The association between the level of body mass index (BMI) and the mortality of patients with critical liver disease remains unclear. This study aimed to examine the association between BMI and hospital mortality of patients with acute-on-chronic liver failure (ACLF).
MethodsClinical data from 146 ACLF patients were collected and analyzed. BMI was categorized into three groups: lower BMI (<18.5kg/m2), normal BMI (18.5–24.9kg/m2), and overweight (25.0–32.0kg/m2). BMI and laboratory parameters were measured one day before, or on the day of the start of the treatment. Values of BMI and laboratory parameters were compared between survivors and non-survivors, and then hospital mortality rates were compared among patients with different BMI levels.
ResultsThe prognosis of ACLF patients was significantly correlated with international normalized ratio (INR), albumin and BMI. The ACLF patients with low albumin level and high INR values tend to have a high mortality rate. Also, survival time was significantly shorter in the ACLF patients with lower BMI, while patients with normal and overweight values had longer survival time.
ConclusionsA graded association between BMI and hospital mortality with a strong significant trend was found in ACLF patients in China.
Liver failure is caused by a variety of precipitating factors, among which the commonest is untreated hepatitis B virus infection in China and excessive alcohol use in Europe respectively. The clinical syndrome of liver failure is usually manifested as coagulation dysfunction, jaundice, ascites and hepatic encephalopathy, and is characterized by a high short-term mortality rate (>15% at 28d) and by an acute deterioration of liver function [1]. Acute-on-chronic liver failure (ACLF), firstly introduced and described by Ohnishi [2], has been attracting attention globally. It was defined as “Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or misdiagnosed chronic liver disease” according to consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) [3]. However, up till now, there is a lack of uniform definition and diagnostic criteria for ACLF in the world. It is found that European and American standards emphasize cirrhosis basis with accompanying multiple organ failure, renal and lung may is the most commonly affected organs, in order to predict the prognosis of patients as early as possible through a CLIF-SOFA scoring system [4]. While, Asia-Pacific consensus emphasizes the emergence of liver failure on the basis of chronic liver disease, mainly focuses on the performance of liver failure to get early diagnosis and intervention of disease. Although a relatively high mortality has been observed in ACLF patients, well-established prognostic indicators are not available for mortality [5].
The intrahepatic causes of liver failure include HBV activation, chronic liver disease with accompanying acute Hepatitis A/E, heavy alcohol consumption or the use of hepatotoxicity drugs, and so on. Extrahepatic triggers include acute gastrointestinal hemorrhage, hepatocellular carcinoma intervention or surgical treatment. Gastrointestinal dysfunction was commonly caused by endotoxemia in ACLF, and endotoxemia was produced by intestinal flora alternation and translocation [6]. It has been shown that early onset of ACLF is usually accompanied with significant loss of appetite and with disorder of nutrient absorption. Besides, progressive muscle consumption and malnutrition, which are characteristics of most chronic liver diseases in general, especially liver cirrhosis, were also found to be associated with ACLF disease [7]. Therefore, early implementation of nutritional intervention has become an important part of the treatment for ACLF patients.
Currently, nutrition assessment is widely used in Oncology, Infection and Gerontology, where it shows a good evaluative power on the prognosis of diseases. Recently, class III obesity is a newly identified risk factor for ACLF development in patients with decompensated cirrhosis which underscore the importance of weight management in cirrhosis to reduce the risk of ACLF [8]. Except that, there have been few studies evaluate the association between nutrition level and the prognosis of ACLF [8,9]. Body mass index (BMI) is considered by the World Health Organization (WHO) to be a standard and useful population measure of weight status in large-scale surveys of nutritional status in adults. BMI is a common indicator and widely used in clinical nutrition assessment as well. Therefore, it may be meaningful to investigate the feasibility of using BMI as a prognostic indicator in ACLF patients.
2Methods2.1Eligibility/study subjectsAll patients diagnosed with ACLF according to the APASL definition in 2014 who subsequently hospitalized and treated at the Department of Liver Research Center of the First Affiliated Hospital of Fujian Medical University, China from December 2014 to July 2017 were recruited in this study. Patients were excluded if they had the following conditions: liver ascites, denovo tumors, diabetes mellitus, with the use of hormone and with missing values in demographics, clinical laboratory parameters or BMI. The total number of patients was 213 before exclusion and only 12 patients were in Intensive Care Unit. There were few patients chose to stay in the ICU because of the high cost. Patients with respiratory failure or kidney failure, who need to do trachea intubation or CRRT, were more possible to give up treatment due to the inability to do liver transplantation. After exclusion, a total of 146 patients were recruited, hepatitis B virus infection 127 patients, alcoholic liver disease 11 patients, other disease 8 patients, among which 36 patients with liver cirrhosis and 51 deaths were observed. Referring to the CLIF-SOFA scoring criteria, renal and lung were the most commonly affected organs in this study. This prospective study was approved by the ethics committee at the First Affiliated Hospital of Fujian Medical University, China. Informed consent were obtained from all individuals prior to donation of blood samples and statistical analysis of their clinical records.
2.2Measurement of parameters/data collectionPatients were classified into survivors and non-survivors’ group based on the prognosis of treatment. Common risk factors including age, gender, hospital stays, and BMI were collected from the electronic medical record system. The following clinical laboratory parameters were collected before the start of the treatment including total bilirubin (TBIL), prothrombin time(PT), international normalized ratio (INR), albumin (ALB), total cholesterol (CHO), triglyceride (TG) and cholinesterase (CHE), leukocyte count (WBC), erythrocyte count (RBC), hemoglobin (HB), platelet count (PLT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γ-GTP), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), alpha fetoprotein (AFP), fasting blood-glucose (FBG), blood urea nitrogen (BUN), and serum creatinine (Scr) and ammonia (AMON).
2.3Statistical analysisStatistical analyses were performed using SPSS 24.0. Continuous variables were presented as mean±standard deviation which were further evaluated by Student's t-test. Continuous variables if not normally distributed were presented as median (interquartile range) which were further evaluated by Mann–Whitney U test. Categorical variables were described using frequencies and proportions and Pearson's chi-squared test was used to assess the differences of categorical variables.
Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (95%CI) for the association between BMI and all-cause mortality. Participants were followed up from date of hospital admission to date of death or date of discharge, whichever came first. Proportional hazard assumption was tested using Kaplan–Meier survival curves method. BMI was categorized into three groups: lower BMI (<18.5kg/m2), normal BMI (18.5–24.9kg/m2), and overweight (25.0–32.0kg/m2). Covariates included in the regression models were age, gender, causes of disease and clinical or laboratory parameters that were statistically differed based on levels of BMI or mortality. P values less than 0.05 were considered significant.
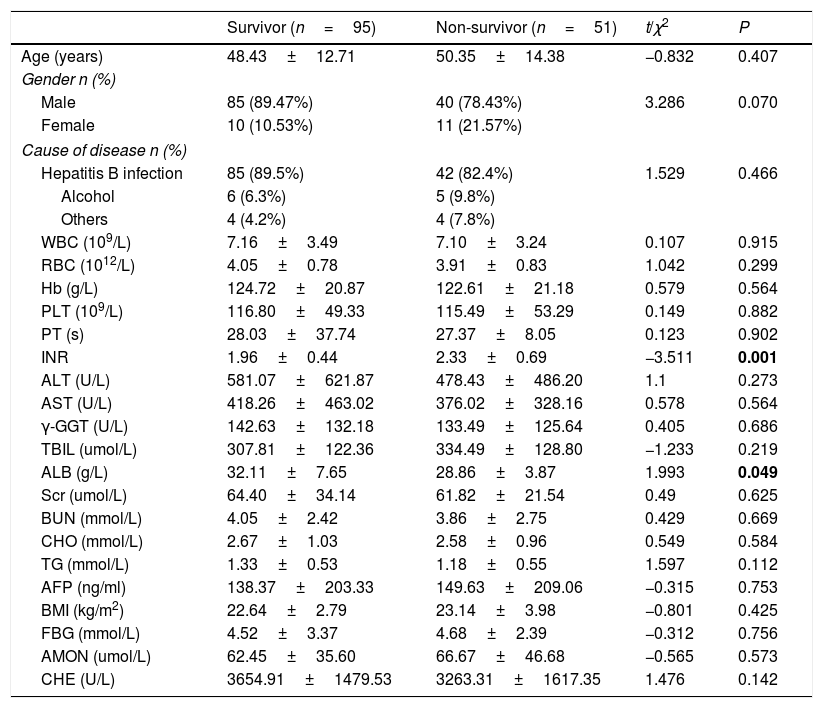
3ResultsDemographics and clinical laboratory parameters between survivors and non-survivors were summarized in Table 1. The level of INR was significantly higher in non-survivors than survivors (non-survivors: 2.33 units, survivors: 1.96 units, P=0.001). In contrast, the level of ALB was higher in survivors (survivors: 32.11g/L, non-survivors: 29.96g/L, P=0.049). Compared reference interval, the levels of blood ammonia were increased which can be used as a reference index of clinical treatment, but there was no difference between the survivors and non-survivors (survivors: 62.45umol/L, non-survivors: 66.67umol/L, P=0.573). Other clinical parameters including age, gender and laboratory tests which were not significantly different between survivors and non-survivors were presented in Table 1.
Characteristics of patients between survivors and non-survivors.
| Survivor (n=95) | Non-survivor (n=51) | t/χ2 | P | |
|---|---|---|---|---|
| Age (years) | 48.43±12.71 | 50.35±14.38 | −0.832 | 0.407 |
| Gender n (%) | ||||
| Male | 85 (89.47%) | 40 (78.43%) | 3.286 | 0.070 |
| Female | 10 (10.53%) | 11 (21.57%) | ||
| Cause of disease n (%) | ||||
| Hepatitis B infection | 85 (89.5%) | 42 (82.4%) | 1.529 | 0.466 |
| Alcohol | 6 (6.3%) | 5 (9.8%) | ||
| Others | 4 (4.2%) | 4 (7.8%) | ||
| WBC (109/L) | 7.16±3.49 | 7.10±3.24 | 0.107 | 0.915 |
| RBC (1012/L) | 4.05±0.78 | 3.91±0.83 | 1.042 | 0.299 |
| Hb (g/L) | 124.72±20.87 | 122.61±21.18 | 0.579 | 0.564 |
| PLT (109/L) | 116.80±49.33 | 115.49±53.29 | 0.149 | 0.882 |
| PT (s) | 28.03±37.74 | 27.37±8.05 | 0.123 | 0.902 |
| INR | 1.96±0.44 | 2.33±0.69 | −3.511 | 0.001 |
| ALT (U/L) | 581.07±621.87 | 478.43±486.20 | 1.1 | 0.273 |
| AST (U/L) | 418.26±463.02 | 376.02±328.16 | 0.578 | 0.564 |
| γ-GGT (U/L) | 142.63±132.18 | 133.49±125.64 | 0.405 | 0.686 |
| TBIL (umol/L) | 307.81±122.36 | 334.49±128.80 | −1.233 | 0.219 |
| ALB (g/L) | 32.11±7.65 | 28.86±3.87 | 1.993 | 0.049 |
| Scr (umol/L) | 64.40±34.14 | 61.82±21.54 | 0.49 | 0.625 |
| BUN (mmol/L) | 4.05±2.42 | 3.86±2.75 | 0.429 | 0.669 |
| CHO (mmol/L) | 2.67±1.03 | 2.58±0.96 | 0.549 | 0.584 |
| TG (mmol/L) | 1.33±0.53 | 1.18±0.55 | 1.597 | 0.112 |
| AFP (ng/ml) | 138.37±203.33 | 149.63±209.06 | −0.315 | 0.753 |
| BMI (kg/m2) | 22.64±2.79 | 23.14±3.98 | −0.801 | 0.425 |
| FBG (mmol/L) | 4.52±3.37 | 4.68±2.39 | −0.312 | 0.756 |
| AMON (umol/L) | 62.45±35.60 | 66.67±46.68 | −0.565 | 0.573 |
| CHE (U/L) | 3654.91±1479.53 | 3263.31±1617.35 | 1.476 | 0.142 |
The p values which less than 0.05 are written in bold.
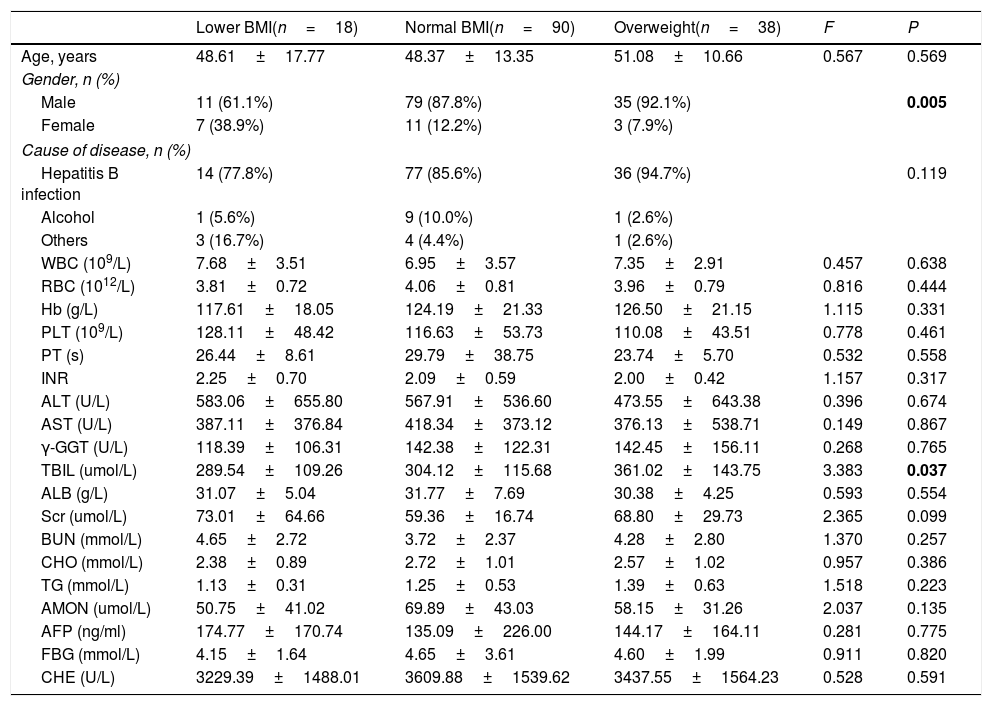
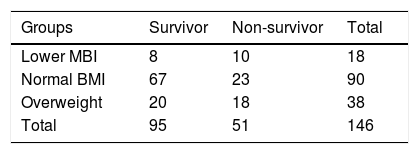
BMI was categorized into three groups: lower BMI group (<18.5kg/m2), normal BMI group (18.5–24.9kg/m2) and overweight group (25.0–32.0kg/m2). Characteristics of study participants with different BMI levels were summarized in Table 2. Compared to female participants, male participants had a higher level of BMI (P=0.005). Meanwhile, the level of TBIL was significantly distinguished among different BMI stages (P=0.037) and overweight patients had highest level of TBIL (361.02umol/L). Overweight patients were commonly complicated with fatty liver which would aggravate their liver function damage and finally lead to the increase of bilirubin.
Characteristics of patients with ACLF by BMI levels.
| Lower BMI(n=18) | Normal BMI(n=90) | Overweight(n=38) | F | P | |
|---|---|---|---|---|---|
| Age, years | 48.61±17.77 | 48.37±13.35 | 51.08±10.66 | 0.567 | 0.569 |
| Gender, n (%) | |||||
| Male | 11 (61.1%) | 79 (87.8%) | 35 (92.1%) | 0.005 | |
| Female | 7 (38.9%) | 11 (12.2%) | 3 (7.9%) | ||
| Cause of disease, n (%) | |||||
| Hepatitis B infection | 14 (77.8%) | 77 (85.6%) | 36 (94.7%) | 0.119 | |
| Alcohol | 1 (5.6%) | 9 (10.0%) | 1 (2.6%) | ||
| Others | 3 (16.7%) | 4 (4.4%) | 1 (2.6%) | ||
| WBC (109/L) | 7.68±3.51 | 6.95±3.57 | 7.35±2.91 | 0.457 | 0.638 |
| RBC (1012/L) | 3.81±0.72 | 4.06±0.81 | 3.96±0.79 | 0.816 | 0.444 |
| Hb (g/L) | 117.61±18.05 | 124.19±21.33 | 126.50±21.15 | 1.115 | 0.331 |
| PLT (109/L) | 128.11±48.42 | 116.63±53.73 | 110.08±43.51 | 0.778 | 0.461 |
| PT (s) | 26.44±8.61 | 29.79±38.75 | 23.74±5.70 | 0.532 | 0.558 |
| INR | 2.25±0.70 | 2.09±0.59 | 2.00±0.42 | 1.157 | 0.317 |
| ALT (U/L) | 583.06±655.80 | 567.91±536.60 | 473.55±643.38 | 0.396 | 0.674 |
| AST (U/L) | 387.11±376.84 | 418.34±373.12 | 376.13±538.71 | 0.149 | 0.867 |
| γ-GGT (U/L) | 118.39±106.31 | 142.38±122.31 | 142.45±156.11 | 0.268 | 0.765 |
| TBIL (umol/L) | 289.54±109.26 | 304.12±115.68 | 361.02±143.75 | 3.383 | 0.037 |
| ALB (g/L) | 31.07±5.04 | 31.77±7.69 | 30.38±4.25 | 0.593 | 0.554 |
| Scr (umol/L) | 73.01±64.66 | 59.36±16.74 | 68.80±29.73 | 2.365 | 0.099 |
| BUN (mmol/L) | 4.65±2.72 | 3.72±2.37 | 4.28±2.80 | 1.370 | 0.257 |
| CHO (mmol/L) | 2.38±0.89 | 2.72±1.01 | 2.57±1.02 | 0.957 | 0.386 |
| TG (mmol/L) | 1.13±0.31 | 1.25±0.53 | 1.39±0.63 | 1.518 | 0.223 |
| AMON (umol/L) | 50.75±41.02 | 69.89±43.03 | 58.15±31.26 | 2.037 | 0.135 |
| AFP (ng/ml) | 174.77±170.74 | 135.09±226.00 | 144.17±164.11 | 0.281 | 0.775 |
| FBG (mmol/L) | 4.15±1.64 | 4.65±3.61 | 4.60±1.99 | 0.911 | 0.820 |
| CHE (U/L) | 3229.39±1488.01 | 3609.88±1539.62 | 3437.55±1564.23 | 0.528 | 0.591 |
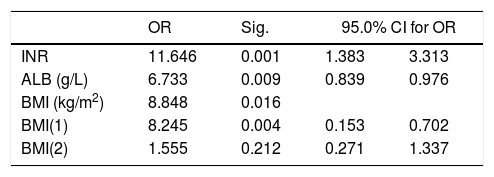
To explore which factors influence survival time of ACLF patients, the multi-factor Cox regression analysis was performed. In Table 3, it is showed that INR, ALB and BMI were independent factors affecting the survival time of ACLF patients (P<0.05).
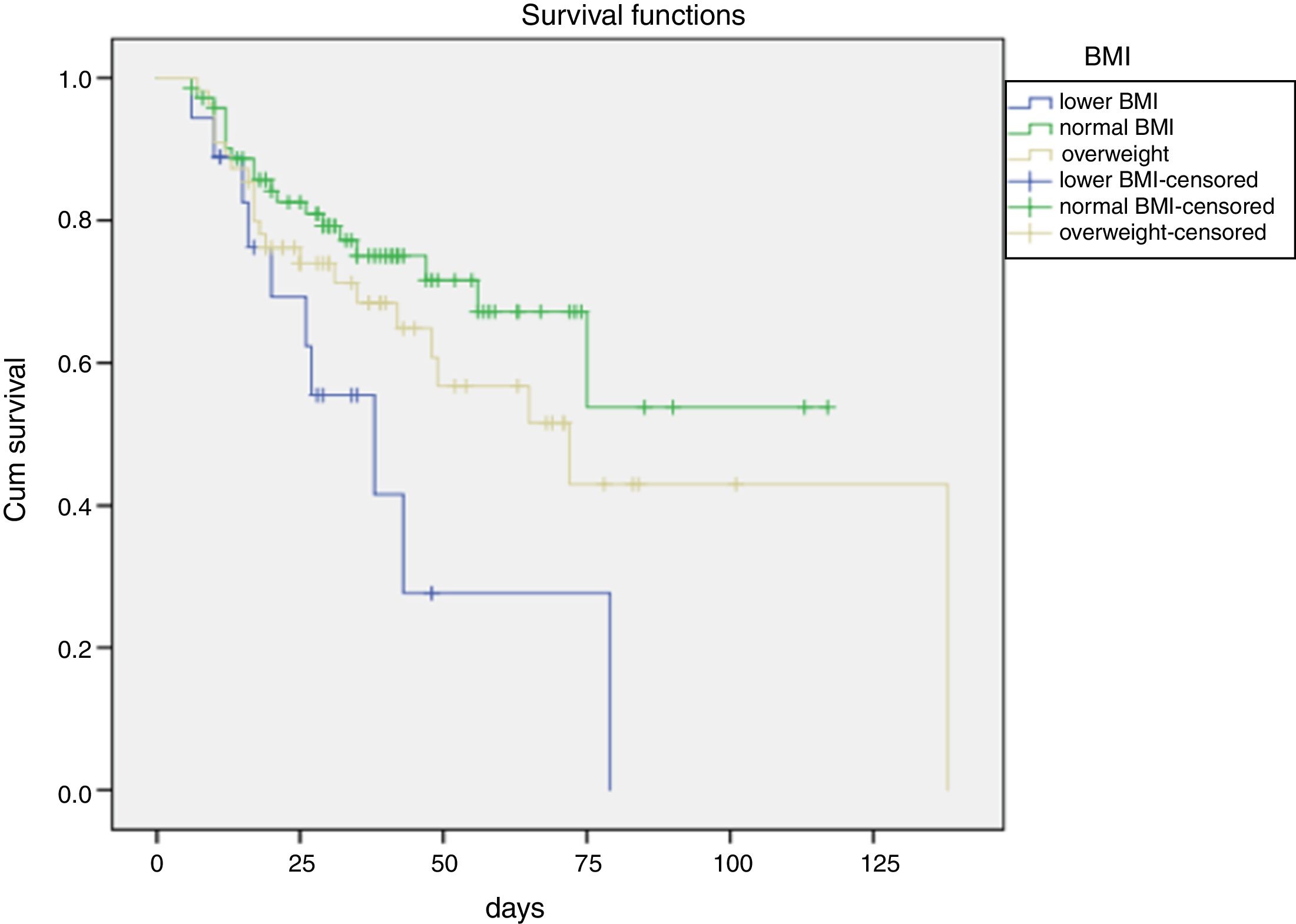
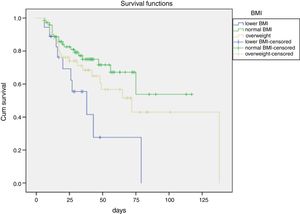
Further, it is showed that the cumulative survival rate of ACLF patients was significantly distinguished among different BMI stages. Meanwhile, the survival time of ACLF patients was gradually increased from lower BMI, overweight to normal BMI (Fig. 1).
It is showed that the survival rate of ACLF patients analyzed using Chi-squared tests was statistically significant different among different BMI stages (χ2=9.435, P=0.009) (Table 4). Further comparisons between two groups showed that there was significant difference in the survival rate between normal BMI and lower BMI or overweight. However, there was no significant difference in survival between lower BMI and overweight.
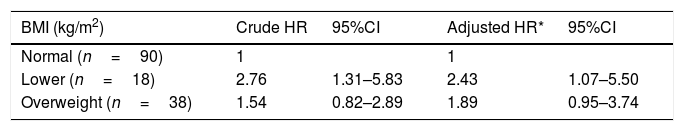
Even more significantly, compared to patients with normal BMI, increasing risk of all-cause mortality was observed in patients with lower BMI (HR=2.43, 95%CI: 1.07–5.50). In addition, it is worth to be mentioned that overweight was also associated with 89% increase in all-cause mortality, despite that the association was only marginally significant (HR=1.89, 95%CI: 0.95–3.74) (Table 5).
Hazard ratios for BMI and all-cause mortality.
| BMI (kg/m2) | Crude HR | 95%CI | Adjusted HR* | 95%CI |
|---|---|---|---|---|
| Normal (n=90) | 1 | 1 | ||
| Lower (n=18) | 2.76 | 1.31–5.83 | 2.43 | 1.07–5.50 |
| Overweight (n=38) | 1.54 | 0.82–2.89 | 1.89 | 0.95–3.74 |
The patients of liver failure carry a high mortality rate, and Lin et al. found that patients who survived for more than 3 months had a good prognosis. Therefore, it's very important to study the prognostic factors for early intervention [10,11]. Liver failure was a hyper-metabolism state, due to inflammatory response enhancement that led to accelerated decomposition of protein, and high consumption of the body energy. Meanwhile, patients with liver failure were usually in the hyper-dynamic circulation, therefore, there is increasing needs for various nutrients in patients with liver failure [12,13]. However, gastrointestinal dysfunction and gastrointestinal mucous edema were both often observed in patients with ACLF, causing severe gastrointestinal symptoms and affecting nutrient absorption. Therefore, malnutrition is common in patients with ACLF. BMI is commonly used as a nutritional evaluation index which can predict the nutritional risk of patients with ACLF and further guide clinical nutritional intervention treatment. Further, Flegal et al. suggested that the BMI was relevant to all-cause mortality based on a large-scale systematic review and meta-analysis [14].
Consistent with the previous studies, our study also showed that different BMI values were associated with different prognosis in patients with ACLF and while compared with normal BMI, lower BMI and overweight were associated with elevated in-hospital mortality of ACLF patients with medical treatments (Fig. 1). Additionally, the multivariate analyses and survival curve showed that underweight patients showed a higher mortality than either normal BMI or overweight patients. Meanwhile, this study excluded the effects of ascites and edema on BMI before admission. Therefore, it is suggested to give adequate nutritional support in early stage of liver failure, especially for patients with lower BMI.
In our study, it is found that overweight patients showed a higher mortality rate than normal BMI patients which may attributed to higher non-alcoholic fatty liver within overweight patients. It has been reported that hepatic steatosis in patients with chronic hepatitis B was associated with host metabolic factors (such as BMI and TG) [15,16]. Zheng et al. found that the patients with chronic hepatitis B combined with fatty liver would aggravate liver inflammation and fibrosis development as well as accelerate the progress of liver disease [17]. Overweight patients could have high concurrency rate of fatty liver and liver failure combined with fatty liver may increase patient's mortality rate [8]. Therefore, patients with chronic hepatitis B should control their weight and avoid overweight or abdominal obesity. While calculating the total intake amount of nutrition of patients with liver failure, we should not only maintain the energy supply of the body, but also minimize the burden of the liver. Based on these, gradually increasing energy intake may be more conducive to the recovery of patients with liver failure [18,19].
As patients suffer from liver failure, the function of protein synthesis is significantly reduced and the main energy supply of human body would convert from glucose to fat and protein [12,20]. Therefore, serum albumin has become an effective indicator for nutritional surveillance. When the concentration of albumin is less than 30g/L, it indicates that the patient is in malnutrition situation. The lower the value of albumin, the worse the malnutrition [21]. In this study, it is also found that serum albumin is associated with prognosis in ACLF patients, the patients who have lower albumin tend to have a higher mortality rate. While patients with ACLF have higher protein breakdown rate, the negative nitrogen balance is more prone to occur. Therefore, it is particularly important to guide the intake of clinical protein in the process of therapy in patients with ACLF. On one hand, it is necessary to avoid aggravating malnutrition because of insufficient protein intake and the protein content in food should be high quality, such as fish and bean products [22,23]. On the other hand, it is necessary to avoid excessive intake of protein which might cause liver burden and even induce hepatic encephalopathy. Protein supply should be based on patients’ tolerance, maintaining positive nitrogen balance, promoting liver regeneration. Therefore, dietary protein, which is rich in branched-chain amino acids, is advocated for patients with ACLF [24,25]. According to the latest research, sarcopenia is also related to nutrition factor. The balance between protein synthesis and degradation decides the amount of muscle mass [26]. Malnutrition and inadequate protein intake lead to the decreased muscle synthesis. Therefore, in order to prevent the sarcopenia to happen, appropriate daily protein intake is recommended [27].
Several large cohort studies have showed a U-shaped association between BMI and the risk of all-cause mortality [28,29]. Consisted with previous research, a U-shaped association between BMI and the risk of mortality was also observed in our present study. The risk of death was higher in ACLF patients with lower BMIs and Over-weigh BMIs than those with normal BMIs. In conclusion, this study found that prognosis of liver failure is associated with BMI and albumin which are commonly used as measures for assessing nutritional status. Above all, accurate assessment of nutritional status in ACLF patients can greatly help clinicians to understand the nutritional status of these patients and predict the prognosis of ACLF patients. According to the nutritional needs of patients, an effective nutritional intervention measure was proposed to improve liver function and to increase the survival rate.
5ConclusionsNutritional therapy may be considered as an adjunction to clinical therapies, especially for ACLF patients. Accurate assessments of nutritional status and adequate intervention are prerequisites for nutritional treatment in ACLF patients. Therefore, BMI, the commonly used nutritional evaluation index, can play a pivotal role. Through this study, we found that BMI can predict the prognosis of the ACLF patients and further guide the nutritional support for patients with ACLF. Given the limitations of current research, additional studies are needed to quantify the association between BMI and ACLF prognosis in the future. What's more, except for BMI, the relationship between the prognosis of ACLF and other nutritional status assessments, for example mid-arm muscle circumference, triceps skinfold thickness, and handgrip strength, should also be considered in the further research.
Conflict of interestThe authors have no conflicts of interest to declare.
This work was supported by the Fujian Medical University Sailing Fund Program (2017XQ1077), National Natural Science Foundation of China (81702073), and Youth scientific research subject of Fujian province health and family planning commission (2016-1-58). All patients participated in this study were appreciated.