Background and aim. Metabolic syndrome is recognised as a potential risk factor for the development of hepatocellular carcinoma (HCC). The association between metabolic factors and hepatitis C (HCV)-related HCC has not yet been well clarified. This study was conducted to elucidate the role of metabolic factors in HCV-related HCC.
Material and methods. We recruited 147 HCC patients and compared them with 147 matched CHC patients and 320 controls. The plasma levels of homeostasis model assessment-IR (HOMA-IR), adiponectin and lipids for all participants were assessed.
Results. The HCC group showed significantly hig-her levels of insulin, glucose, HOMA-IR and adiponectin as well as lower levels of total cholesterol, HDL-C, LDL-C, and triglycerides compared with the matched CHC patients and controls. HOMA-IR did not correlate with pathologic features of HCC, whereas serum adiponectin levels correlated positively with the size of tumour nodules (P = 0.009). Based on stepwise logistic regression analysis, age (OR: 1.456, 95% CI: 1.0721.979, P < 0.01), HOMA-IR (OR: 2.50, 95% CI: 1.70-3.69, P = 0.001), and adiponectin (OR: 1.585, 95% CI: 1.2691.980, P = 0.001) were independently associated with HCC.
Conclusions. Metabolic abnormalities are closely associated with the occurrence and development of HCV-related HCC. Patients with CHC and high serum adiponectin levels face a higher risk of developing liver cancer. Insulin resistance, as measured by HOMA-IR, is significantly associated with HCV-related HCC.
Hepatocellular carcinoma (HCC) is the fifth most common tumour and the third most common cause of cancer-related deaths worldwide.1,2 It is well known that the high prevalence of hepatitis B and C gives rise to the high incidence of HCC.3 Recently, obesity and metabolic syndrome (MS) were shown in several epidemiologic studies to increase the risk of HCC. Understanding the risk factors for HCC development in patients infected with HCV is of great importance to help in elucidating novel modalities of management. The relationship between metabolic factors and chronic liver diseases, such as chronic hepatitis C (CHC) and HCC, has recently become a rapidly growing topic.4 MS is a combination of problems that include obesity, dyslipidaemia, insulin resistance (IR) and type 2 diabetes mellitus (T2DM) as potentially modifying factors.5 The prevalence of MS is increasing and parallels the obesity epidemic proportions worldwide.6 Moreover, HCV infection per se is now considered to be a special type of MS. HCV interacts with lipid metabolism, leading to steatosis and impairs glucose metabolism, leading to IR and T2DM.7
The mechanism by which obesity and MS promote hepatocarcinogenesis is still not fully understood. However, obesity-induced dysregulation of adipoki-nes, cytokines secreted by adipose tissues, is considered to play a key role.8 Adipose tissue controls the functions of other organs through the secretion of various adipokines, such as leptin, adiponectin, tumour necrosis factor α (TNF-α) and interleukin-6 (IL-6). Adiponectin, one of the major adipokines, possesses insulin-sensitising, anti-steatotic,7 antiinflammatory9 and antifibrotic effects.10 To date, the action of adiponectin CHC is still controversial. Our recent findings show that among BMI, age, and gender-matched HCV-negative controls, chronic HCV-4 infected patients have hyperadiponecti-naemia,11 and that adiponectin correlated with the different stages of liver injury (steatosis, inflammation and fibrosis).12 However, the role of adiponec-tin in HCV-related hepatocarcinogenesis remains unclear.
Taking into consideration the high prevalence of HCV-related HCC in Egypt,1,2 we designed a prospective case-controlled study to elucidate the association between metabolic abnormalities and the serum adiponectin levels and risk of hepatocarcino-genesis in patients with CHC.
Material and MethodsThis prospective case-controlled, hospital-based study was conducted between February 2009 and January 2010. A series of patients with HCV-related HCC was compared with two other groups: patients with CHC and healthy controls. CHC was diagnosed based on the presence of anti-HCV and detectable serum HCV-RNA for 6 months or more in combination with a liver biopsy obtained within 12 months of enrollment, with findings compatible with CHC using the Metavir score.13 Informed consent was obtained from each patient, and the protocol was approved by the institutional ethics committee and conducted in accordance with the international ethical guidelines.
Hepatocellular carcinoma group (group 1)This group consisted of 147 consecutive patients with HCC [114 (77.5%) patients were male, and 33 (22.5%) patients were female]. The mean age was 43.9 ± 4.7 (38-61) years. The diagnosis of HCC was based on either the histopathological findings of tumour tissue, one typical HCC feature on a dynamic image or alpha-fetoprotein (AFP) > 200 ng/mL if the nodule was > 2 cm in a cirrhotic liver, or two typical HCC features of dynamic images if the nodule was between 1 and 2 cm in a cirrhotic liver.14 Although histological data on the severity of liver diseases are not available for all HCC patients, we have histological data for 96 patients. Of the 96 patients, 43/96 (44.7%) were patients with advanced fi-brosis (F3-4).
Chronic hepatitis C (CHC) group (group 2)During the same period, 147 consecutive patients [115 (78.2%) patients were male, and 32 (21.8%) patients were female; mean age: 41.6 ± 6.6 (21-57) years] with CHC who consulted our clinic for possible IFN-based therapy were studied, including 86 with chronic hepatitis (F0-2) and 61/147 (42%). with advanced fibrosis (F3-4). Therefore, we do not believe that there is any obvious bias.
Healthy control group (group 3)The control group was comprised of 320 control subjects [201 (62.8%) patients were male, and 119 (37.1%) patients were female; mean age: 42.9 ± 10.3 (20-60) years]. To evaluate and compare the data to patients in the HCC and CHC groups, health check examinees from the same hospital who showed no abnormalities in laboratory examinations were included.
Patients who were younger than 18 years of age, were admitted for malignancies, received prior cancer therapy, and had a history of previous resection of HCC alcohol-related disorders, hepatic viral infections other than HCV, concurrent human immunodeficiency virus infection, significant changes in body weight (≥ 3 kg within 3 months), interferon-based therapy for HCV, or T2DM were excluded from our study.
Clinical and laboratory assessmentsInformation on weight, height, blood pressure, tumour size and whether there were any violations of metastasis, medical history, lifestyle characteristics and other related information was reported. Body mass index (BMI) was calculated by dividing the body weight in kilograms by the square of the patient’s height in meters. Data on Child-Pugh class, tumour-nodes metastases (TNM) stage of HCC determined by The American Joint Committee on Cancer/United International Consensus Committee (AJCC/UICC) staging system for HCC (6th edition),15 Okuda stage, and the Cancer of the Liver Italian Program (CLIP) stage were obtained by reviewing the medical records and radiological studies. Staging was determined by the Barcelona Clinic Liver Cancer staging system (BCLC).15
- •
Laboratory tests. Venous blood was drawn after a 12-hour overnight fast to determine the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, bili-rubin, platelet count, international normalised ratio, total cholesterol, HDL cholesterol (HDL-C), triglycerides (TG), LDL cholesterol (LDL-C), glucose, insulin, adiponectin, hepatitis B surface antigen (HBsAg), hepatitis B core antibody (HBc-Ab), anti-HCV, quantitative PCR (VER-SANT HCV RNA 3.0 Assay) for serum HCV-RNA levels, and HCV genotyping using INNO-LiPA III provided by (Inno-Lipa, Innogenetics, Genetics, Belgium). Serum glucose, ALT, AST, total and HDL cholesterol, LDL-C and TG were determined using automated procedures. Serum insulin was determined by electrochemiluminescence im-munoassays (Elecsys 2010; Roche Diagnostics, Indianapolis, Indiana, USA). IR was then investigated in all patients by the homeostasis model for the assessment of IR (HOMA-IR) using the standard formula: HOMA-IR = fasting insulin (uU/mL) x fasting glucose (mmol/L)/22.5. Serum adiponectin levels were measured in duplicate using Luminex xMAP technology and a multiplex assay (Linco Research, St. Charles, MO). The mean values of duplicate measurements were used in the analyses. For HCC patients, serum samples were collected prior to any tumour treatments.
All analyses were carried out using the statistical software package SPSS (Statistical Package for the Social Sciences) for Windows, version 14 (SPSS, Chicago, IL). Continuous variables were summarised as the mean ± SD, and categorical variables were stated as the frequency and percentage, unless otherwise indicated. Logarithmic transformation was performed for variables that were non-normally distributed. The Student’s t-test was used to evaluate the differences in serum adiponectin levels and HOMA-IR between groups. Correlations between variables were analysed using the Spearman’s rank correlation coefficient. Categorical variables were compared using a Mann-Whitney U test. For multi-variate analysis, a multivariate logistic regression analysis was applied. The Odds Ratio (OR) was used to estimate the relative risk of HCC. P values less than 0.05 were considered significant.
ResultsCharacteristics of the patientsThe baseline socio-demographic data and functional status of all the subjects are listed and summarised in table 1. All patients were infected with HCV genotype 4. There were significant differences in levels of bilirubin, albumin, ALT, AST and platelet count among HCC patients, CHC patients and healthy controls (P < 0.05).
Baseline demographics of the hepatocellular carcinoma, liver cirrhosis and healthy control groups.
| Variable Mean ± SD (range) | HCC (n =147) | CHC (n =147) | Controls (n = 320) | * P value (1 vs. 2) | ‡ P value (1 vs. 3) | ‡ P value (2 vs. 3) |
|---|---|---|---|---|---|---|
| Age (years) | 43.9 ± 4.7 (38-61) | 41.6 ± 6.6 (21-57) | 42.9 ± 10.3 (20-60) | 0.1 | 0.1 | 0.2 |
| Sex114 (77.5%) | 115 (78.2%) | 201 (62.8%) | 0.9 | 0.05 | 0.01 | |
| Male/Female (%) | 33 (22.5%) | 32 (21.8%) | 119 (37.1%) | |||
| BMI (kg/m2) | 24.9 ± 2.5 (21-30) | 25.08 ± 3.5 (20-35) | 25.3 ± 3.5 (20-35) | 0.2 | 0. 1 | 0.5 |
| AST (IU/L) | 135.1 ± 113.6 (31-707) | 46.6 ± 31.9 (11-220) | 15.7 ± 5.5 (12-21) | 0.001 | 0.001 | 0.002 |
| ALT (IU/L) | 71.7 ± 58.3 (15-342) | 44.1 ± 33.5 (10-300) | 29.6 ± 4.3 (12-35) | 0.001 | 0.001 | 0.01 |
| Albumin (g/dL) | 2.2 ± 0.4 (1.8-2.6) | 3.2 ± 0.3 (2.9-3.5) | 4 ± 0.2 (3.8-4.2) | 0.001 | 0.001 | 0.2 |
| Bilirubin (mg/dL) | 1.5 ± 0.2 (1.3-1.7) | 1 ± 0.3 (0.7-1.3) | 0.6 ± 0.4 (0.5-1) | 0.001 | 0.001 | 0.1 |
| Platelet (x 109/L) | 130.8 ± 52.2 (57-309) | 222.1 ± 50.9 (135-391) | 231.2 ± 60.32 (118-474) | 0.001 | 0.001 | 0.2 |
| Insulin (μU/mL) | 15.6 ± 4.3 (8.1-24.3) | 11.9 ± 4.05 (4.4-27.3) | 6.3 ± 0.8 (3.7-9) | 0.001 | 0.001 | 0.001 |
| Fasting glucose (mmol/L) | 7.2 ± 1.2 (4.07-9.6) | 4.3 ± 0.6 (2.4-5.8) | 3.5 ± 0.6 (2.3-4.5) | 0.001 | 0.001 | 0.001 |
| HOMA-IR | 5.3 ± 2.1 (2.1-9.8) | 2.2 ± 0.7 (1.5-5.04) | 1.02 ± 0.2 (0.6-1.5) | 0.001 | 0.001 | 0.001 |
| Adiponectin (μg/mL) | 21.7 ± 3.8 (14-30) | 15.4 ± 1.5 (11.5-17.5) | 8.1 ± 1.5 (5-12) | 0.001 | 0.001 | 0.001 |
| Cholesterol (mg/dL) | 144.9 ± 19.1 (110-200) | 185.7 ± 81.3 (103-474) | 202.8 ± 15.4 (156-236) | 0.001 | 0.05 | 0.001 |
| Triglycerides (mg/dL) | 95.9 ± 19.1 (49-140) | 103.7 ± 8.1 (44.6-190) | 119.4 ± 24.8 (64-178) | 0.05 | 0.001 | 0.01 |
| HDL-C (mg/dL) | 41.2 ± 2.6 (36-62) | 43.1 ± 3.7 (37-53) | 48.6 ± 6.9 (38-62) | 0.03 | 0.001 | 0.001 |
| LDL-C (mg/dL) | 110.6 ± 22 (70-183) | 122.2 ± 1.4 (72-126) | 122.9 ± 21.9 (80-180) | 0.001 | 0.001 | 0.5 |
| TNM stage † (I/II/III/IV) | 20/48/47/32 | |||||
| Okuda stage 1/2/3 | 54/59/34 |
TNM (tumour-nodes-metastases) stage for HCC was determined according to AJCC/UICC, 6th. edition. AFP: α-fetoprotein. ALT: alanine aminotransferase. BMI: body mass index. CI: confidence interval. DM2: type 2 diabetes mellitus. HCC: hepatocellular carcinoma. CHC: chronic hepatitis C. SD: standard deviation. t-test and ANOVA with pot-hoc test were used.
The metabolic parameters of the three groups are displayed in figures 1 and 2. The HCC group showed significantly lower levels of total cholesterol, HDL-C, and TG as well as higher levels of insulin, glucose, HOMA-IR and adiponectin than both the CHC patients and healthy controls (P < 0.01). The HCC group showed significantly lower levels of total cholesterol, LDL-C, HDL-C, and TG.
The correlation between serum adiponectin levels, and liver fibrosis was evaluated in patients with CHC. We elucidated that serum serum adiponectin level is directly proportionate with the stages of the fibrosis (16.4, 14.7, 12.9 and 11.8 μg/mL for stages of fibrosis F4, F3, F2 and F1).
Correlation of serum adiponectin, HOMA-IR and clinical parameters in patients with HCCThe correlation between serum adiponectin levels and other clinical factors was evaluated to elucidate the clinical relevance of serum adiponectin levels in 147 patients with HCC (Table 2). Adiponectin levels correlated positively with age and albumin and negatively with platelet count, male gender and BMI.
Correlations between serum adiponectin levels, HOMA-IR and other parameters in patients with hepatocellular carcinoma (HCC).
| Variable | HOMA-IR | Adiponectin | ||
|---|---|---|---|---|
| r | P | r | P | |
| Age | 0.13 | 0.2 | 0.32 | 0.001 |
| Male sex | 0.01 | 0.8 | -0.18 | 0.02 |
| BMI (kg/m2) | 0.30 | 0.02 | -0.30 | 0.001 |
| Cholesterol (mg/dL) | 0.01 | 0.2 | -0.05 | 0.6 |
| Triglycerides (mg/dL) | 0.08 | 0.6 | 0.06 | 0.4 |
| HDL (mg/dL) | -0.23 | 0.02 | 0.12 | 0.1 |
| LDL (mg/dL) | 0.18 | 0.08 | -0.08 | 0.2 |
| Fasting glucose (mmol/L) | 0.91 | 0.001 | -0.12 | 0.2 |
| Insulin (μU/ml) | 0.96 | 0.001 | -0.04 | 0.6 |
| HOMA-IR | - | - | -0.21 | 0.1 |
| Serum adiponectin (μg/mL) | -0.21 | 0.1 | - | - |
| ALT (IU/L) | 0.14 | 0.1 | 0.17 | 0.7 |
| AST (IU/L) | 0.13 | 0.2 | 0.01 | 0.8 |
| Haemoglobin (g/dL) | 0.02 | 0.8 | 0.07 | 0.8 |
| WBCs | 0.12 | 0.2 | 0.09 | 0.2 |
| Platelet (X 109/L) | 0.14 | 0.1 | -0.16 | 0.05 |
| Albumin (g/L) | 0.09 | 0.3 | 0.19 | 0.01 |
| Bilirubin (g/L) | 0.02 | 0.8 | 0.08 | 0.3 |
The HOMA-IR score correlated positively with BMI, serum insulin and glucose and negatively with HDL-C (Table 2).
The correlation between serum adiponectin levels and other clinical factors was evaluated to elucidate the clinical relevance of serum adiponectin levels in patients with CHC. In male subjects, the serum adi-ponectin level was correlated positively with age and negatively with platelet count and BMI. In female subjects, the serum adiponectin level was positively correlated with age. Platelet count showed a weak negative correlation. In the healthy control; adipo-nectin correlated with age and BMI in both males and females (Data not showed).
Stepwise logistic regression analysis for factors associated with development of HCCBased on stepwise logistic regression analysis, significant factors associated with development of HCC in patients with CHC were age (OR: 1.456, 95% CI: 1.072-1.979, P<0.01), HOMA-IR (OR: 2.50, 95% CI: 1.70-3.69, P = 0.001), and adiponectin (OR: 1.585, 95% CI: 1.269-1.980, P = 0.001) (Table 3).
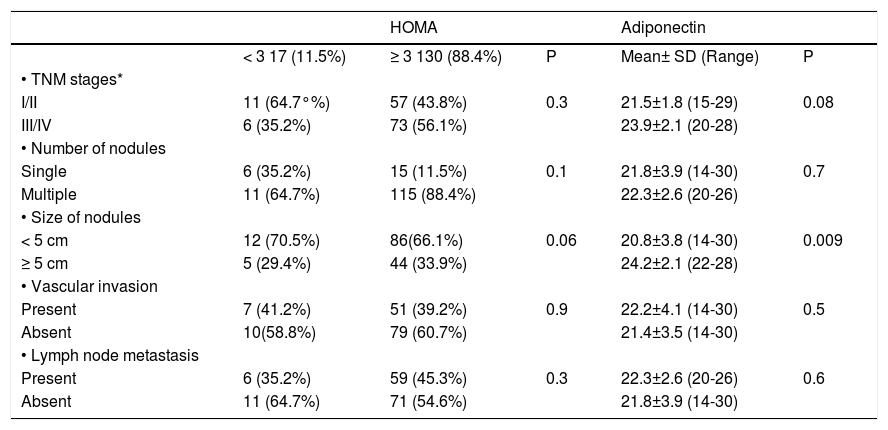
Correlation of serum adiponectin, HOMA-IR and pathologic features in patients with HCCAdiponectin levels and HOMA-IR score in HCC group according to various pathological parameters are summarised in table 4. Adiponectin levels correlated positively with the size of tumour nodules (P = 0.009) but not with the number of nodules (P = 0.7), vascular invasion (P = 0.5), lymph node metastasis (P = 0.6) or TNM staging (P = 0.08).
Relationship between adiponectin levels and HOMA and pathologic features in patients with hepatocellular carcinoma (HCC).
| HOMA | Adiponectin | ||||
|---|---|---|---|---|---|
| < 3 17 (11.5%) | ≥ 3 130 (88.4%) | P | Mean± SD (Range) | P | |
| • TNM stages* | |||||
| I/II | 11 (64.7°%) | 57 (43.8%) | 0.3 | 21.5±1.8 (15-29) | 0.08 |
| III/IV | 6 (35.2%) | 73 (56.1%) | 23.9±2.1 (20-28) | ||
| • Number of nodules | |||||
| Single | 6 (35.2%) | 15 (11.5%) | 0.1 | 21.8±3.9 (14-30) | 0.7 |
| Multiple | 11 (64.7%) | 115 (88.4%) | 22.3±2.6 (20-26) | ||
| • Size of nodules | |||||
| < 5 cm | 12 (70.5%) | 86(66.1%) | 0.06 | 20.8±3.8 (14-30) | 0.009 |
| ≥ 5 cm | 5 (29.4%) | 44 (33.9%) | 24.2±2.1 (22-28) | ||
| • Vascular invasion | |||||
| Present | 7 (41.2%) | 51 (39.2%) | 0.9 | 22.2±4.1 (14-30) | 0.5 |
| Absent | 10(58.8%) | 79 (60.7%) | 21.4±3.5 (14-30) | ||
| • Lymph node metastasis | |||||
| Present | 6 (35.2%) | 59 (45.3%) | 0.3 | 22.3±2.6 (20-26) | 0.6 |
| Absent | 11 (64.7%) | 71 (54.6%) | 21.8±3.9 (14-30) |
The HOMA-IR score showed a tendency for correlation with the size of tumour nodules but did not yield statistical significant results (P = 0.06) and showed no correlation with the number of nodules (P = 0.1), vascular invasion (P = 0.9), lymph node metastasis (P = 0.3) or TNM staging (P = 0.3).
DiscussionIn our prospective case-controlled study, we assessed the association between metabolic factors and HCV-related HCC in a hospital-based setting. To our knowledge, this is the first single study to report a positive association between adiponectin levels, HOMA-IR and the presence of HCC. Our data demonstrated higher levels of glucose, insulin, HOMA-IR, and adiponectin in HCC patients than in CHC patients and healthy matched controls. An independent association between IR, adiponectin and HCC development was reported. No correlation was detected between the HOMA-IR score and adiponectin.
First, this work elucidated an independent association between IR and the development of HCC, regardless of T2DM. IR and obesity, the major components of MS linked with different cancers. Previous reports also showed a link between T2DM and HCC.16,17 Although, our work was not designed to clarify the pathogenic interaction between IR and the development of HCC, a few hypotheses can be stated. IR and compensatory hyperinsulinaemia can promote the synthesis and biological activity of insulin-like growth factor 1 (IGF-1),18 which stimulates cell proliferation and inhibits apoptosis, and has been shown to have strong mitogenic effects on a wide variety of cancer cell lines, including HCC.19 Excess insulin might also affect the development of cancer indirectly by downregulating the level of IGF-binding protein 1, which increases the level and bioavailability of total circulating IGF-1.19
Second, we found that patients with HCC tended to have hyperadiponectinaemia. Adiponectin was an independent risk factor of hepatocarcino-genesis. Albeit, in vitro data demonstrate that adi-ponectin has the molecular potential to inhibit the oncogenic actions of leptin in hepatocellular carci-nogenesis by blocking downstream effector mole-cules,20 our clinical findings are consistent with data from another prospective longitudinal study that reported a positive association between adipo-nectin levels and later HCC development,21 and with data from another study in chronic hepatitis B patients.22
Exploring the role of adiponectin in HCC may be more complex because of its underlying chronic hepatitis infection. Previous studies have demonstrated that circulating adiponectin levels are inversely associated with the risk of malignancies associated with IR, including endometrial, breast, colon and gastric cancers.23-25 Although adiponectin is often considered to have anti-inflammatory properties, hy-peradiponectinaemia has recently been associated with inflammation in diseases such as arthritis,26 preeclampsia27 and end-stage renal disease.28 In this study, the adiponectin level was elevated in patients with CHC compared with the healthy controls, which is consistent with previous data both from our unit and those reported by others.10,11 Accordingly, adiponectin may play different roles in CHC are in contrast to observations in patients with obesity-related (non-alcoholic fatty liver disease; NAFLD and non-alcoholic steatohepatitis (NASH) in whom necroinflammatory activity was associated with decreased levels of adiponectin. While hypoadiponecti-nemia may initiate the pathogenesis of NASH, adiponectin may be elevated in CHC secondary to fi-brosis progression and subsequently modulates disease progression. An alternative explanation for the association of hyperadiponectinemia with degree of fibrosis and HCC development could be adiponec-tin resistance caused by downregulation of adipo-nectin receptors.
In this study, serum adiponectin levels exhibited a positive correlation with tumour size; these interesting observations may suggest an intimate relationship between metabolic disorders and HCV-related HCC.
Third, patients with HCC showed significantly lower levels of total cholesterol, LDL-C, HDL-C, and TG. Potential pathogenic mechanisms of hypolipida-emia include pro-inflammatory cytokines (as IL-6, TNF-α), inhibition of TG synthesis29 and change in general nutritional status. These findings are consistent with a previous study in HCC patients31,32 and in other malignancies.30,31 Ultimately, the contradictory associations, i.e., the inverse and positive correlations between lipid metabolism and HCC, reported here and elsewhere, further demonstrate the complicated process of dyslipidaemia involved in the pathogenesis of HCC31,32 and deserve further evaluation.
There are several limitations to our study. First, the analysis was carried out in a case-controlled, hospital-based cohort drawn from a clinical series of patients, and not from the community. However, the cases were carefully matched or stratified by age, gender, BMI, time of hospital admission and aetiology to minimise any confounding factors. Second, it would be interesting to apply more stringent methodology in order to dissect the temporal relationship between IR/adipocytokines and HCC in longitudinal follow-up studies and to evaluate the serial changes in adiponectin levels in HCC patients who showed tumor growth or underwent treatment which may be useful in better understanding of the role of adi-ponectin in the pathogenesis of HCC.
In conclusion, this study demonstrated an independent association between IR/serum adiponectin levels and HCV-related HCC. The predictive value of metabolic parameters and the pathogenesis of abnormal metabolism in HCC require further validation in future studies. These findings may have potentially crucial prognostic and therapeutic implications in the management of CHC patients. Because MS is a potentially modifiable factor, the influence of therapeutic intervention aimed at decreasing IR on the natural history of HCV and risk of HCV-related HCC deserves further evaluation.











![The comparison of lipid profiles of the three groups [hepatocellular carcinoma (HCC), chronic hepatitis C (CHC) and the control group]. The comparison of lipid profiles of the three groups [hepatocellular carcinoma (HCC), chronic hepatitis C (CHC) and the control group].](https://static.elsevier.es/multimedia/16652681/0000001100000004/v1_201906191936/S1665268119314620/v1_201906191936/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




