Severe liver dysfunction during pregnancy implies a serious risk for both mother and fetus, and represents a technical and ethical challenge for treating physicians. We report a case of a previously healthy 32-year old woman who was admitted to our hospital with idiopathic fulminant hepatic failure and underwent successful orthotopic liver transplantation (OLT) at gestation week 21. Patient’s and fetus’ immediate postoperative course were relatively uneventful until week six after OLT, when the mother developed oligohydramnios and preeclampsia. At pregnancy week 27, after inducing baby’s lung maturation, a cesarean section was performed with the delivery of an otherwise healthy girl. After 3 years of follow-up, mother and child are leading normal lives with no complications related either to pregnancy or to OLT. We describe the case of a successful emergency liver transplant in a woman during the second trimester of pregnancy, demonstrating that OLT can be a viable option to preserve the life of the mother and an otherwise unviable fetus. Intrauterine baby’s growths until the attainment of a viable gestational age was feasible despite the mother’s fulminant hepatic failure and liver transplant surgery.
Acute liver failure (ALF) during pregnancy usually occurs in the third trimester and is usually related to HELLP syndrome (hemolysis, elevated liver enzymes and low platelets) with eclampsia, and to acute fatty liver of pregnancy (AFLP).1 However, when ALF develops in the first or second trimester of gestation, the fetus is unable to survive if delivery is induced early on. Furthermore, when liver transplantation is performed, special consideration should be taken on the effects of pre transplant X-ray examinations, hemodynamic instability during surgery, and potential negative impact of the exposure to immunosuppressive and various other drugs upon fetus development. Few case reports on orthotopic liver transplantation (OLT) during the first or second trimester of pregnancy have been published.2–9 Most of these reports describe induced or spontaneous fetal abortions. In the present report, we describe a case of a 21-week pregnant woman who underwent an emergency OLT for idiopathic ALF. Subsequently, the pregnancy progressed successfully until the child reached a viable age. We discuss the perioperative management of both mother and the fetus, and also review literature searching for similar cases.
Clinical CaseA 32-year old Hispanic woman in the 21st week of pregnancy arrived at the emergency room with history of asthenia and jaundice for the past 4 weeks. Her past medical history included an ectopic pregnancy and a preterm cesarean section for preeclampsia. On physical examination the patient was icteric, lethargic with slurred speech and asterixis. Initial vital signs were stable except for elevated blood pressure of 160/80 mmHg. There were no stigmata of chronic liver disease. The liver and spleen were not palpable and obstetrical examination demonstrated an enlarged uterus with normal fetal vital signs, and no uterine activity or bleeding. Abdominal ultrasonography did not reveal ascites, liver or spleen enlargement, fatty infiltration or any ductal dilatation and the Doppler study showed patency of the hepatic vessels.
Her initial laboratory evaluation was remarkable for total bilirubin 28.3 mg/dL, direct bilirubin 13.7 mg/dL, aspartate aminotransferase (AST) 375 U/L, alanine aminotransferase (ALT) 456 U/L, alkaline phosphatase 129 U/L, total protein 5.7 gr/dL, serum albumin 2.8 gr/dL, glucose 49 mg/dL, hemoglobin 13.2 gr/dL, leukocyte count 11,000 cell/mm3, platelet count 225,000/mm3, prothrombin concentration 7%, International Normalized Ratio (INR) 14.02, Factor V 5%, blood ammonium level 327 μgr/dL (normal range 40-98), BUN 25 mg/dL and serum creatinine 0.78 mg/dL. Further evaluation excluded acute hepatitis A, hepatitis B, hepatitis E, cytomegalovirus, herpes simplex virus and Epstein-Barr virus infection. Ceruloplasmin was normal. Anti-nuclear antibodies, anti-smooth muscle antibodies and anti-LKM1 antibodies were all negative. No new drug exposure was documented.
Twenty-four hours after admission, having thoroughly investigated all major causes of liver failure a transjugular liver biopsy was performed revealing massive hepatic necrosis with no evidence of acute fatty liver of pregnancy. The patient was listed for emergency liver transplant with a MELD score of 38. She remained two days on the waiting list before she underwent an OLT from a healthy 37 year-old male ABO-identical donor with normal liver function tests that died from massive intracranial hemorrhage. The OLT was performed with inferior vena cava (IVC) preservation, vascular anastomoses were completed following standard procedure, and a T tube left in place after bile duct reconstruction. Cold ischemia lasted 5 h and warm ischemia time was 35 min. During surgery, the patient required 6 units of packed RBCs, 10 units of fresh frozen plasma and 8 units of cryoprecipitate. The patient and fetus remained hemodinamically stable during the whole procedure.
Immunosuppression was based on methylpredni-solone, receiving 1 gr IV during the anhepatic phase and then slowly tapered off. Tacrolimus, was introduced on postoperative day 2 to achieve a trough level of 10-12 ng/mL. The patient was extubated 60 h after surgery. On postoperative day 3 she presented with abdominal bleeding requiring an exploratory laparotomy during which no active bleeding sites were found and some clots were removed. On postoperative day 6 the patient evolved with pneumonia that responded favorably to antibiotics and mild decrease of immunosuppression dosing. On postoperative day 13 she presented with elevated ALT and AST, and after a biopsy confirmed acute cellular rejection diagnosis, she was successfully treated with pulse of corticosteroids.
The fetus was followed with daily obstetric ultrasounds that showed normal fetal growth and anatomy. However, at 27 weeks of pregnancy, the mother developed oligohydramnios and preeclampsia, then fetal lung maturation was induced with corticosteroids and a cesarean section with a midline longitudinal incision was performed with a delivery of an otherwise healthy girl weighting 830 g. The baby required treatment with ibuprophen for a patent ductus arteriosus and respiratory support with continuous positive airway pressure for one day. She required total parenteral nutrition for 17 days since partum until she started with enteral feeding. Plasma electrolyte and renal function remained stable during the early postnatal days. Four days after delivery, the mother was discharged, while her daughter remained under standard care for premature infants for 10 weeks, until she achieved an adequate weight gain. After more than 3-years of follow-up both mother and child are in excellent conditions leading normal lives, and no pregnancy or transplant related complications have been observed.
DiscussionALF during pregnancy has been described at any moment of gestation, but when due to liver diseases unique to pregnancy, it typically occurs in the third trimester. However, non-pregnancy related causes of ALF can affect women at any stage of pregnancy with severe consequences to both, the mother and fetus. After an exhaustive examination, the etiology of ALF in our patient was not elucidated. Although rarely reported during the second trimester of pregnancy, careful attention was paid to exclude AFLP. We promptly ruled out this disorder with a transjugular liver biopsy that did not show microvesicular fatty infiltration of the liver.
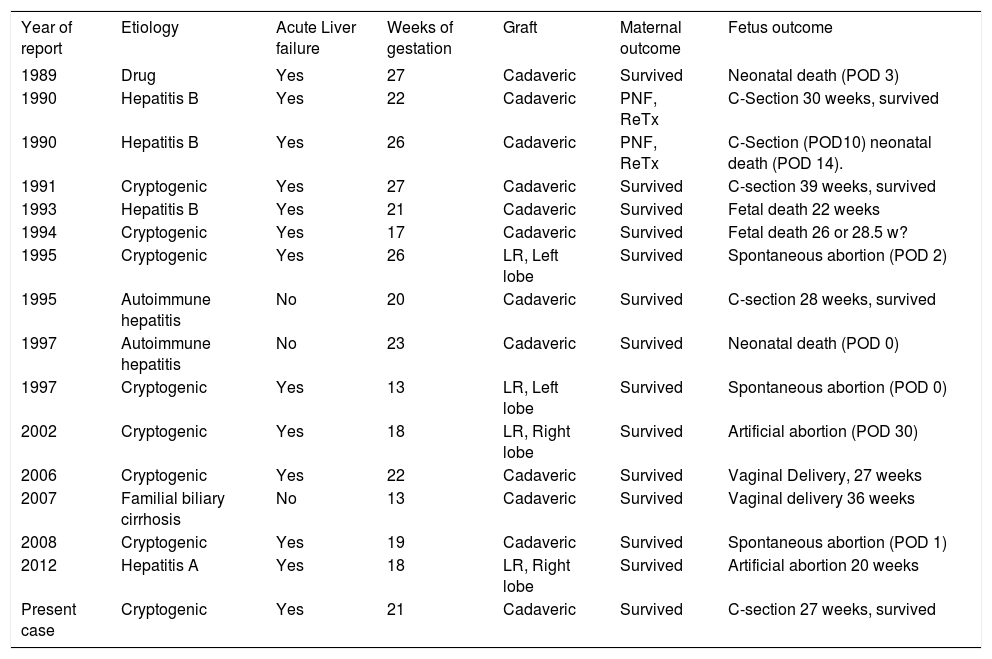
OLT during the early term of pregnancy is a challenging scenario. Ten years ago Eguchi, et al.5 described 11 reported cases, since then, to our knowledge only 5 more cases have been published in the literature (Table 1). The different case reports described outstanding maternal outcomes using cadaveric or living-related grafts.2–9 On the other hand, fetal outcomes are dismal, with only 4 preterm deliveries and 1 full-term delivery. The outcomes of the other 10 pregnancies were 3 fetal deaths, 2 artificial abortions, 2 spontaneous abortions and 3 neonatal deaths. In our case, interruption of pregnancy at an early stage with an unviable fetus was not contemplated as an option, due to ethical considerations. We discussed this with the mother’s family and they also agreed to perform a liver transplant and try to complete pregnancy. However, we explained possible fetal and maternal risks in a pregnant liver transplanted recipient such as preeclampsia, cesarean section and preterm delivery.10 We also considered that our patient presented a higher risk of developing complications given the mother’s past medical history of preeclampsia and the fact that the American Society of Transplantation recommends that liver transplant recipients should wait a minimum of 1 year before conception to stabilize graft function.11,12 The use of anesthetics or drugs that would possibly have toxic effects on the fetus were avoided. Furthermore, X-ray examinations were restricted, and when necessary, X-ray proof equipment was used to protect the fetus. The indication of standard immunosuppression with tacrolimus was used based on the reported evidence that shows a lower incidence of renal function impairment, acute cellular rejection and hypertension compared with cyclosporine during pregnancy.13,14 Mycophenolate mofetil was not added due to its association with increased risk of pregnancy loss and congenital malformations characterized by orofacial clefts and microtia/anotia.15,16
Orthotopic liver transplantation during the second trimester of pregnancy.
| Year of report | Etiology | Acute Liver failure | Weeks of gestation | Graft | Maternal outcome | Fetus outcome |
|---|---|---|---|---|---|---|
| 1989 | Drug | Yes | 27 | Cadaveric | Survived | Neonatal death (POD 3) |
| 1990 | Hepatitis B | Yes | 22 | Cadaveric | PNF, ReTx | C-Section 30 weeks, survived |
| 1990 | Hepatitis B | Yes | 26 | Cadaveric | PNF, ReTx | C-Section (POD10) neonatal death (POD 14). |
| 1991 | Cryptogenic | Yes | 27 | Cadaveric | Survived | C-section 39 weeks, survived |
| 1993 | Hepatitis B | Yes | 21 | Cadaveric | Survived | Fetal death 22 weeks |
| 1994 | Cryptogenic | Yes | 17 | Cadaveric | Survived | Fetal death 26 or 28.5 w? |
| 1995 | Cryptogenic | Yes | 26 | LR, Left lobe | Survived | Spontaneous abortion (POD 2) |
| 1995 | Autoimmune hepatitis | No | 20 | Cadaveric | Survived | C-section 28 weeks, survived |
| 1997 | Autoimmune hepatitis | No | 23 | Cadaveric | Survived | Neonatal death (POD 0) |
| 1997 | Cryptogenic | Yes | 13 | LR, Left lobe | Survived | Spontaneous abortion (POD 0) |
| 2002 | Cryptogenic | Yes | 18 | LR, Right lobe | Survived | Artificial abortion (POD 30) |
| 2006 | Cryptogenic | Yes | 22 | Cadaveric | Survived | Vaginal Delivery, 27 weeks |
| 2007 | Familial biliary cirrhosis | No | 13 | Cadaveric | Survived | Vaginal delivery 36 weeks |
| 2008 | Cryptogenic | Yes | 19 | Cadaveric | Survived | Spontaneous abortion (POD 1) |
| 2012 | Hepatitis A | Yes | 18 | LR, Right lobe | Survived | Artificial abortion 20 weeks |
| Present case | Cryptogenic | Yes | 21 | Cadaveric | Survived | C-section 27 weeks, survived |
POD: postoperative day. PNF: primary non-function. ReTx: retransplantation. C-section: cesarean section. LR: living related.
The pre-operative assessment included the coagulation profile, glycemia, renal function and most importantly, cerebral edema. Given the progressive nature of ALF the coagulation profile was conducted as close as possible to the time of surgery. Measurements included prothrombin time, INR, activated partial thromboplastin time, fibrinogen and platelet level. A complete correction of coagulopathy is probably unachievable but a platelet count > 50,000/ mm3, fibrinogen level > 100 mg/dL and an INR < 1.5 are realistic goals.17 Coagulation parameters were continuously checked during surgery and 24 blood product units were transfused to our patient during the whole procedure. Hypoglycemia was avoided with continuous infusion of hypertonic glucose. Fluid balance was evaluated with urine output, blood pressure and central venous pressure trends. An adequate fluid replacement was assessed closely to avoid any maneuver that could potentially aggravate cerebral edema. Although we usually monitor cerebral edema with an intracranial pressure catheter (ICP), in this particular case an ICP catheter was not placed given that the patient underwent endothracheal intubation not for progression of encephalopathy to coma but to perform a transjugular liver biopsy. During the time the patient remained on the waiting list she did not show any clinical signs of cerebral edema and no abnormalities were found with transcranial Doppler ultrasonography.
The surgical technique employed was a piggyback, which maintained patency of the IVC during the whole procedure. The venovenous bypass technique was avoided due to its risk of reducing pressure in the uterine veins and cause uterus and fetal hypoperfusion. Reperfusion phase is a crucial moment in pregnant patients were transient hypotension can cause fetal death.18 The distended uterus displaced the bowel into the operative field so retraction on the uterus was necessary but was well tolerated by both fetus and mother. Our patient presented with ALF but in those patients with acutely decompensated liver function on a background of known advanced liver cirrhosis with portal hypertension, special consideration when planning surgery and anaesthesia is mandatory. Abdominal surgery can be technically challenging due to the large collateral circulation and preparation for potentially significant blood loss is required. During surgery continuous monitoring of the fetal heart tones were checked. Following OLT, fetal surveillance was based on ultrasound biometry, fetal heart rate monitoring and Doppler blood flow studies of fetal and uteroplacental circulation. Interestingly, amniotic fluid was dark brown in color, we speculated to be related to jaundice during pregnancy.
In summary, we report a case in which OLT was employed in a pregnant woman who presented with ALF of unclear origin during the second trimester. She subsequently was able to carry her pregnancy for six more weeks and delivered a pre-term otherwise healthy infant. Three years later, both mother and child continue to be in good health with no neurological impairment and overall excellent clinical condition. With careful planning and cooperation between the transplant team, anesthetists, obstetricians and neonatologists, the risks to the fetus can be minimized. Finally, ethical advice from an ethical committee and prenatal counseling evaluating fetal and maternal risks may be needed for an adequate management of pregnant patients with ALF.19