of patients with ALD.
Alcoholic liver disease (ALD) related to alcohol abuse and non-alcoholic fatty liver disease (NAFLD) related to metabolic syndrome,are leading causes of liver cirrhosis and indications for liver transplantation after hepatitis C virus (HCV) infection.1,2 Both these diseases share the liver disease pathophysiology with seemingly similar histologic finding of steatohepatitis. However, the clinical phenotype and risk factors of these diseases are different.3–5
The spectrum of liver disease from ALD or NAFLD-may range from fatty liver, asymptomatic elevation of liver enzymes, cirrhosis with its complications, and hepatocellular carcinoma.5,6 The worldwide mortality from alcoholic liver disease (ALD) is about 4% of mortality and about 5% of disability.7 With rising epidemic of obesity, about 12-30% of NAFLD patients may have non-alcoholic steatohepatitis (NASH) with a potential for development of cirrhosis in about 25% of these patients.3,8–10
Early referral with management and control of underlying risk factors(alcohol abstinence for ALD and obesity with metabolic syndrome for NAFLD) is crucial in disease prevention and improve outcomes.3,5,11 However, data are scant comparing ALD and NAFLD patients for initial presentation and natural history on follow-up. To test our hypothesis that ALD and NAFLD differ in presentation and natural history, we performed this retrospective analysis of patients with steatohepatitis related liver disease seen and managed at our center.
Material and MethodsStudy populationThe study was approved by the Institutional Review Board at our center. Patients seen in the liver clinic during at a single tertiary referral center (2007-2011) with the diagnosis of ALD or NAFLD were retrospectively analyzed. Of 607 patients in our database seen for a diagnosis of ALD (ICD-09 code 571.0-571.3) or NAFLD (ICD-09 code 571.5, 571.8, 571.9), 36 obese patients with metabolic syndrome and consuming alcohol in excess of 10 g/d were excluded from further analysis.3,12
Definitions- •
Alcoholic liver disease. Exclusion of other liver diseases (except HCV) and documenting history of alcohol abuse with minimum intake of > 40 g/d in women and > 60 g/d in men for over 5 years.7,13
- •
Non-alcoholic fatty liver disease. Presence of fatty liver on liver imaging and/or elevated liver enzymes along with exclusion of other liver diseases and documented alcohol use of < 10 g/d.3,12 Patients undergoing liver biopsy and showing features of steatohepatitis were diagnosed with NASH.
- •
Alcohol use.Defined in g/d as calculated from average number of drinks consumed per day in mL. One drink defined as 12 ounces of beer, 5 ounces of wine, and 1.25 ounces of hard liquor was considered equivalent to 15 g of pure alcohol.7
Medical charts of 571 patients (365 NAFLD) were reviewed for patient demographics (age, gender and race, body mass index or BMI); dates of onset of symptoms and of diagnosis; comorbidities (diabetes, hypertension, dyslipidemia, cholecystectomy); metabolic syndrome; and alcohol intake in g/d. BMI was calculated using patient’s height and weight at the time of presentation was classified based on WHO criteria as normal (BMI 19 - 24.9), overweight (25 - 29.9), mild obesity (30 - 34.9), moderate obesity (35 - 39.9), and morbid obesity (≥ 40). Cholecystectomy was defined with history of cholecystectomy and/ or absent gall bladder on ultrasound examination of the abdomen. Metabolic syndrome was defined with presence of 3 or more of the following comorbidities: diabetes (patient being on medications or fasting blood sugar level >110 mg/dL), hypertension (patient being on antihypertensive medications or BP > 130/85 mm Hg), abdominal obesity (waist circumference > 40 inches in men or > 36 inches in women), low HDL (< 45 mg/dL in men or < 55 mg/dL in women), elevated triglycerides (>150 mg/dL).14 As body mass index (BMI) is linearly associated with increasing prevalence of metabolic syndrome, we used obesity (BMI ≥ 30) as surrogate for waist circumference in defining the metabolic syndrome.15 Data were also recorded for laboratory values: liver chemistry with model for end-stage disease (MELD) score; endoscopic findings: portal hypertensive gastropathy and esophageal varices; and liver histology: steatosis, lobular inflammation, hepatocyte ballooning, and fibrosis. Steatosis was graded based on proportion of hepatocytes containing fat as grade 0 (up to 5%); grade I (5 to < 33%); grade II (33 to < 66%); and grade III (> 66%). Similarly, lobular inflammation and hepatocyte ballooning were graded as none, mild to moderate, or severe. The stages of fibrosis were recorded as follows:16
- •
Stage 0: no fibrosis.
- •
Stage 1: portal fibrosis.
- •
Stage 2: peri-portal fibrosis.
- •
Stage 3: bridging fibrosis.
- •
Stage 4: cirrhosis.
NAFLD Activity Scorewas calculated using respective scores for steatosis, lobular inflammation, and hepatocyte ballooning.17 In the absence of liver biopsy, ALD and NAFLD were diagnosed based on the clinical criteria detailed in the definitions section and exclusion of other liver diseases.
Study outcomes- •
Disease stage at presentation. Diagnosis at presentation was stratified based on presence or absence of cirrhosis or its complications. Cirrhosis was defined using clinical, hematological, and imaging criteria and/ or biopsy when available.18
- •
Development of cirrhosis or its complications. Follow-up information was collected for development of cirrhosis and liver disease complications (ascites, hepatic encephalopathy, variceal bleeding, or HCC). Patients diagnosed with cirrhosis at or within first 6 months of their presentation were excluded in order to consider for time gap in coding for cirrhosis diagnosis. Similarly, for assessing development of complications of cirrhosis, patients diagnosed with respective complication at or within 1 month of their presentation were excluded. Cirrhosis was diagnosed based on clinical or imaging evidence(ultrasonography, computed tomography, or magnetic resonance imaging) or biopsy for uncertain clinical diagnosis. HCC was diagnosed based on AASLD guidelines and criteria using CT/ MRI scan and/or biopsy.19
- •
Patient survival.Charts were reviewed for the survival status and confirmed with National Death Index using the social security number.
Time to development of specific outcome was calculated from date of its occurrence and date of diagnosis of respective disease (ALD or NAFLD).
Statistical analysesBaseline characteristics were compared for patients with ALD or with NAFLD using χ2 and Student’s t tests for categorical and continuous variables respectively. Logistic regression analysis was used to assess whether liver disease etiology (ALD vs. NAFLD) was predictive of cirrhosis presentation. Factors different between the two groups and clinically relevant were entered into the model. Cumulative curves were generated comparing ALD and NAFLD for development of outcomes (cirrhosis, complications of liver disease, and patient survival) after adjusting for age, gender, and MELD score from stratified cox proportional hazard models. Patients lost to follow-up and those without the event at the time of their last follow-up were censored. P values < 0.05 were considered significant. Analyses were performed using the Statistical Analyses Software (SAS Institute, Cary, NC).
ResultsComparison at initial presentation: ALD vs. NASH.- •
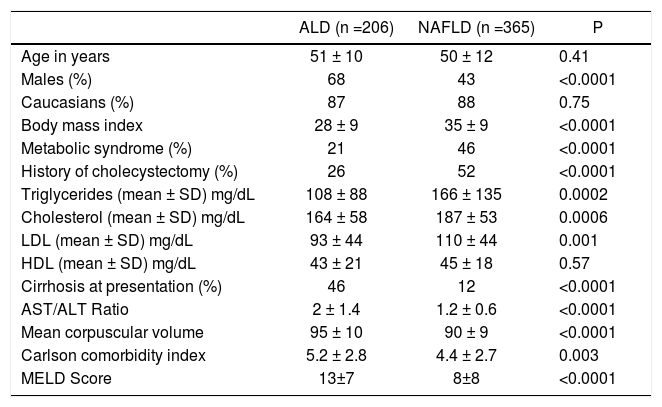
Baseline characteristics. On analysis of 571 patients, patients with NAFLD (n = 365) compared to those with ALD differed for being older, females, and higher BMI (Table 1). About 94% of NAFLD patients were either overweight or obese compared to 62% of ALD patients (P < 0.0001). Prevalence of metabolic syndrome was higher among NAFLD as compared to ALD patients with a higher proportion of diabetes mellitus (53 vs. 24%, P < 0.0001) and hypertension (60 vs. 49%, P = 0.011). Prevalence of cholecystectomy at the time of presentation was 2 fold higher among NAFLD as compared to ALD patients (52 vs. 26%, P < 0.0001). A total of 23 (12 ALD) patients had concomitant HCV infection. Compared to ALD, NAFLD patients had higher levels of serum triglycerides and total cholesterol with no differences on serum HDL levels (Table 1). On the other hand, ALD patients as compared to NAFLD had higher AST/ALT ratio and mean corpuscular volume (MCV) (Table 1).
Table 1.Baseline characteristics at presentation comparing alcoholic liver disease (ALD) patients with non-alcoholic fatty liver disease (NAFLD).
ALD (n =206) NAFLD (n =365) P Age in years 51 ± 10 50 ± 12 0.41 Males (%) 68 43 <0.0001 Caucasians (%) 87 88 0.75 Body mass index 28 ± 9 35 ± 9 <0.0001 Metabolic syndrome (%) 21 46 <0.0001 History of cholecystectomy (%) 26 52 <0.0001 Triglycerides (mean ± SD) mg/dL 108 ± 88 166 ± 135 0.0002 Cholesterol (mean ± SD) mg/dL 164 ± 58 187 ± 53 0.0006 LDL (mean ± SD) mg/dL 93 ± 44 110 ± 44 0.001 HDL (mean ± SD) mg/dL 43 ± 21 45 ± 18 0.57 Cirrhosis at presentation (%) 46 12 <0.0001 AST/ALT Ratio 2 ± 1.4 1.2 ± 0.6 <0.0001 Mean corpuscular volume 95 ± 10 90 ± 9 <0.0001 Carlson comorbidity index 5.2 ± 2.8 4.4 ± 2.7 0.003 MELD Score 13±7 8±8 <0.0001 LDL: low density lipoprotein. HDL: high density lipoprotein. SD: standard deviation. MELD: Model for End-Stage Disease.
- •
Disease status at initial presentation. Patients with ALD compared to NAFLD were more likely to have cirrhosis at initial presentation, as evaluated on imaging (ultrasound, computerized tomography, or magnetic resonance imaging) or biopsy when available, with a higher comorbidity index and MELD score (Table 1). Proportion of patients at the time of initial presentation were higher in ALD patients as compared to NAFLD for ascites (36 vs. 10%; P < 0.0001), hepatic encephalopathy (7 vs. 3%; P = 0.03), and variceal hemorrhage (10 vs. 4%; P = 0.004).On logistic regression analysis, diagnosis of ALD was associated with the presence of cirrhosis by over three fold (3.3 [1.3 - 8.2]). Other associations of cirrhosis at presentation were presence of metabolic syndrome and AST/ALT ratio (Table 2). Data remained unchanged after excluding 23 patients (12 ALD) with concomitant HCV infection.
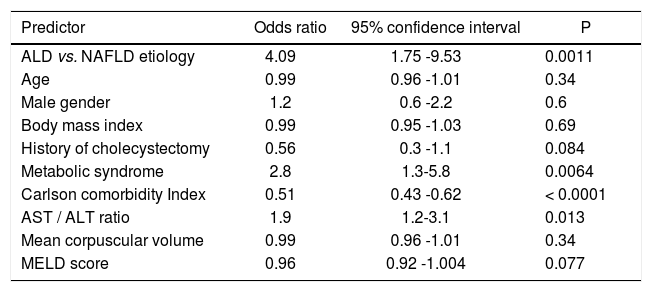
Table 2.Predictors of presence of cirrhosis among patients with steatohepatitis related liver disease.
Predictor Odds ratio 95% confidence interval P ALD vs. NAFLD etiology 4.09 1.75 -9.53 0.0011 Age 0.99 0.96 -1.01 0.34 Male gender 1.2 0.6 -2.2 0.6 Body mass index 0.99 0.95 -1.03 0.69 History of cholecystectomy 0.56 0.3 -1.1 0.084 Metabolic syndrome 2.8 1.3-5.8 0.0064 Carlson comorbidity Index 0.51 0.43 -0.62 < 0.0001 AST / ALT ratio 1.9 1.2-3.1 0.013 Mean corpuscular volume 0.99 0.96 -1.01 0.34 MELD score 0.96 0.92 -1.004 0.077 ALD: alcoholic liver disease. NAFLD: non-alcoholic fatty liver disease. MELD: Model for End-Stage Disease.
- •
Endoscopic findings. Endoscopic findings in 269 (137 NAFLD) showed higher prevalence in patients with ALD compared to NAFLD for portal hypertensive gastropathy (53 vs. 33%; P = 0.007) and esophageal varices (75 vs. 55%; P = 0.004). Prevalence of large varices was similar between ALD and NAFLD patients (6.8 vs. 8%; P = 0.36).
- •
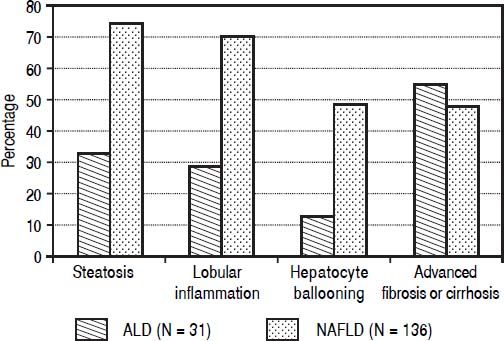
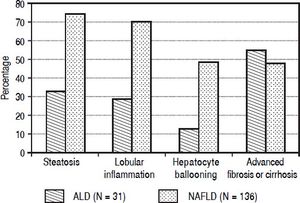
Histological findings. Histological data was available on 167 patients (136 NAFLD). These patients compared to 404 not subjected to liver biopsy differed for higher proportion with NASH diagnosis (81 vs. 57%, P < 0.0001) and history of cholecystectomy (49 vs. 29%, P = 0.0001). A higher proportion of patients with NAFLD compared to ALD patients had steatosis (75 vs. 33%, P < 0.0001). Steatosis prevalence was higher for grades I - II (51 vs. 19%, P < 0.0001) and similar for grade III (16 vs. 13%, P = 0.87). NAFLD patients compared to ALD had higher prevalence of obular inflammation (70 vs. 29%, P < 0.0001), and hepatocyte ballooning (49 vs. 13%, P = 0.0002) (Figure 1) with a higher NAFLD activity score (3.7 ± 1.5 vs. 2.8 ± 1.9; P = 0.045). Prevalence of fibrosis was similar between NAFLD and ALD patients (77 vs. 71%; P = 0.34). However, a higher proportion of ALD patients as compared to NAFLD had advanced fibrosis or cirrhosis (55 vs. 48%; P = 0.022) (Figure 1).
Figure 1.Histological findings comparing 31 alcoholic liver disease (ALD) and 136 non-alcoholic fatty liver disease (NAFLD) patients. Results show that ALD patients have higher prevalence of advanced fibrosis or cirrhosis compared to NAFLD patients (55 vs. 48%, P = 0.022). On the other hand NAFLD patients have higher prevalence of a) steatosis (75 vs. 33%, P < 0.0001) and b) inflammatory activity with lobular inflammation (70 vs. 29%, P < 0.0001) and hepatocyte ballooning (49 vs. 13%, P = 0.0002).
(0.04MB).
- •
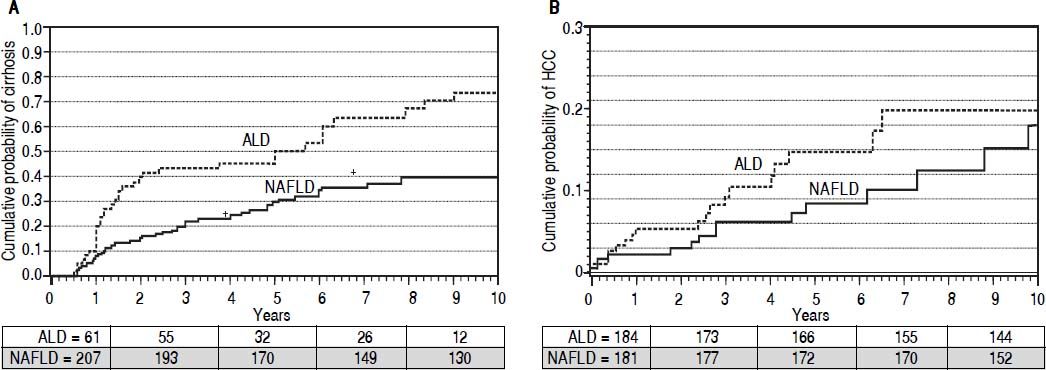
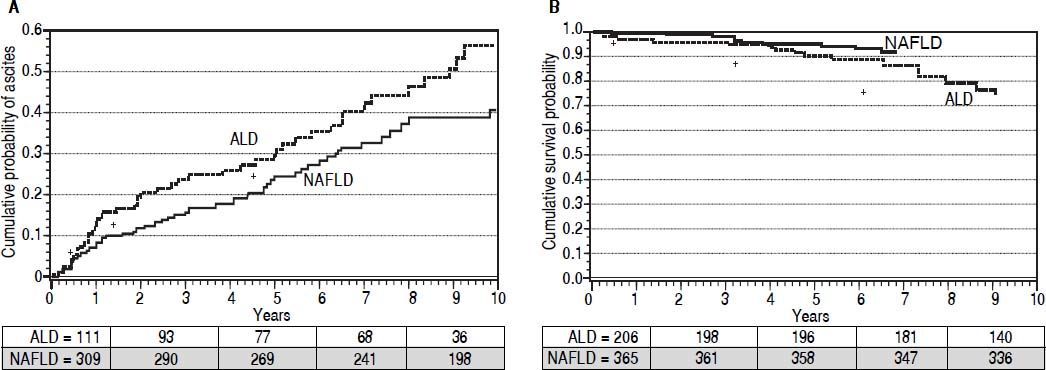
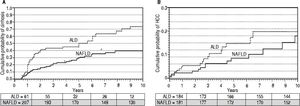
Development of cirrhosis and its complications. Patients were followed for up to 10 years from the time of disease diagnosis for development of cirrhosis or its complications. Cumulative curves were generated after adjusting for MELD score, age, and gender using cox proportional hazard regression model. In a cohort of 268 patients (201 NAFLD) without cirrhosis at or within 6 months of presentation, cumulative probability of cirrhosis development was higher in ALD patients (67 vs. 34%; P < 0.0001) (Figure 2A). A total of 33 cirrhotic (20 ALD) developed HCC with higher cumulative probability in ALD patients (22 vs. 16%; P < 0.0001) at 10 years of follow-up (Figure 2B). In a cohort of 420 patients without ascites at or within a month of presentation, 113 (51 ALD) developed ascites. Cumulative probability of ascites was higher among ALD patients (49 vs. 24%; P = 0.003) at 10 years of follow-up (Figure 3A). A total of 122 (55 ALD) and 43 (20 ALD) patients developed hepatic encephalopathy and variceal bleeding respectively in out of 511 patients without hepatic encephalopathy and 508 patients without variceal bleeding at or within month of initial presentation. Although, probability of development of both these complications was also higher at 10 years among ALD patients compared to NAFLD, MELD adjusted cumulative probability was similar comparing ALD and NAFLD patients for hepatic encephalopathy(30 vs. 25%; P = 0.22)and variceal bleeding (13 vs. 10%; P = 0.12)(adjusted curves not shown).
Figure 2.Adjusted * curves comparing alcoholic liver disease (ALD, black line) and non-alcoholic fatty liver disease (NAFLD, gray line) for cumulative probability of development of cirrhosis (A) and hepatocellular carcinoma or HCC (B). For assessment of liver cirrhosis on follow up, patients with cirrhosis at or within 6 months of diagnosis were excluded. Over median follow-up periods of 2 and 3.4 years for ALD and NAFLD patients respectively, ALD patients have higher probability of development of cirrhosis (67vs.34%, P < 0.0001). Similary, over median follow-up periods of 2.8 and 3.5 years respectively for ALD and NAFLD, cumulative probability of HCC was higher among ALD patients (13vs.9%, P = 0.001). Numbers in the table below each curve represent the number of patients at 0, 3, 5, 7, and 10 years respectively. * Estimated from the stratified cox proportional hazard regression adjusted for etiology (ALD or NAFLD), age, gender, and MELD score.
(0.06MB).Figure 3.Adjusted * curves comparing alcoholic liver disease (ALD, black line) and non-alcoholic fatty liver disease (NAFLD, gray line) for cumulative probability of development of ascites (A) and for patient survival (B). For assessment of ascites on follow up, patients with cirrhosis at or within a month of diagnosis were excluded. Over median follow-up periods of 3 and 4 years for ALD and NAFLD patients respectively, ALD patients have higher probability of development of ascites (49vs.24%, P = 0.003). Similarly, over median follow-up periods of 2.8 and 3.9 years respectively for ALD and NAFLD, probability of survival was lower among ALD patients (90vs.95%, P = 0.038). Numbers in the table below each curve represent the number of patients at 0, 3, 5, 7, and 10 years respectively. * Estimated from the stratified cox proportional hazard regression adjusted for etiology (ALD or NAFLD), age, gender, and MELD score.
(0.06MB). - •
Overall survival and need for liver transplantation. A total of 35 patients (21 ALD) died on follow-up of up to 10 years with worse survival in ALD patients after adjusting for baseline MELD score (90 vs. 95%; P = 0.038) (Figure 3B). A total of 35 patients received liver transplant (19 ALD) with no difference between the groups (P = 0.26). Five year post-transplant survival was also similar in the two groups (75 vs. 86%; respectively = 0.46).
- •
Subgroup analysis among ALD patients. Compared to 78 non-obese patients with ALD, 128 obese ALD patients had higher prevalence of esophageal varices (91 vs. 68%, P = 0.001) with similar prevalence of cirrhosis (56 vs. 51%, P = 0.56) at the time of initial presentation. On follow-up, there were no differences on the disease progression comparing the obese and non-obese alcoholics.
Main findings from this study are that ALD patients as compared to NAFLD.
- •
Present at an advanced stage of liver disease with cirrhosis and/or its complications.
- •
Have higher prevalence forportal gastropathy and/ orvarices on endoscopy and advanced fibrosis or cirrhosis on liver biopsy.
- •
Progress faster after adjusting for MELD score with more frequent development of cirrhosis and/or its complications; and
- •
Have worse patient transplant free survival.
Many studies have compared ALD and NAFLD for clinical and histological features with results similar to what we observed in our current analysis.1,20–23 To our knowledge, comparison of these two diseases for initial liver disease stage and natural history on follow-up has not been examined before. Single center retrospective medical chart review with strict case definitions and detailed information is also a strength of our study. However, our study suffers from limitations of a retrospective study design including lack of follow-up information on alcohol abstinence, body mass index, and control of comorbidities such as diabetes, hypertension, and dyslipidemia. Our study findings also need external validation given the potential referral bias with about 2/3rd of our cohort of steatohepatitis related liver disease due to NAFLD. Further, liver biopsy was more often performed among NAFLD compared to ALD patients. This is likely to be biased due to NAFLD patients more often presenting without cirrhosis compared to ALD patients and cholecystectomy more often performed among NAFLD patients, at which time surgeons took the liver biopsy from abnormal appearing liver.
Interpretation of study resultsIncreasing awareness on NAFLD may partly explain less prevalent advanced disease in NAFLD patients. Whether higher prevalence of advanced disease in ALD is secondary to provider or patient referral bias remains a testable hypothesis. Faster progression of ALD in comparison to NAFLD may be speculated to be due to variations in the natural history of the two diseases or differences in control of risk factor/s for respective liver disease etiology. Lack of follow-up information on alcohol abstinence, patient compliance, and control of metabolic syndrome limited this analysis. Concomitant hepatitis C virus (HCV) infection is known to synergistically act with alcohol in causing higher prevalence of cirrhosis and faster progression of liver disease.24 Although, concomitant HCV tended to be more prevalent in ALD compared to NAFLD (4 vs. 2.8%, P = 0.1), in a cohort after excluding concomitant HCV, odds for presence of cirrhosis at the time of initial clinic visit were over 3 fold higher with diagnosis of ALD compared to NAFLD. ALD patients compared to NAFLD had worse transplant free survival. However, post-transplant survival was similar as reported in previous studies.1,20
Potential implications for clinicians and policy makersALD contributes to significant morbidity and mortality worldwide among young and middle aged population with a huge economic burden and impact on the quality adjusted life years.7Alcohol related liver disease compared to other liver diseases had remained under researched for many years.25 Our study findings could be related to factors such as barriers to referral of ALD patients and to alcohol abstinence. There is an urgent need for well-designed prospective studies to identify referral barriers of ALD patients and develop strategies to overcome these. The future seems bright and encouraging with the decision of National Institute of Alcohol and Alcoholism to preserve clinical and translational research on ALD.
Conflicts of InterestNone of the authors have any financial, professional, or personal conflicts to disclose.
Funding SourceFaculty development award by the American College of Gastroenterology to Ashwani K. Singal.