Spirulina platensis is a blue-green alga used as a dietary supplement because of its hypocholesterolemic properties. Among other bioactive substances, it is also rich in tetrapyrrolic compounds closely related to bilirubin molecule, a potent antioxidant and anti-proliferative agent. The aim of our study was to evaluate possible anticancer effects of S. platensis and S. platensis-derived tetrapyrroles using an experimental model of pancreatic cancer. The anti-proliferative effects of S. platensis and its tetrapyrrolic components [phycocyanobilin (PCB) and chlorophyllin, a surrogate molecule for chlorophyll A] were tested on several human pancreatic cancer cell lines and xenotransplanted nude mice. The effects of experimental therapeutics on mitochondrial reactive oxygen species (ROS) production and glutathione redox status were also evaluated. Compared to untreated cells, experimental therapeutics significantly decreased proliferation of human pancreatic cancer cell lines in vitro in a dose-dependent manner (from 0.16 g-L-1 [S. platensis], 60 μΜ [PCB], and 125 μΜ [chlorophyllin], p<0.05). The anti-proliferative effects of S. platensis were also shown in vivo, where inhibition of pancreatic cancer growth was evidenced since the third day of treatment (p < 0.05). All tested compounds decreased generation of mitochondrial ROS and glutathione redox status (p = 0.0006; 0.016; and 0.006 for S. platensis, PCB, and chlorophyllin, respectively). In conclusion, S. platensis and its tetrapyrrolic components substantially decreased the proliferation of experimental pancreatic cancer. These data support a chemopreventive role of this edible alga. Furthermore, it seems that dietary supplementation with this alga might enhance systemic pool of tetrapyrroles, known to be higher in subjects with Gilbert syndrome.
Spirulina platensis is a blue-green freshwater alga widely used as a dietary supplement. It is rich in proteins, carotenoids, essential fatty acids, vitamin B complex, vitamin E, and minerals such as copper, manganese, magnesium, iron, selenium, and zinc.1 S. platensis has garnered much attention not only because of its high nutritional value; but also, it is a source of potent antioxidants including spirulans (sulphated polysaccharides), selenocompounds, phenolic compounds, and phycobiliproteins (C-phycocyanin and allophycocyanin).1 In fact, numerous studies have demonstrated that dietary supplementation of S. platensis is helpful in the prevention and treatment of atherosclerosis, diabetes, and/or cancers (for review see Gershwin, 20082).
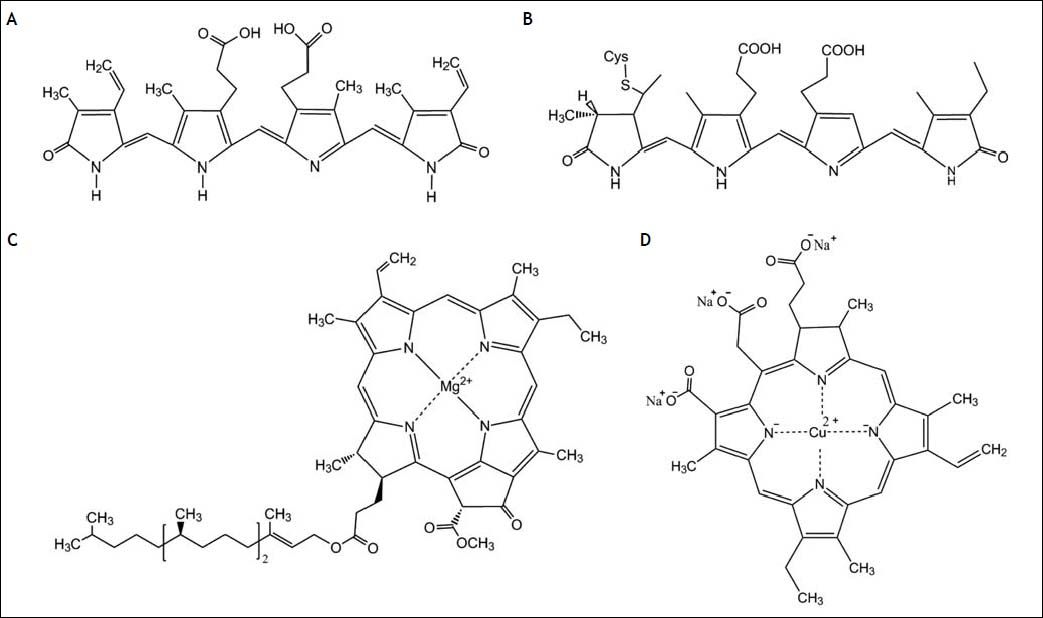
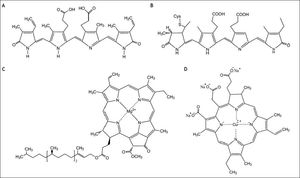
C-phycocyanin is a light-harvesting biliprotein possibly implicated in biological effects of S. platensis.2 C-phycocyanin contains a covalentlylinked chromophore called phycocyanobilin (PCB),3 a linear tetrapyrrolic molecule structurally resembling biliverdin, an antioxidant bile pigment (Figure 1). PCB can be metabolized by biliverdin reductase to phycocyanorubin similarly as biliverdin is reduced to bilirubin in the human body. Bilirubin is a known major antioxidant in blood and negative associations between serum levels and numerous oxidative stress-mediated diseases including cardiovascular, certain cancer and autoimmune diseases have been published in recent years.4–6 In fact, hyperbilirubinemic subjects with Gilbert syndrome were shown to have substantially lower risk of colon cancer,5,7 and iatrogenic increase of serum bilirubin levels was proposed as a plausible approach to prevent oxidative stress-mediated diseases.8 In addition, bilirubin has been reported to inhibit mitochondrial cytochrome c oxidase activity,9 which causes cells to undergo premature apoptosis mediated through mitochondrial depolarization, caspase-3 activation and increased expression of mitochondria-associated pro-apoptotic Bax protein,10 or even more profound changes in mitochondrial membrane integrity.11 Simultaneously, bilirubin was demonstrated to substantially inhibit NADPH oxidase activity,12,13 and the same inhibitory action was described also for phycocyanin and phycocyanobilin.13
Bilirubin is the major product of the heme catabolic pathway in the intravascular compartment, and its production is dependent on heme oxygenase activity (HMOX), the rate-limiting enzyme of this pathway. The inducible HMOX isoform (HMOX1, OMIM*141250), a member of the heat-shock protein HSP32 family, is activated by a number of oxidative stress-provoking stimuli. HMOX is also believed to be implicated in carcinogenesis, although our understanding of its exact role is still far from complete.14 It is also important to note that various tetrapyrrolic compounds are potent modulators of HMOX1.15
In addition to PCB, S. platensis is also rich in another bioactive tetrapyrrole compound, which is chlorophyll. Despite being one of the most abundant biomolecules on Earth, only scarce data exist on the possible anti-proliferative effects of chlorophyll. These reports, nevertheless, propose the potential use of chlorophyll as a chemopreventive agent,16 although detailed data are lacking. Chlorophylls, nonetheless, exert potent reactive oxygen species scavenging effects, as described in detail for chlorophyllin, a water-soluble analog of chlorophylls.17 Since the cell proliferation is strongly influenced by redox signaling,18 this antioxidant action of chlorophylls might account for their presumed anti-proliferative properties.
The aim of our study was to evaluate possible anticancer effects of S. platensis and its tetrapyrrolic components on the model of experimental pancreatic cancer. More specifically, our focus was placed on their potential impact on the heme catabolic p athway and mitochondrial redox metabolism.
Material and MethodsChemicalsS. platensis was purchased from Martin Bauer GmbH (Vestenbergsgreuth, Germany). Bilirubin and hemin were from Frontier Scientific (Logan, UT, USA), C-phycocyanin was purchased from ProZyme (Hayward, CA, USA), chlorophyllin (a water soluble, semi-synthetic derivate of chlorophyll commonly used in food industry, was employed in all in vitro studies as a surrogate for non-polar chlorophylls) and other chemicals were from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of a water extract of S. platensisDistilled water was added to freeze-dried S. platensis powder (30 mL-g-1 of alga). The suspension was sonicated for 15 min, incubated for 10 min at room temperature, centrifuged (8,000 x g, 30 min, 10 °C) and finally freeze-dried overnight. Prior to use, the extract was dissolved in culture medium and sterile-filtered.
PCB isolation from S. platensisPCB was isolated from freeze-dried S. platensis according to the method of Terry.19 Identification of PCB was confirmed by HPLC (Luna C8 column, 4.6-150 mm, 3m/100A, Phenomenex, Torrance, CA, USA; isocratic mobile phase with methanol/water/ TBA, 59:40:1 w/w/w, flow rate of 0.5 mL-min-1), and mass spectrometry.
Cell linesThe following pancreatic cancer cell lines were used for the in vitro studies: PA-TU-8902 (DSMZ, Braunschweig, Germany), Mia PaCa-2 and BxPC-3 (ATCC, Manassas, VA, USA). All cell lines were maintained in a humidified atmosphere (containing 5% at 37 °C) and in the following media supplemented with 10% fetal bovine serum (FBS): PA-TU- 8902 and Mia PaCa-2 in DMEM, BxPC-3 in RPMI. Authentication of PA-TU-8902 cell line used in majority of described studies was confirmed by independent laboratory.
MTT assayViability of cancer cell lines was examined by MTT assay. The cells were cultured in a 96-well plate with tested therapeutics for 24 h. Then the cells were incubated for 2 h with the new medium containing MTT. The absorbance of formazan-reduction product of MTT corresponding to the relative viable cell number was measured at 545 nm with standard ELISA reader. Experimental therapeutics were found not to interfere with the reduction of MTT.
Selenium analysisTo assess possible contribution of selenium and/ or selenocompounds to the biological effects of S. platensis, its content in the water extract of S. platensis used in our in vitro studies was determined by atomic absorption spectrometry using a standard clinical chemistry procedure.
Peroxyl radical scavenging capacityPeroxyl radical scavenging capacity was measured fluorometrically as a proportion of antioxidant consumption relative to that of Trolox, as described previously.20
Determination of heme oxygenase enzyme activityPA-TU-8902 cells were incubated for 24 h with tested therapeutics. After incubation, the cells were quickly washed with phosphate buffered saline (PBS, 0.1 M, pH 7.4), harvested, centrifuged, and each pellet was dispersed in PBS, and then sonicated. HMOX activity was determined by measurements of CO production using gas chromatography with a reduction gas analyzer (Peak Instruments, Menlo Park, CA, USA) as described previously.21 HMOX activity was calculated as pmol CO/h/mg of protein.
Determination of mitochondrial reactive oxygen species productionThe production of reactive oxygen species (ROS) in the mitochondrial matrix was determined as a time-dependent increase in the fluorescence intensity of selective MitoSOX (Life Technologies, Pleasanton, CA, USA) dye in the cells exposed to tested therapeutics using flow cytometry (BD LSRII, BD Biosciences, San Jose, CA, USA). After 24 h, Mito-SOX was added for 15 min to cells. Immediately before each measurement, cells were treated with rotenone (10 μM) to enhance the production of mitochondrial ROS. The rate of fluorescence intensity increase was determined in 1-min intervals for 16 min by flow cytometry.
Determination of GSH/GSSGThe cells exposed to tested therapeutics for 24 h were re-suspended in distilled water, and then lyzed with chloroform. The lysates were centrifuged at 1,000 x g for 5 min, upper aqueous phase containing glutathione was collected, snap-frozen in liquid nitrogen, and stored at liquid nitrogen until analysis. Samples (in 40 mM PBS, pH 7.0) were analyzed by capillary electrophoresis (voltage = 30 kV, detection at 195 nm, Agilent 7100, Agilent, Santa Clara, CA, USA) equipped with a polyimidecoated fused silica capillary (68 cm x 50 μm) as described previously.22
High-resolution respirometryThe respiration of intact and treated cells in the complete medium was measured with the Oroboros Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria). Oxygen consumption was measured in the basal state, after treatment with oligomycin (2 mg-L-1), a Complex V inhibitor used to determine the relative coupling of respiration and phosphorylation, and FCCP (5 μΜ), a ionophore and mitochondrial uncoupling agent used to determine maximal respiratory capacity of the cells.
Fluorescence microscopy determination of PCB and C-phycocyanin within PA-TU-8902 cellsTo determine the uptake of PCB and C-phycocyanin, PA-TU-8902 cells (105-well-1) were seeded on microscopic dishes and left to adhere overnight (16 h). Attached cells were incubated with PCB and Cphycocyanin (0.2 to 5.0 μΜ) dissolved in the complete phenol red-free medium at 37°C for 1, 3, 6, and 20 h. In an additional set of experiments that focused on the determination of intracellular localization of pigment uptake, cells were exposed to C-phycocyanin (0.2 μΜ) for 1 and 20 h, and MitoTracker® Green FM (100 nM) and LysoTracker® Green DND-26 (75 nM) (Molecular Probes, Life Technologies, Carlsbad, CA, USA) for staining of mitochondria and lysosomes, respectively.
Intracellular localization was assessed by realtime live-cell fluorescence microscopy. The images were acquired by an inverse fluorescent microscope Olympus IX-81 with a confocal unit Cell® System (Olympus, Tokyo, Japan) using high-stability 150 W xenon arc burner and EM-CCD camera C9100-02 (Hamamatsu, Ammersee, Germany).
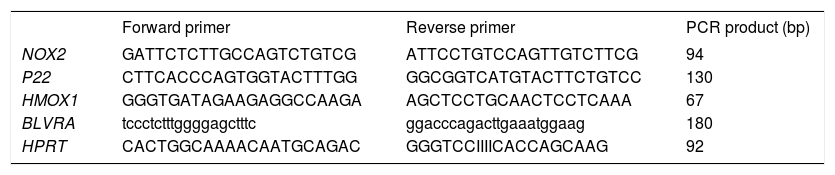
Reverse Transcription quantitative Polymerase Chain Reaction (RT-qPCR)PA-TU-8902 cells were incubated for 24 h before treatment. Chlorophyllin (30 μM), unconjugated bilirubin (10 μM), PCB (30 μM), and S. platensis extract (0.3 g-L) were added and RNA was isolated after 1 h (PerfectPure RNA Cultured Cell Kit, 5PRIME, Hamburg, Germany). Primers (sequences in table 1) were designed using Primer 3 software http://frodo.wi.mit.edu/primer3/) and synthesized by Generi Biotech (Hradec Kralove, Czech Republic). RT-qPCR were performed in 20-μL reaction volumes, containing 4 μL of 10-fold diluted cDNA template from a completed reverse transcription
Primer sequences for RT-qPCR target genes.
| Forward primer | Reverse primer | PCR product (bp) | |
|---|---|---|---|
| NOX2 | GATTCTCTTGCCAGTCTGTCG | ATTCCTGTCCAGTTGTCTTCG | 94 |
| P22 | CTTCACCCAGTGGTACTTTGG | GGCGGTCATGTACTTCTGTCC | 130 |
| HMOX1 | GGGTGATAGAAGAGGCCAAGA | AGCTCCTGCAACTCCTCAAA | 67 |
| BLVRA | tccctctttggggagctttc | ggacccagacttgaaatggaag | 180 |
| HPRT | CACTGGCAAAACAATGCAGAC | GGGTCCIIIICACCAGCAAG | 92 |
NOX2: NADPH oxidase. p22: p22phox NADPH oxidase. HMOX1: heme oxygenase 1. BLVRA: biliverdin reductase A. HPRT: hypoxanthine phosphoribosyl-transferase (a control gene). reaction, 1x SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), and 200-1000 nM of forward and reverse primers. All RT-qPCR were run on a ViiATM™ (Applied Biosystems).
In vivo experiments
In vivo studies were performed on nude mice (strain CD-1, Charles River WIGA, Sulzfeld, Germany) xenotransplanted subcutaneously with PA-TU-8902 cells (107 per mouse; n = 6 for each treatment group). After initiation of tumor growth (7-10 days after xenotransplantation), mice received oral treatment (intragastrically once daily via a gastric tube) with a placebo (water) or a water suspension of freeze-dried S. platensis (0.5 g-kg-1). An assessment of subcutaneous tumor size was performed by measurements of the 2 greatest perpendicular diameters, measured every 3 days with a caliper.23 After 14 days of treatment, mice were sacrificed.
Blood and tumor tissue specimens were sampled and stored at -80 °C until analyzed.
To confirm antioxidant effects of S. platensis treatment (10 g-kg-1 BW for 4 days), Wistar rats (n = 9) were used in a separate study using the same protocol as described above. To determine the total peroxyl scavenging activity in sera, blood was sampled from the ocular sinus prior to initiation of the feeding and from vena cava at sacrifice.
Ethics statementAll aspects of the animal studies and all protocols met the accepted criteria for the care and experimental use of laboratory animals, and were approved by the Animal Research Committee of the 1st Faculty of Medicine, Charles University in Prague (under registration No. 356/10). All procedures were performed under lege artis conditions and all efforts were made to minimize animal suffering.
Statistical analysesDifferences between variables were evaluated by the Mann-Whitney Rank Sum test. Group mean differences in tumor size were measured by repeated measures analysis of variance (RM ANOVA) with Holm-Šidák posthoc testing, when p-values were significant. When needed, log transform values of tumor size were used for comparisons to comply with normality and equal variance requirements. Linear regression analyses were used to compare the effects of S. platensis treatment on total peroxyl scavenging activity in vitro. Paired t-tests were used to compare the effects of S. platensis on total peroxyl scavenging activity in vivo. Depending on their normality, data are presented as the mean ± SD or the median and 25%-75% range. Differences were considered statistically significant when p-values were less than 0.05.
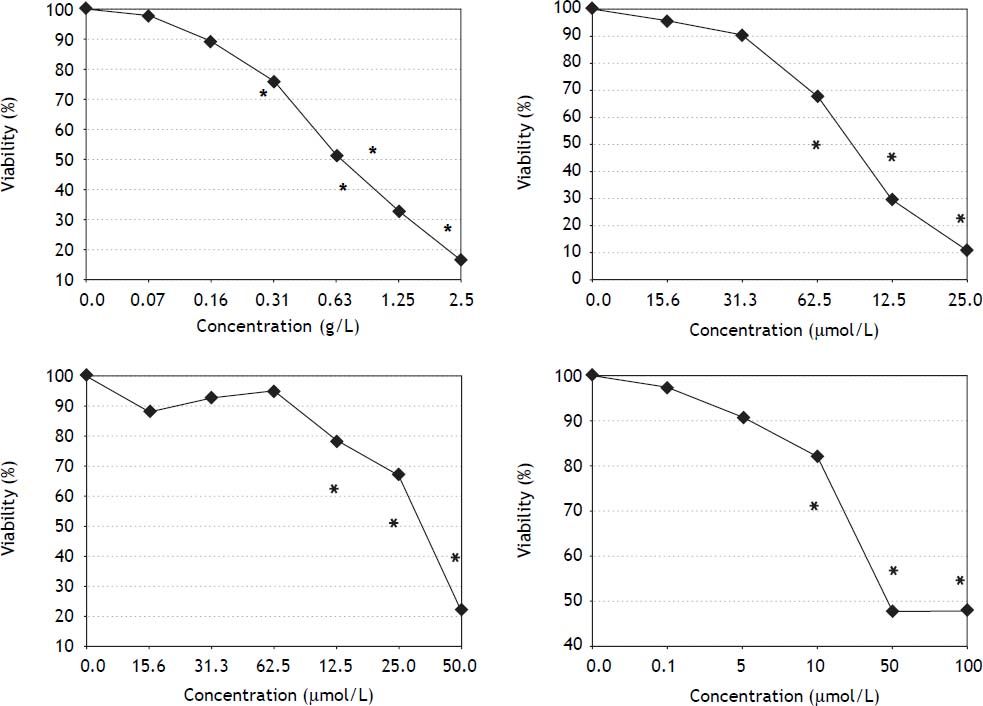
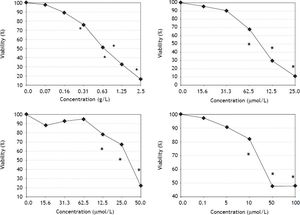
ResultsEffects of S. platensis extract and its tetrapyrrolic components on pancreatic cancer cell viabilityAll therapeutics efficiently decreased pancreatic cancer cell viability in a dose-dependent manner. The most sensitive cancer cell line was PA-TU-8902, whose viability was substantially decreased following treatment with S. platensis (from 0.16 g-L-1, p < 0.05) (Figure 2). As low as 10 μM concentrations of bilirubin (corresponding to its levels in human serum) substantially suppressed PA-TU-8902 cell viability (p < 0.05); whereas, doses of approximately 60 μM PCB, and 125 μM chlorophyllin were needed to reach the same effect (Figure 2). For other pancreatic cancer cell lines (Mia PaCa-2 and BxPC-3), higher concentrations of all compounds (~3 x) were required to reduce cell viability to 50% (data not shown). No selenium was detected in the S. platensis extract (detection limit of the method = 9 ng-mL) indicating that selenoproteins were not responsible for observed anti-proliferative effects of S. platensis treatment.
Effects of S. platensis extract and related tetrapyrrolic compounds on PA-TU-8902 pancreatic cancer cell viability. A. S. platensis extract. B. PCB. C. Chlorophyllin. D. Bilirubin. Viability was measured using the MTT assay after 24 h exposure to each compound. Experiments were performed in quadruplicates, data presented as mean. *p < 0.05.
Based on these findings, the PA-TU-8902 cell line was used for further studies.
Effects of S. platensis extract and its tetrapyrrolic components on HMOX in PA-TU-8902 cellsThe HMOX pathway was investigated for two reasons:
- •
HMOX has been reported to affect tumor cell proliferation24 and tetrapyrrolic compounds used in our study might modulate HMOX in a feedback mechanism; and
- •
HMOX is a potent effector of the antioxidant defense system.
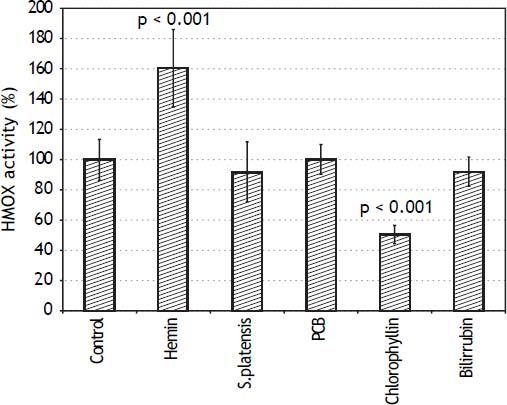
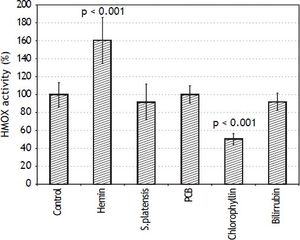
Therefore, the effects of S. platensis extract and its related tetrapyrrolic compounds on HMOX in the PA-TU-8902 cells were analyzed. Neither the S. platensis extract, PCB, nor bilirubin had any effect on HMOX activity (Figure 3) or HMOX1 mRNA expression. However, only chlorophyllin (30 μM) significantly inhibited HMOX activity (to 51 ± 6% of control values, p < 0.001) (Figure 3) and mRNA expression (to 55 ± 32% of control values, p = 0.016, data not shown). Interestingly, the same inhibitory effect of chlorophyllin on HMOX activity was also observed for Mia PaCa-2 pancreatic cancer cells (data not shown). Expression of biliverdin reductase (OMIM * 109750), another important gene in the heme catabolic pathway, was not affected by any of these compounds (data not shown).
Effects of S. platensis extract and related tetrapyrrolic compounds on HMOX enzyme activity. PA-TU-8902 pancreatic cancer cells were incubated with S. platensis extract (0.3 g•L-1), hemin (30 μM), PCB (30 μM), chlorophyllin (30 μM), and bilirubin (10 μM) for 24 h. Heme oxygenase activity expressed as percentage of control (100%).
Proliferation of normal and cancer cells is strongly influenced by redox signaling.18 Because PCB is structurally related to the potent antioxidants bilirubin and biliverdin, its effects on the cellular redox balance were investigated. The main source of intracellular free radicals under basal conditions is from a leakage of superoxide from the mitochondrial respiratory chain.25 Even more importantly, NADPH oxidase-derived superoxide has an important function in pancreatic cancer cell proliferation due to Kras/Rac1-dependent NADPH oxidase induction26 and the importance of this pathway was demonstrated also for liver carcinogenesis.27 Therefore, we were interested whether S. platensis and/or S. platensis-derived tetrapyrroles could influence mitochondrial production of ROS.
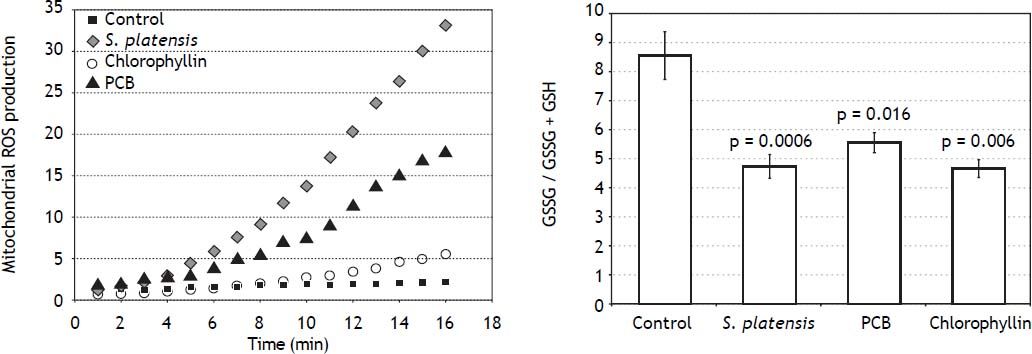
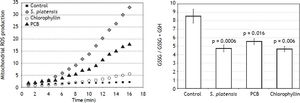
Indeed, we found that 24 h after exposure of PA-TU-8902 cells to S. platensis extract, PCB, and chlorophyllin resulted in significant decreases in intramitochondrial ROS production (Figure 4A), despite the fact that mRNA expressions of NOX2 and p22 NADPH oxidase subunits were not affected by 1 h incubation of cells with either S. platensis extract, PCB, or chlorophyllin (data not shown).
Effects of S. platensis and related tetrapyrrolic compounds on (A) mitochondrial production of ROS and (B) glutathione redox status. PA-TU-8902 pancreatic cancer cells were incubated with S. platensis extract (0.3 g•L-1), PCB (50 μM), and chlorophyllin (50 μM) for 24 h. ROS: reactive oxygen species. GSH: reduced glutathione. GSSG: oxidized glutathione.
We then investigated whether this effect is robust enough to modify the cellular redox balance by determination of the oxidized and reduced glutathione ratio. We found that pre-incubation of cells with S. platensis extract, PCB, or chlorophyllin shifted the ratio towards increased reduction (p = 0.0006; 0.016; and 0.006, respectively) (Figure 4B).
Finally, we investigated whether a decreased leakage of mitochondrial ROS was due to direct antioxidant properties of tested therapeutics, or whether these compounds influence the ROS leakage by modulation of activity and efficiency of mitochondrial respiratory chain.25 We measured mitochondrial respiration of the cells maintained in the complete medium in a basal state and during maximal stimulation with an uncoupling agent FCCP, and basal proton leakage during inhibition of Complex V with oligomycin. Interestingly, we found no changes in the respiration of the PA-TU-8902 cells after exposure to S. platensis extract, PCB, or chlorophyllin (data not shown) suggesting that decreased leakage of ROS was due to direct antioxidant properties of S. platensis and its tetrapyrroles, PCB, and/or chlorophyllin.
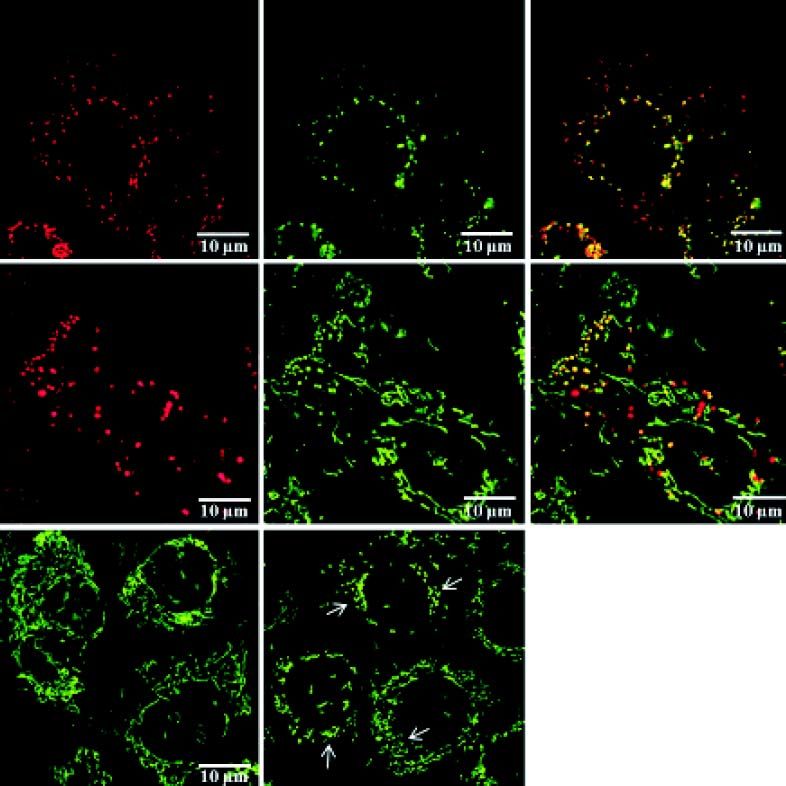
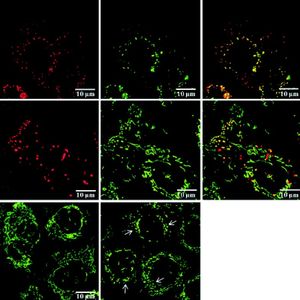
Uptake and localization of C-phycocyanin and PCB in PA-TU-8902 cellsLive cell imaging studies demonstrated that both C-phycocyanin and PCB incubated with PA-TU-8902 cells can easily enter the cells (Figure 5.1, data for PCB not shown). Accumulation of both pigments within the cells was apparent within 1 h of treatment, and gradually increased in time- and concentration-dependent manners. Localizations of C-phycocyanin and PCB in the cells were observed mainly in the form of vesicles localized in perinuclear region. The co-localization of LysoTracker Green with C-phycocyanin was seen in most of the observed vesicles within the cells (Figure 5.1). Co-localization study with a mitochondrial marker MitoTracker Green revealed that C-phycocyanin was absent in the mitochondria as shown by their typical elongated shape (mitochondrial network). In contrast, both dyes co-localized in aberrant-looking vesicles of mitochondrial origin (fragmented mitochondria) (Figure 5.2), disrupting the usual mitochondrial network (Figure 5.3).
C-phycocyanin uptake and its localization within PA-TU-8902 cells. 1. Lysosomal staining of PA-TU-8902 cells with LysoTracker Green exposed to C-phycocyanin (0.2 μM, 20 h incubation). A. C-Phycocyanin. B. Lyso-Tracker Green. C. Merge of A and B. 2. Mitochondrial staining of PA-TU-8902 cells with MitoTracker Green exposed to C-phycocyanin (0.2 μM, 20 h incubation). A. C-Phycocyanin. B. MitoTracker Green. C. Merge of A and B. 3. Mitochondrial fragmentation in PA-TU-8902 cells exposed to C-phycocyanin (0.2 μM, 20 h incubation). A. Mitochondria of untreated cells stained with MitoTracker Green. B. Fragmented mitochondria of cells treated with C-phycocyanin (mitochondrial fragmentation depicted by arrows).
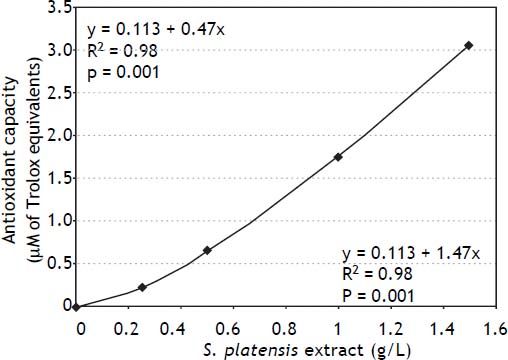
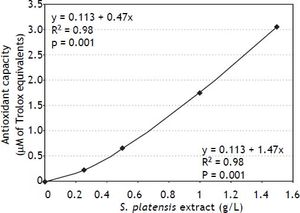
To demonstrate whether the antioxidant effects observed on the cellular level may influence total antioxidant capacity, the total peroxyl scavenging activity was measured in S. platensis extracts demonstrating an approximately linear relationship between antioxidant activity and the concentration of the S. platensis extract (Figure 6).
Effects of S. platensis extract on total peroxyl scavenging activity in vitro. Peroxyl radical scavenging capacity was measured fluorometrically as a proportion of antioxidant consumption present in serum relative to that of Trolox (a reference and calibration antioxidant compound).20
Biological relevance of these findings was confirmed in our in vivo study, which demonstrated a significant increase (132 ± 22%, p = 0.002) in antioxidant capacity of rats fed S. platensis for 5 days.
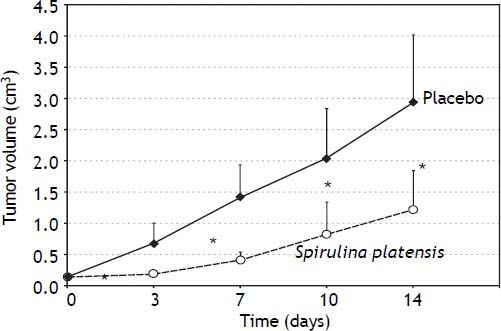
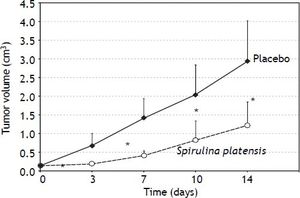
In vivo anticancer effects of S. platensisAthymic nu/nu mice xenotransplanted with PA-TU-8902 cells, which were treated orally with S. platensis extract, exhibited much slower tumor progression compared to the placebo-treated mice. Tumor sizes were significantly smaller as early as the third day after initiation of the treatment (p < 0.01) (Figure 7).
DiscussionS. platensis has been used by humans for centuries as evidenced by reports from Mexico and Central Africa.2 Currently, it is widely used as a nutraceutical namely for the prevention of diabetes, although numerous other biological effects have been ascribed to this alga.2 Among many potentially-bioactive substances, PCB and chlorophylls, represent compounds of particular interest due to their structural resemblance to bilirubin, a potent antioxidant, atheroprotective, and anti-proliferative agent.24 In fact, the anticancer action of bilirubin has been attributed to its effects on mitochondria28 as well as intracellular signalization.29 In addition, bilirubin was found to be a widespread inhibitor of protein phosphorylation,30 which might account for these biological properties. Importantly, these data are reflected by results from human studies demonstrating lower risk of colon5,7 as well as lung31 cancer in Gilbert syndrome subjects. In our study, we demonstrated that S. platensis and related tetrapyr-roles have potent anti-proliferative effects on experimental human pancreatic cancer. We found that tested therapeutics exerted strong antioxidant effects with substantial reductions of mitochondrial ROS production, which may improve overall cellular redox status as indicated by an observed shift in glutathione redox parameters. These findings seem to parallel the inhibitory effects of bilirubin on superoxide production32 and on overall mitochondrial metabolism.33 In fact, pyrrole groups of bilirubin, present also in our compounds, have been suggested to compete with NADH on the active sites of mitochondrial dehydrogenases indicating possible mechanisms of these effects.33 This conclusion is also in accord with a report by Boloor, et al., who described marked protective effects of chlorophyllin on mitochondria-induced lipid peroxidation.34 It seems that observed anti-carcinogenic effects are mediated, at least partially, by direct inhibition of mitochondrial ROS generation.
Collectively, our data suggest that the primary target of algal tetrapyrroles is the mitochondria of the pancreatic cancer cells. This is based on the results of our topological as well as functional studies. The former observation is supported by our finding of co-localization of C-phycocyanin/PCB with fragmented mitochondria and lysosomes. It is generally believed that the observed mitochondria-related cytoplasmic vesicles result from the lysosomal apoptotic pathway mediated by mitochondria.35 The formed vesicles might also be autophagosomes of mitochondrial origin, which after maturation, fuse with lysosomes.36 It is of note that a similar preferential intracellular co-localization phenomenon was described for phtalo-cyanine, a related porphyrin-like molecule.37 This data are also in line with the results of the functional studies. Although we did not see changes in mRNA expressions of NADPH oxidase subunits, our results on inhibition of mitochondrial ROS production are consistent with previous reports indicating that bilirubin, biliverdin, and PCB are potent inhibitors of NADPH oxidase.12,13,38 In fact, Lanone, et al. showed potent inhibitory effects of bilirubin towards NADPH oxidase activity, although mRNA expressions were unchanged.12 Mitochondrial NADPH oxidase-derived superoxide represents an important carcinogenic factor affecting redox-sensitive cell survival, cell cycle as well as multiple metabolic pathways18,39 and this factor is implicated not only in pancreatic,26 but also liver carcinogenesis.27 Consistent with this data is the algal tetrapyrroles-induced improvement in glutathione redox status known to be associated with inhibition of tumor promotion.40 Interestingly, complementary antioxidant and cytoprotective roles of glutathione and bilirubin have been reported previously.41
Interestingly, we also observed a marked inhibitory effect of chlorophyllin on both HMOX1 mRNA expression and total HMOX enzyme activity, while the other tetrapyrroles did not have any effect. This inhibitory action, which is in accord with opposing effects of various metalloporphyrins on HMOX,15 might contribute to anti-carcinogenic effects observed in our study and also, at least in part, account for chemopreventive effects of chlorophyll-rich nutrients.
One of the limitations of our study is that we have not directly identified all particular components in the S. platensis extract responsible for the observed biological effects, and focused only on the algal tetrapyrroles. However, it should be emphasized that exactly the same preparation, i.e. pulverized S. platensis, is commonly used in a global scale. Our data thus provide evidence for all the consumers on the anti-cancer potential of this alga.
In conclusion, S. platensis, as well as its tetrapyrrolic components, substantially decreased proliferation of pancreatic cancer. These effects were at least partially due to their potent antioxidant activity, inhibition of mitochondrial production of ROS and subsequent changes in intracellular redox status. These data support a chemopreventive role of this edible alga with promising potential for its broad use in the chemoadjuvant treatment of cancer diseases. This assumption of more generalized use of S. platensis in cancer chemoprevention is supported also by recent data demonstrating anti-cancer potential of this alga on liver39 as well as breast42 carcinogenesis. It also should be noted, that dietary supplementation with this alga might enhance systemic pool of tetrapyrroles in a more natural way, as compared to xenobiotic-induced hyperbilirubinemia approach proposed recently.43
Financial SupportThis work was supported by grants Nos. 55210, 438911, LH11030, PRVOUK-P25/LF1/2, and RVO-VFN64165/2013 given by the Granting Agency of the Charles University in Prague, Czech Ministry of E <ducation and Czech Ministry of Health, respectively. The granting agencies had no influence upon the collection, analysis, interpretation of data, writing of the report, nor on the decision to submit the paper for publication.
AcknowledgementsAuthors thank to Drs. Pavel Coufal and Tomáš Kř¿žek from Department of Analytical Chemistry, Faculty of Science of the Charles University in Prague for kindly providing us with capillary electro-phoresis equipment, Magdalena Kadlecovâ and Olga Svejdovâ for excellent technical assistance.