Background. Transforming growth factor alpha (TGFα) is an important mitogen that binds to epidermal growth factor receptor and is associated with the development of several tumors.
Aims. Assessment of the immunoexpression of TGFα in hepatocellular carcinoma (HCC) and in non-neoplastic liver tissue and its relationship to morphological patterns of HCC.
Material and methods. The immunohistochemical expression of TGFα was studied in 47 cases of HCC (27 multinodular, 20 nodular lesions). Five lesions measured up to 5 cm and 15 lesions above 5 cm. Thirty-two cases were graded as I or II and 15 as III or IV. The non-neoplastic tissue was examined in 40 cases, of which 22 had cirrhosis. HBsAg and anti-HCV were positive in 5/38 and 15/37 patients, respectively. The statistical analysis for possible association of immunostaining of TGFα and pathological features was performed through chi-square test.
Results. TGFα was detected in 31.9% of the HCC and in 42.5% of the non-neoplastic. There was a statistically significant association between the expression of TGFα and cirrhosis (OR = 8.75, 95% CI = [1.93, 39.75]). The TGFα was detected more frequently in patients anti-HCV(+) than in those HBsAg(+). The immunoexpression of TGFα was not found related to tumor size or differentiation. In conclusion the TGFα is present in hepatocarcinogenesis in HBV negative patients. Further analysis is needed to examine the involvement of TGFα in the carcinogenesis associated with HCV and other possible agents. In addition, TGFα has an higher expression in hepatocyte regeneration and proliferation in cirrhotic livers than in HCC.
The heterogeneity in the worldwide incidence of hepatocellular carcinoma (HCC) clearly reflects the risk factors to which populations are exposed, particularly in regard to the hepatitis viruses B (HBV) and C (HCV). Other conditions predisposing to HCC, such as the alcohol intake and food contamination by aflatoxin B1 also contribute to this varia-tion.1 Recent publications have shown that the incidence of HCC has increased worldwide, even in regions considered to have historically low incidence. This fact is most probably a reflection of HCV infection and the increasing number of obesity in many countries.2–4
Additionally, advances in imaging and surgical techniques, as well as more clearly established morphological criteria, have led to increased detection of precancerous lesions and early HCC.5–7
HCC arises mainly in liver that presents with chronic injuries and, consequently, cirrhosis. Therefore, surgical human samples including regenerative, macroregenerative and dysplastic nodules and HCC may serve as models for the assessment of several aspects of hepatocarcinogenesis.8 Molecular studies have demonstrated genetic heterogeneity in human and animal hepatocarcinogenesis, with the involvement of suppressor and promoters genes as well as of adhesion molecules and growth factors.9,10
Growth factors are polypeptides that, upon binding to specific receptors on the cell membrane, elicit several intracellular signals which, among other functions, regulate cell proliferation, survival to apoptosis, neoangiogenesis, invasion and metastasis. Insulin growth factor, hepatocyte growth factor, platelet-derived growth factor, and transforming growth factor β are among the most studied factors in hepatocarcinogenesis.11,12
The association between growth factors and onco-genes plays an important role in the development of several tumors. In HCC, co-expression of transforming growth factor alpha (TGFα) and c-myc has been found.13 Transforming growth factor β inacti-vation also appears to cooperate with TGFα overex-pression to promote liver cancer in mouse.14 Under physiological conditions in the liver, TGFα acts as an important mitogen for hepatocytes and can stimulate liver regeneration in rats.15 The identification of HCC molecular pathways also contribute to new therapeutic targets and may offer the patients a possible tailored treatment as blockage of TGFα surface receptor.16,17 However, only a few publications have examined the expression of TGFα in HCC, most of them approaching experimental models.18–22 In this study we assess the immunoex-pression of TGFα in HCC and adjacent non-neoplastic tissue and the relationship of this growth factor to morphological patterns of HCC.
Material and MethodsForty seven samples of HCC from Department of Pathology, Federal University of Rio de Janeiro were studied. The tissues were obtained from surgery, biopsy and autopsy. Grossly, 27 were multino-dular and 20 were single nodule, of which 5 measured up to 5 cm and 15 measured above 5 cm. Histologically, 22 cases were trabecular, 18 pseudo-glandular (admixed with trabecular pattern) and 7 compact. Thirty-two cases were graded as I or II (well-differentiated) and 15 as III or IV (moderately and poorly differentiated).23
The adjacent non-neoplastic tissue was studied in 40 cases, of which 22 had cirrhosis. Among the patients enrolled in this study the serological markers of hepatitis B and C infection were investigated in 38 and 37 patients, respectively. Of these, 5 were positive for HBsAg and 15 for anti-HCV.
ImmunohistochemistryThe monoclonal antibody used in the immunohis-tochemical study was anti-TGFα (AB2, clone 2134.4, Oncogene Science Inc, USA) at a dilution of 1:100. The sections were incubated with this antibody in a moist chamber at 4 °C for 18 h (overnight). This was followed by incubation of biotinylated secondary antibody, in a moist chamber at 37 °C for 30 minutes. Between incubations, the slides were washed in PBS, pH 7.4. The reaction was detected by incubation with the streptavidin-biotin-peroxidase system (Strep AB complex/HRP Duet Mouse/Rabbit, Dako A/S Denmark) at a dilution of 1:500. The development was performed with a chromogen substrate (diaminobenzidine) for 5 min at 37 °C. The sections were stained with Harris hematoxylin. The immuno-detection was assessed as positive when > 5% of the cells were labeled.
Statistical analysisPearson’s chi-square test was employed in the analysis of associations between variables considering a 5% significance level or calculating an odds ratio (OR) and their respective confidence interval at 95%. All statistical analyses were performed using the program Statistical Package for the Social Sciences 17.0.
ResultsTGFα was detected in 31.9% (15/47) of HCC and 42.5% (17/40) in adjacent non-neoplastic tissue. This difference in expression was not statistically significant (p = 0.308). The immunoreactivity was cyto-plasmic and diffuse in the HCC and in non-neoplastic tissue. The perinuclear pattern was observed only in four HCC. Tumors that expressed TGFα were observed in 40% (6/15) of patients with positive serology for HCV and in only one patient out of the 5 positive for HBsAg.
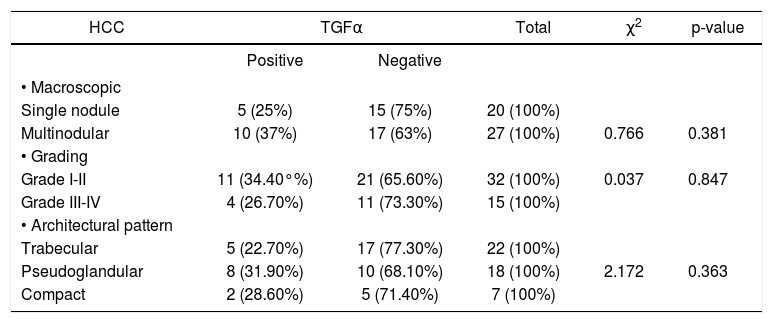
TGFα immunoexpression was observed in 44.4% (4/13) of the nodules > 5 cm and only in 14.2% (1/7) of the smaller ones. Meanwhile, the multinodular lesions displayed TGFα in 37%. When comparing the multinodular and single tumors (including both those greater than and < 5 cm), the expression of TGFα is more frequent in the multinodular lesions, although not reaching statistical significance. The same was found regarding the tumor differentiation. TGFα was positive in 34.3% (grade I and II) and in 26.6% (grade III and IV) (OR = 1.44), also there were no differences of TGFα immunoexpression among the different histological patterns (Table 1).
Hepatocellular carcinoma. Morphological data and TGFα immunoexpression.
| HCC | TGFα | Total | χ2 | p-value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| • Macroscopic | |||||
| Single nodule | 5 (25%) | 15 (75%) | 20 (100%) | ||
| Multinodular | 10 (37%) | 17 (63%) | 27 (100%) | 0.766 | 0.381 |
| • Grading | |||||
| Grade I-II | 11 (34.40°%) | 21 (65.60%) | 32 (100%) | 0.037 | 0.847 |
| Grade III-IV | 4 (26.70%) | 11 (73.30%) | 15 (100%) | ||
| • Architectural pattern | |||||
| Trabecular | 5 (22.70%) | 17 (77.30%) | 22 (100%) | ||
| Pseudoglandular | 8 (31.90%) | 10 (68.10%) | 18 (100%) | 2.172 | 0.363 |
| Compact | 2 (28.60%) | 5 (71.40%) | 7 (100%) | ||
TGFα: transforming growth factor alpha. HCC: hepatocellular carcinoma.
The non-neoplastic tissues were TGFα positive in 17 cases, of which 14 were cirrhotic parenchyma and 3 were non-cirrhotic, but with chronic hepatitis. Thus, patients with cirrhosis had a chance of TGFα expression 8 times greater than cases without cirrhosis, and this association was statistically significant at 5% (OR = 8.75, 95% CI = [1, 93, 39,75]).
DiscussionTGFα is a polypeptide composed of 50 amino acids residues with 30-40% homology to epidermal growth factor and binds the its membrane receptor.24 The synthesis of TGFα occurs during fetal development and in some transformed cells.25,26 TGFα mRNA has been reported to be over expressed in rat livers after partial hepatectomy9 and in those with malignant transformation arising from chemical carcinogene-sis.26 In acute and chronic liver damage (hepatitis and cirrhosis), TGFα stimulates hepatocyte proliferation and differentiation.27
In human malignancies, TGFα is expressed in hepatic, ovarian, breast, colonic, and brain carcino-mas.28–30 HCC patients showed increased serum and urinary levels of TGFα,31–33 which has also been detected by immunohistochemistry in neoplastic cells.16–18 The present study showed the immunohis-tochemical expression of TGFα in 31.9% of HCC. Others have described positivity percentages ranging from 28 to 96%.18–24,31 However, the highest described incidences have been in Asian patients and are associated with HBV infection.19,20 Percentages that are similar to ours were found in studies conducted with Caucasians.18 The low frequency of TGFα expression detected in our study may be explained by the small number of patients with a positive serological marker for HBV in our sample set. Some authors have suggested that the expression of TGFα could be involved in a series of HBV-associated hepatocarcinogenetic events.19 However, the levels of TGFα mRNA are high in HCC and cirrhosis patients with chronic hepatitis C as well as hepatitis B.34 In patients with HCV cirrhosis, viral replication seems to mediate the expression TGFα and IGF-II, which may allow these factors to contribute to the initiation of hepatocarcinogenesis.35 In this study 40% of HCC patients positive for anti-HCV were also positive for expression of TGF and the expression of TGFα was not related to any specific morphological pattern, such as the macroscopic pattern and degree of tumor differentiation, which are considered to be prognostic factors. These results are consistent with those of other authors.19,21 However, our results differ from others in relation to tumor differentiation because, in some studies, TGFα decreases as the tumor becomes less differentiated.20 Although the relationship between the degree of tumor differentiation and the expression of TGFα has not been found significant herein (which may be due to sample size), the measurement of estimated odds ratio (odds ratio > 1) indicated that the chance of expression of TGFα tends to be higher in well-differentiated lesions. A comparative analysis between the frequencies of cases positive for TGFα showed that its occurrence in HCC is lower than in non-neoplastic parenchyma, a finding also found by others.36
In regarding to non-neoplastic tissue analysis, our results corroborate previous investigations, which have suggested a greater involvement of TGFα in cirrhosis than in HCC.21 Therefore, this greater involvement in early steps of hepatocyte proliferation and regeneration may serve as a hint for a possible role of TGFα in early stages of hepatocarcinogenesis. Other study reinforces this possibility, because the immunoreactivity of TGFα is more intense in the regenerative nodules and low and high grade dysplastic nodules than in HCC.33
In conclusions, our findings show that TGFα is present in hepatocarcinogenesis, even in HBV negative patients, and that further analysis is needed to examine the involvement of TGFα in the carcinoge-nesis associated with HCV and other possible agents. Furthermore, our data suggest that TGFα has a greater expression in hepatocyte regeneration and proliferation in cirrhotic livers than in HCC.
Abbreviations- •
TGFα: transforming growth factor alpha (TGFα).
- •
HCC: hepatocellular carcinoma.
- •
HBsAg: hepatitis B surface antigen.
- •
HCV: hepatitis C virus.
- •
EGF: Epidermal growth factor.
- •
IGF: Insulin growth factor.
- •
OR: Odds ratio.
CAPES (PROAP).