A 3 year 6 month-old child with high fever for 4 days, bilateral non-purulent conjunctivitis, rash in the upper and lower limbs regressed within 72 h, lymphadenitis in the left mandibular corner, edema on the soles of her feet and limping, was admitted to the Pediatric Medical Department of our hospital. Blood tests showed abnormal values for C-reactive protein (CRP) [14.11 mg/dL; normal level (nl): 0-0.5 mg/dL], white blood cell (WBC) (17.13 x 103/uL; nl: 4-14 x 103/uL), Neutrophil % (82.7%; nl: 10-74 %), Platelet count (467 x 103/uL; nl: 150-450 x 103/uL), Cholesterol (107 mg/dL; nl: 120-200 mg/dL), Triglycerides (156 mg/dL; nl:40-150 mg/dL); normal vascular parameters for haemoglobin (Hb) (11,4 g/dL; nl: 9-16 g/dL), Albumin (3.8 g/dL; nl: 3,5-5,5 g/dL), glutamic pyruvic transaminase (GPT) (15 UI/L; nl: 5-40 UI/L) and glutamic oxaloacetic transaminase (GOT) (24 UI/L; nl: 5-40 UI/L). The diagnosis of typical Kawasaki’s disease was made based on persistent fever associated with 4 positive diagnostic clinical criteria. Echocardiogram was found to be negative for coronary dilatation, and treatment with intravenous Immunoglogulins (IVIG) (2 g/kg) and acetylsalycilic Acid (ASA) (80 mg/kg) was started. Because of the persistence of the hyperpyrexia after 48 h a second IVIG bolus was performed and after another 48 h 3 boluses of steroids were delivered, after which all symptoms regressed. Therefore, the child was discharged in good general health conditions on a regimen with ASA at antiaggregant doses (5 mg/kg). Four weeks later, fever appeared again with arthralgia in the lower limbs. The patient was re-hospitalized and submitted to level III tests: follow-up echocardiogram (no coronary dilatations) and blood tests [erythrocyte sedimentation rate (ESR) 89 mm/h, WBC: 9,540 x 103/uL, neutrophils: 66.3%, platelect count: 793,000 x 103/uL, Hb: 9.2 g/dL, ferritin 283 ng/mL; nl = 9-290, CRP: 16.12 mg/dL, triglycerides: 87 mg/dl, cholesterol: 99 mg/dL)]. GPT, GOT; autoimmune tests, bone marrow aspiration, colture and virology tests were negative. Chest x-ray and abdominal ultrasound (US) examinations were unremarkable. Steroid (metilprednison at 2 mg/kg) treatment was restarted. After favourable initial response, fever and progressive coronary dilatations appeared, so anti-TNF-alpha monoclonal antibody was administered. Because the clinical condition worsened (persistence of the fever and the further worsening of the coronary dilatations), it was decided to start anti Il-1 therapy associated with anticoagulation (Warfarin), added to ASA. Clinical condition improved progressivly, although repeated echocardiograms showed a worsening of coronary lesions and the formation of multiple giant aneurysms (about 7-8 mm diameter both in the left and right coronary arteries). Contrast-enhanced (CE) multidetector-row computed tomography (MDCT) of total body was performed to investigate the systemic circulation. CE-MDCT examination confirmed the aneurysmatic dilatations in both coronary arteries (Figures 1A, 1B) and also revealed two giant saccular aneurysms in the right hepatic artery (Figures 1C, 1D). Of these last two dilatations the first (maximum diameter: 12 mm) affected the right hepatic artery bifuraction involving also the origin of its posterior branch and the second (maximum diameter: 8 mm) arised in the same vessel more distally, within the liver. Because of the high risk of rupture and complications in patients with multiple and non-artherosclerotic large hepatic artery aneurysms, a radiological intervention was necessary1 whereas surgery had been considered inappropriate due to the anatomy of the lesion. Specifically, the aim of the treatment was try excluding the aneurysms from hepatic arterial circulation through endovascular embolization, and ensuring the patency of the main arteries and thereby the peripheral arterial vascularization of the liver. Before starting the procedure, a trans-abdominal color-Doppler US examination of the abdomen was repeated, showing a significant reduction of color-flow signals within the distal aneurysm, as for partial intra-luminal thrombosis (Figure 2). The hepatic arteriography confirmed the previous imaging findings and revealed also an occlusion of intra-parenchymal tract of left hepatic artery, distally revascularized by microvascular anastomosis (Figure 3A). The treatment started by excluding the smaller (intrahepatic) aneurysm. Three coils (MicroPlex 10 Platinum Coil System, 6 mm X 18 cm, 4 mm X 10 cm, 6 mm X 8 cm, MicroVention, Terumo) were released after coaxial superselective catheterization (Headway 17 Advanced, 150 cm X 11 cm, MicroVention, Terumo) of its collar (Figure 3B). The coils appeared well compacted within the aneurysmal sac, suggesting that the previous observation (absent color-flow signals at US study and poor opacification at angiography) were probably related to poor filling of the aneurysm rather than partial thrombosis. As a second step, stent-assisted coil embolization technique was used to treat the largest aneurysm. Self-expand-ing nickel titanium stent [Low-profile Visualized Intraluminal Support Junior (LVIS Jr.), MicroVention, Terumo] was deployed across the lesion as a scaffold. Unfortunately, the device appeared slighly deformed in its central portion after it had been released. Therefore, a microcatheter (Excelsior SL-10, 150 cm X 6 cm, Boston Scientific) was advanced between the meshes of the device to allow the release of two coils (GDC-10 UltraSoft, 2 mm x 3 cm, 4 mm x 8 cm, Boston Scientific) within the aneurysmal sac (Figure 3C-3E). At the end of the procedure, the two aneurysm appeared completely excluded and peripheral hepatic arterial branches were preserved (Figure 3F). Actually, the patient is in good clinical condition and kept under periodic follow up. Last ultrasound examination revealed slight size reduction of the coronary arteries (approximately 6 mm) and patency of the hepatic artery circulation.
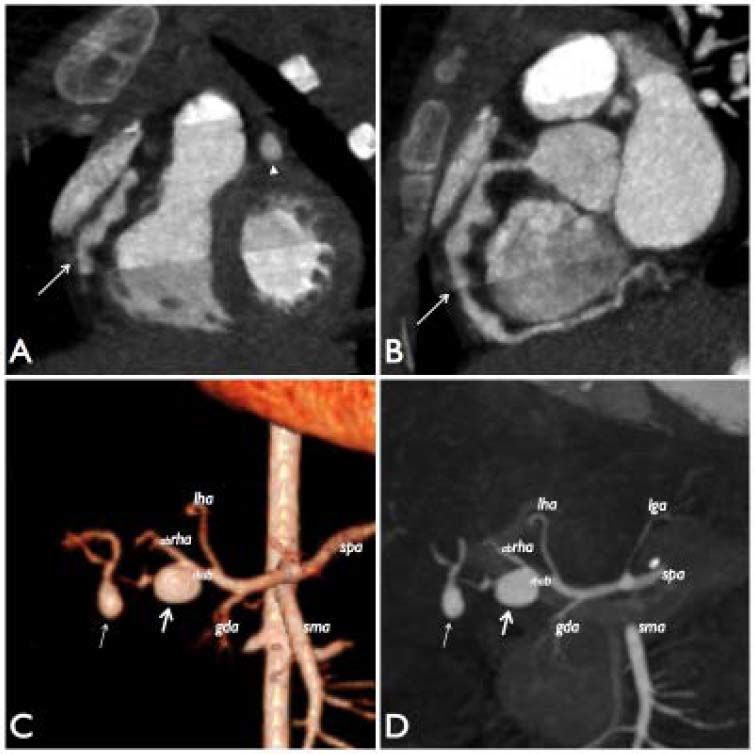
CE-MDCT image of the heart reformatted on frontal (A) and sagittal (B) plane, showing multiple aneurysmatic dilatations with rosary-like appearance in the proximal and middle tract of right coronary artery (arrows) and a fusiform aneurysm in the middle part of left coronary artery (arrow head in A). C-D. 3D volume-rendered (C) and multi-intensity projection (D) images of celiac trunk reformatted on coronal plane from abdominal CE-MDCT shows the first saccular aneurysm (thick arrow) at bifurcation of right hepatic artery (rhab) affecting the proximal part of its posterior branch and the second aneurysmal dilatation (thin arrow) distally to the first. lha: left hepatic artery; lga: left gastric artery; abrha: anterior branch of right hepatic artery; gda: gastroduodenal artery; sma: superior mesenteric artery; spa: splenic artery.
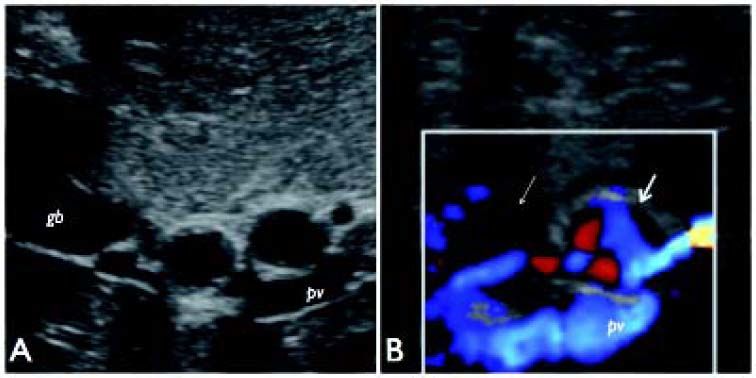
Trans-abdominal gray-scale (A) US image of hepatic hilum on subcostal view shows the two consecutive aneurysms of right hepatic artery as rounded anechoic structures. Color-Doppler examination (B) displays turbulent flow represented by red and blue color signals within the proximal aneurysm (thick arrow) and significant reduction flow with absent color-flow signals in the distal arterial dilatation (thin arrow), as for intra-aneurysmal thrombosis. gb: gallbladder; pv: portal vein.
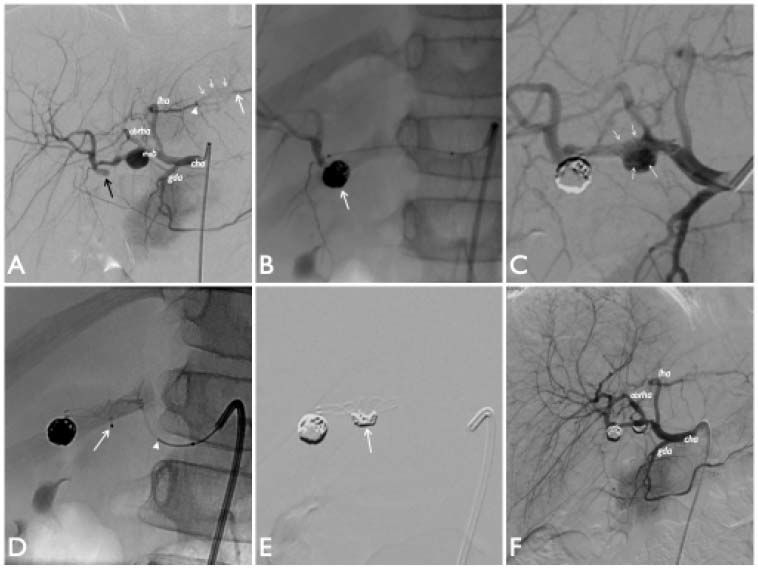
Hepatic arteriograms performed after catheterization of common hepatic artery (cha) on postero-anterior projection [digital subtraction angiograms (DSA) in A, C, E and F]. A. The angiography confirms the MDCT features showing poor opacification of the second aneurysm (black arrow) most likely due to unorganized intraluminal thrombotic material, as suspected by US examination. Note the obstruction of intra-parenchymal tract of left hepatic artery (arrow head) and its distal revascularization (white arrow) by thin collateral circulation (white short arrows). B-F. Steps of the endovascular treatment. B. Distal hepatic arteriogram performed through the micro-catheter after coils embolization of the distal aneurysm (arrow) shows complete exclusion of the aneurysm sac from hepatic arterial circulation and good opacification of the peripheral branches. C-E. Stent-assisted coil embolization of the proximal aneurysm. C. Angiographic check after stent placement across the aneurysmatic sac. The stent ensures opacification both of the left hepatic artery and the anterior branch of right hepatic artery. Note that the device appears slightly deformed within the sac (arrows). D. Super-selective micro-catheterization of the aneurysm sac through the meshes of the stent (arrow) to allow releasing coils (arrow head: tip of coil). E. Check after the stent-assisted coil embolization of the sac. Coils (with crescent-shaped appearance) appear positioned within the aneurysm sac immediately below the lower edge of the stent (arrow). F. Final check at the end of the procedure shows complete exclusion of aneurysms from right hepatic arterial vasculature. Vascularization of its peripheral branches is preserved.
Kawasaki disease is an acute multisystemic vasculitis that affects medium-sized vessels in of all areas of the body. It is self-limiting, of unknown etiology, probably multifactorial, and it preferentially affects newborns and infants. It is characterized by fever lasting more than 5 days associated with ≥ 4 of the following clinical signs and criteria: bilateral conjunctival hyperemia, erythema of the lips and oral mucosa, anomalies of the extremities, rash and cervical lymphadenopathy. While the aneurysms of coronary arteries can develop in up to 25 % of patients with Kawasaki disease, systemic involvment occurs only in 2 %, with visceral arteries being the less commonly affected.2 Furthermore, the finding of hepatic artery aneurysm is a very rare complication of the disease. Very little is known about the natural history of hepatic artery aneurysm in Kawasaki disease: Caputo, et al. report that their case was the fifth in the literature.3 Although the treatment of a hepatic artery aneurysm is often indicated in sympomatic patients or if the diameter exceeds 2 cm (guidelines for adults and for the atherosclerotic lesions),4 Abbas et al recommend treatment for all nonatherosclerotic and multiple aneurysm of hepatic artery - as in our case - because of the high risk of rupture and related complications.1 The treatment of visceral artery aneurysms can be surgical or endovascular. The second approach is particularly suitable for aneurysms involving the distal and/or the intra-parenchymal branches of the hepatic artey. Different techniques and technologies used for endovascular treatment of intracranial aneurysms may be applicable to the management of visceral aneurysms. In our case, the choice of stent-assisted coil embolization for the proximal aneurysm was imposed by the need to preserve the perfusion of the two main branches of right hepatic artery and thereby the vascularization of the biliary system: this was successfully achieved by inserting a stent through the aneurysm to preserve the flow, and secondarily excluding the aneurysmal sac with the coils. Interestingly, the latter coils, as they were “compacted” within the sac around the stent, helped to support and stabilise at long-term the overlying stent, especially as it had been slightly over-dilated in its central portion.
DisclosuresNone.







![Hepatic arteriograms performed after catheterization of common hepatic artery (cha) on postero-anterior projection [digital subtraction angiograms (DSA) in A, C, E and F]. A. The angiography confirms the MDCT features showing poor opacification of the second aneurysm (black arrow) most likely due to unorganized intraluminal thrombotic material, as suspected by US examination. Note the obstruction of intra-parenchymal tract of left hepatic artery (arrow head) and its distal revascularization (white arrow) by thin collateral circulation (white short arrows). B-F. Steps of the endovascular treatment. B. Distal hepatic arteriogram performed through the micro-catheter after coils embolization of the distal aneurysm (arrow) shows complete exclusion of the aneurysm sac from hepatic arterial circulation and good opacification of the peripheral branches. C-E. Stent-assisted coil embolization of the proximal aneurysm. C. Angiographic check after stent placement across the aneurysmatic sac. The stent ensures opacification both of the left hepatic artery and the anterior branch of right hepatic artery. Note that the device appears slightly deformed within the sac (arrows). D. Super-selective micro-catheterization of the aneurysm sac through the meshes of the stent (arrow) to allow releasing coils (arrow head: tip of coil). E. Check after the stent-assisted coil embolization of the sac. Coils (with crescent-shaped appearance) appear positioned within the aneurysm sac immediately below the lower edge of the stent (arrow). F. Final check at the end of the procedure shows complete exclusion of aneurysms from right hepatic arterial vasculature. Vascularization of its peripheral branches is preserved. Hepatic arteriograms performed after catheterization of common hepatic artery (cha) on postero-anterior projection [digital subtraction angiograms (DSA) in A, C, E and F]. A. The angiography confirms the MDCT features showing poor opacification of the second aneurysm (black arrow) most likely due to unorganized intraluminal thrombotic material, as suspected by US examination. Note the obstruction of intra-parenchymal tract of left hepatic artery (arrow head) and its distal revascularization (white arrow) by thin collateral circulation (white short arrows). B-F. Steps of the endovascular treatment. B. Distal hepatic arteriogram performed through the micro-catheter after coils embolization of the distal aneurysm (arrow) shows complete exclusion of the aneurysm sac from hepatic arterial circulation and good opacification of the peripheral branches. C-E. Stent-assisted coil embolization of the proximal aneurysm. C. Angiographic check after stent placement across the aneurysmatic sac. The stent ensures opacification both of the left hepatic artery and the anterior branch of right hepatic artery. Note that the device appears slightly deformed within the sac (arrows). D. Super-selective micro-catheterization of the aneurysm sac through the meshes of the stent (arrow) to allow releasing coils (arrow head: tip of coil). E. Check after the stent-assisted coil embolization of the sac. Coils (with crescent-shaped appearance) appear positioned within the aneurysm sac immediately below the lower edge of the stent (arrow). F. Final check at the end of the procedure shows complete exclusion of aneurysms from right hepatic arterial vasculature. Vascularization of its peripheral branches is preserved.](https://static.elsevier.es/multimedia/16652681/0000001300000002/v1_201906020852/S1665268119308920/v1_201906020852/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




