Background and rationale for the study. Bacterial translocation is an important triggering factor of infection and mortality in cirrhosis. In a rat model using bile duct ligation (BDL), bacterial translocation appears within 24 h after ligation. The dynamic between TH1/TH2/TH17 cytokines and the integrity of the colonic mucosa in the context of cirrhosis is little known. This study aims to determine the link between bacterial translocation and intestinal inflammation in a cholestasis model. Additionally, alterations of the colonic mucus layer and the bacterial load were also addressed.
Results. Bacterial translocation detected by microbiological cultures and MALDI-TOF showed that Escherichia coli predominates in mesenteric lymph nodes of BDL rats. Intestinal bacterial load analyzed by qPCR indicates a dramatic Escherichia/Shigella overgrowth at 8 and 30 days post-BDL. IFN-γ, IL-4, and IL-17 evaluated by Western blotting were increased at 8 and 30 days in the small intestine. In the colon, in contrast, only IFN-γ was significantly increased. The colonic mucus layer and mucin-2 expression determined by Alcian blue staining and immunohistochemistry surprisingly showed an increase in the mucus layer thickness related to increased mucin-2 expression during the entire process of liver damage. Hepatic enzymes, as well as collagen I, collagen III, TNF-α, and IL-6 liver gene expression were increased.
In conclusion, bacterial overgrowth associated with bacterial translocation is linked to the over-expression of IFN-γ, IL-4, IL-17 and mucin-2. These molecules might facilitate the intestinal permeability through exacerbating the inflammatory process and disturbing tight junctions, leading to the perpetuation of the liver damage.
Bacterial translocation (BT) in chronic liver diseases is an important triggering factor of infections and sepsis. Moreover, bacterial infections are responsible for 30-50% of deaths in cirrhosis. Systemic alterations to the immune system with respect to BT have been thoroughly reported in the literature; the most notable of these include decreased phagocytic capacity, lower production of complement molecules, and chronic inflammation.1 However, intestinal alterations that promoted BT have yet to be well explored and should be considered a novel research frontier.
Cytokines characteristic of TH1, TH2, and TH17 immune responses are necessary to support the stability of gut epithelia; the aberrant production of these cytokines, however, has been related to inflammatory bowel diseases.2,4
T-bet is the major transcription factor for the expression of IFN-γ. IFN-γ is a strong activator of macrophages, with a principal function of protecting the host against intracellular infections. Likewise, in order to improve pathogen recognition, this cytokine also induces expression of MHC molecules and IgG isotype switching.4
GATA-3 is a transcription factor that drives TH2 cell differentiation. IL-4 is necessary for the elimination of helminths, production of mucin-2 and intestinal motility.5,6 On the other hand, under inflammatory conditions IL-4 strongly induces the expression of claudin-2, contributing to intestinal permeability.7,8
ROR-γ is the master transcription factor in TH17 cells and is necessary for the production of IL-17. IL-17 induces the intestinal epithelial production of antimicrobial peptides, mucins, and the recruitment of neutrophils.9 Additionally, TH17 cells promote IgA isotype switching in B cells, for the neutralization of gut bacteria.10 Nonetheless, the participation of the above mentioned cytokines in the intestinal inflammatory process during hepatic fibrosis development in humans and rodents is as of yet unknown.
The intestinal mucus layer also can affect BT; this works as a physical barrier to protect the epithelium against noxious agents, viruses, and pathogenic bacteria. Mucin-2 is the predominant secreted mucin in the whole intestine.11
Therefore, the aim of this study was to determine the link between bacterial translocation, intestinal inflammation and mucus layer in a cholestasis model.
Material and MethodsCholestasis induced by bile duct ligationMale Wistar rats weighing 200–250 g, were obtained from Charles River (Boston, MA, USA). Cholestasis was induced by bile duct ligation (BDL) according to the method of Lee, et al., 1988. Ligated animals (n = 17) were divided into two groups, one corresponding to 8 days and the other one to 30 days of BDL evolution. At the conclusion of the experiment, 5 survivor animals remained in each BDL group; meanwhile, all sham animals (control) survived (n = 5). A control group of healthy rats (n = 5) without any manipulation was included. Animals were sacrificed and representative samples of liver, mesenteric lymph nodes (MLNs) and intestine were obtained.
This project was approved to Ethics Committee of the University of Guadalajara based on technical specifications for the production, care, and use of laboratory animals NOM-062-ZOO-1999 and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health established in 1985.
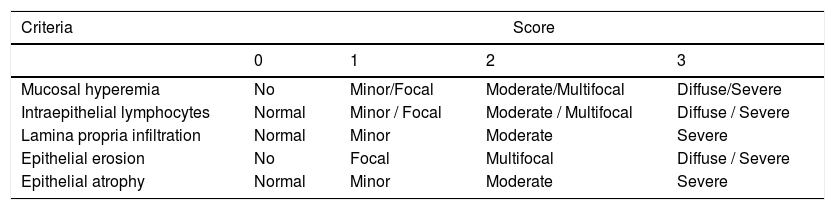
Histological analysisSamples of the middle region of the small intestine and colon were obtained. Fouts, et al., 2012 reported that these regions of the intestines are the most vulnerable to intestinal permeability in the model of BDL.12 Liver sections (5-µm thick) were stained with Masson’s trichrome. The amount of fibrosis was assessed based on the Ishak score.13 Intestinal sections (5-μm thick) were stained with H&E. The severity of epithelial damage was determined following the criteria shown in table 1.14,15
Criteria of intestinal histological score.
| Criteria | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Mucosal hyperemia | No | Minor/Focal | Moderate/Multifocal | Diffuse/Severe |
| Intraepithelial lymphocytes | Normal | Minor / Focal | Moderate / Multifocal | Diffuse / Severe |
| Lamina propria infiltration | Normal | Minor | Moderate | Severe |
| Epithelial erosion | No | Focal | Multifocal | Diffuse / Severe |
| Epithelial atrophy | Normal | Minor | Moderate | Severe |
MLN and liver were removed under aseptic conditions. All the samples were immersed in sterile water and 70% ethanol. Subsequently, the tissues were ground in 15 mL of Trypticase Soy broth and pre-incubated for 12 h at 37°C. Ten μL were plated on Agar with 5% sheep blood. Plates were incubated under aerobic conditions at 37 °C for 24 h.
Single bacterial colonies were carefully spread onto the MALDI target plate, 1 of matrix (α-cyano-4-hydroxycinnamic acid in saturated solution with 50% acetonitrile and 2.5% trifluoroacetic acid) was added, and the mass spectra analysis was performed in a MALDI Biotyper Microflex LT (Bruker Daltonics, Bremen, Germany). Three different identification events were prepared using different colonies from the same isolate. Spectra data with Log score values lower than 1.9 was excluded. The spectra were analyzed using the MALDI BioTyper software (Bruker Daltonics, Germany).
qPCRDNA was isolated from 100 mg of stool, following the manufacturer’s instructions using the ZR Fecal DNA MiniPrep™ kit. Primers 5’-GTTAATACCTTTGCTCATTGA-3’ and 5’-ACCAGGGTATCTAATCCTGTT-3’ were used to amplify the V3 to V4 regions of the 16S rDNA of both Escherichia and Shigella. Reactions were performed with the following conditions: hold 95°C (7 min) followed by 40 cycles of 95 °C (30 s), 60 °C (40 s). Bacterial concentration was calculated by comparing the CT values obtained from the standard curve of Escherichia coli (1 x 103 - 1 × 109 CFU/mL). Bacterial quantity was expressed as log concentration.
Western blotting50 μg of protein were separated in SDS-PAGE 12 % and transferred to PVDF membranes. A 5 % Blotting-Grade Blocker (BioRad Laboratories, Hercules, CA) was added for 45 min. Subsequently, primary antibodies anti-T-bet sc-21763; anti-IFN-γ sc-74106; anti-GATA-3 sc-137152; anti-IL-4 sc-80094; anti-ROR-y sc-28559 and anti-IL-17 sc-374218 (Santa Cruz Biotechnology, Inc., CA, USA) were used at 1:3000 and incubated at 4 °C followed by 1 h with 1:8000 HRP-linked secondary antibodies at 37°C. Chemiluminescence reactions (Immobilion, Merck Millipore®) were performed on Microchemi 4.2. Densitometry was analyzed with the GelQuant 12.2 software.
Alcian blue stainingStaining was performed on 2-μm thick, Carnoy-fixed (for 2.5 hours without removal of fecal content), paraffin-embedded colon sections. Slides were immersed in acetic acid at 3% for 3 min, following by 30 min in alcian blue solution pH 2.5. Counterstaining was performed with nuclear fast red. Intestinal mucus thickness analysis was carried out with the Leica application suite 16.0 software (LASEZ).
ImmunohistochemistryImmunohistochemistry was performed on 1-μm-thick Carnoy-fixed, paraffin-embedded colon sections. Rabbit anti-mucin 2 sc-15334 (Santa Cruz Biotechnology, Inc., CA, USA) at 1:50 dilution was incubated for 2 h at 37 °C, followed by 45 min with Dako EnVision Labelled Polymer. Diaminobenzidine (Dako) was used as a chromogen. Immunostaining was considered positive when cytoplasmic or extra-nuclear mucin-2 staining were observed. The percentage of cells stained was scaled in accordance with their staining intensity: 1 (1–25 %), 2 (2–50 %), 3 (51–75 %) and 4 (76–100 %).
Biochemical analysisSerum hepatic enzymes including total bilirubin, alanine aminotransferase (ALT) and alkaline phosphatase were determined by an automated method.
qRT-PCRRNA was isolated from 100 mg of liver using TRIzol reagent (Invitrogen, Belgium) and reverse transcribed. PCR conditions were 95 °C (10 min) followed by 35 cycles of 95 °C (15 s). Alignment temperature and primer sequences are shown in table 2. Gene expression analysis was performed by the 2- ΔΔCT method.
Primers sequences.
| PrimersPrimers | Sequence | Ta (°c) | Size (bp) |
|---|---|---|---|
| COL-1 α1Rat | F-GAGAGCATGACCGATGGATT R-CTACGCTGTTCTTGCAGTG | 60 | 143 |
| COL-3 α1Rat | F- AGATGGATCAAGTGGACATC R- ATGTTTCTCCGGTTTCCAT | 55 | 449 |
| IL-1β Rat | F- GGCTTCCTTGTGCAAGTGTC R- TGTCGAGATGCTGCTGTGAG | 63 | 202 |
| IL-6 Rat | F- GCCAGAGTCATTCAGAGCAATA R-TTAGGAGAGCATTGGAAGTTGG | 60 | 109 |
| TNF-α Rat | F- CACCACGCTCTTCTGTCTACTG R-AGATAAGGTACAGCCCATCTGC | 60 | 203 |
COL-1α1: collage 1 alpha 1. COL-3 α1: collage 3 alpha 1. IL-1: Interleukin 1. IL-6: Interleukin 6. TNF-α: tumor necrosis factor alpha. bp: bases pairs.
Data were analyzed using the SPSS 10.0 statistical package (SPSS Inc.). Results were expressed as mean ± SD. Comparisons were performed using one-way ANOVA, followed by Bonferroni’s post hoc test. P-values ≤ 0.05 were considered as statistically significant.
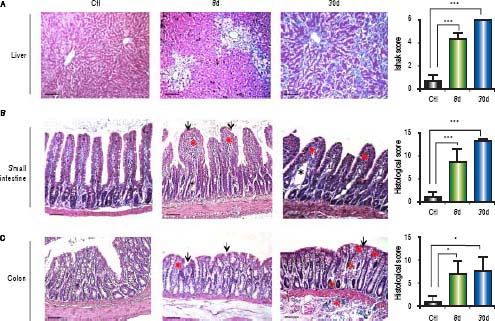
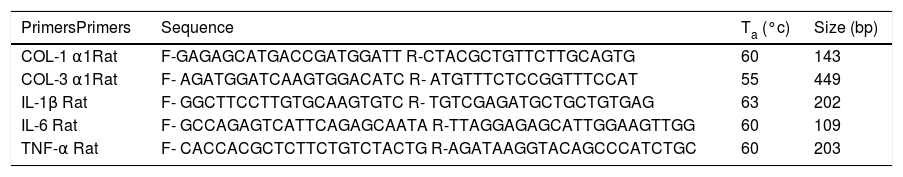
ResultsHistopathological alterations in the liver of BDL ratsTo evaluate liver fibrosis, the tissues were stained with Masson’s trichrome stain. In control animals, hepatic sections exhibited a normal morphology of the portal triad and central vein. In experimental animals, ductal proliferation and extracellular matrix deposits were evident at 8 days of BDL. At 30 days post-BDL, fibrosis is clearly observed throughout the entire parenchyma. The Ishak analysis showed that the liver damage was gradually increased from 8 days (p < 0.001) to 30 days (p < 0.001) post-BDL (Figure 1A).
Histological analysis of liver and bowel sections. A. Representative images of liver sections with Masson’s trichrome staining of control, 8 and 30 days post-BDL and Ishak anaysis. B. Representative images of small intestine sections with Hematoxylin-eosin staining of control, 8 and 30 days post-BDL and histological analysis. C. Representative images of colon sections with Hematoxylin-eosin staining of control, 8 and 30 days post-BDL and histological analysis. Inflammatory cellular infiltrated (red asterisk); dilation of the central lacteal cavity (black asterisk); epithelial atrophy (red arrow); epithelial erosion (black arrow). Bars represent 100 μm. Results were expressed as the mean ± SD. Five rats per group *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Control groups (healthy and sham) revealed the presence of villi (small intestine) and crypts lined with simple columnar epithelium, with goblet cells and a lamina propria of well-vascularized fibroconnective tissue with many lymphocytes. The 8 and 30 days post-BDL groups showed an increase in the number of intraepithelial lymphocytes and lymphocyte infiltration into the lamina propria, as well as erosion and atrophy of the epithelium. In both small intestine and colon, these histological changes were statistically significant (p < 0.001 and p < 0.05, respectively) when compared with the control group. Additionally, in many microscopic fields of the small intestine from the BDL groups, a dilation of the central lacteal cavity was observed (Figures 1B, 1C).
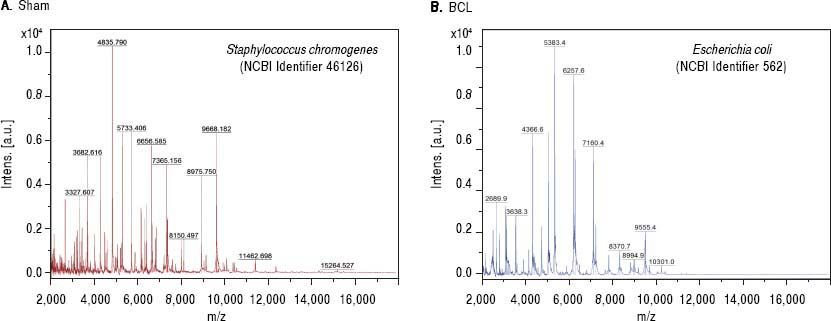
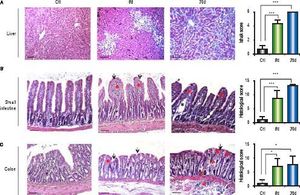
Bacterial translocation to the liver is present at 8 days post-BDLA recurrent and important complication of liver disease is BT. To assess this variable, we performed bacterial cultures of the liver and MLN homogenates. Our results showed negative cultures in healthy rats. However, cultures from the MLNs of the sham control rats were positive. In contrast, cirrhotic rats showed BT in both organs at eight days after BDL. Then, we analyzed single bacterial colonies from mesenteric lymph nodes by MALDI-TOF. Our results show that sham rats were positive for Staphylococcus chromogenes (NCBI Identifier: 46126) (Figure 2A), while cirrhotic rats were positive for Escherichia coli (NCBI Identifier: 562) (Figure 2B).
MALDI-TOF/MS average spectra plots of isolates from mesenteric lymph nodes. A. Representative spectral plot of mesenteric lymph nodes from sham rats shows specific peaks for Staphylococcus chromogenes (red) NCBI identifier 46126. B. Representative spectral plot of mesenteric lymph nodes from BDL rats shows specific peaks for Escherichia coli (blue) NCBI identifier 562. The x-axis shows m/z values and the y-axis indicates the intensities of peaks (expressed in arbitrary intensity units). The analysis was performed in the Burker biotyper platform (n = 5 per group).
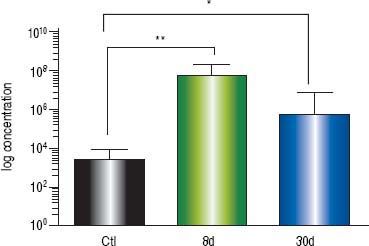
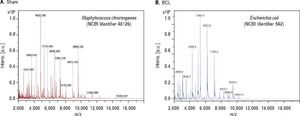
At eight days post liver damage by BDL, the intestinal bacterial load of the genera Escherichia/Shigella increased dramatically, approximately four logarithms when compared with the control groups (p < 0.001). Bacterial overgrowth was maintained at 30 days post-BDL with two logarithms of difference with respect to the control groups, p < 0.05 (Figure 3).
Intestinal bacterial load of Escherichia/Shigella by qPCR. Amplification was carried out by detection of 16S rDNA of Escherichia/Shigella. Bacterial concentration from each fecal sample was calculated by comparing the CT values obtained from the standard curve of E. coli (1 × 103 - 1 × 109 CFU/mL). Results were expressed as the mean ± SD. Five rats per group. * p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
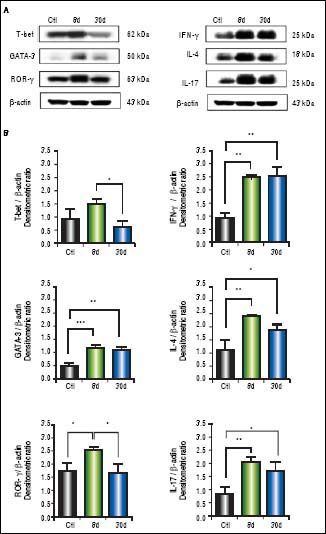
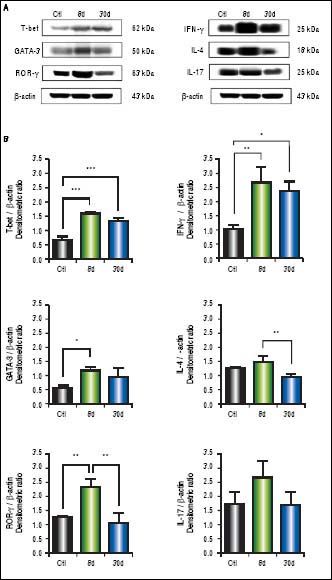
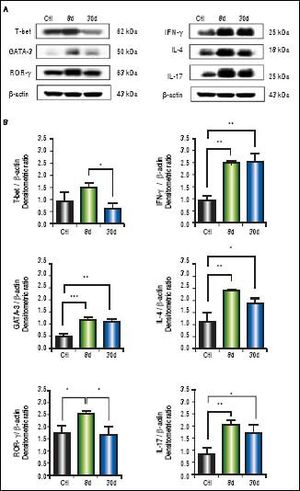
We observed significantly increased levels of IFN-γ (p < 0.01), GATA-3 (p < 0.001), IL-4 (p < 0.01), ROR-γ (p < 0.05) and IL-17 (p < 0.01) (Figure 4B) in the small intestine during early or chronic stages of liver damage by cholestasis, compared to control groups. Interestingly, the expression of T-bet at 30 days post-BDL was down-regulated.
Expression of T-bet, IFN-γ, GATA-3, IL-4,ROR-γ, and IL-17 in small intestine by Western blotting. A. Representative blot of control (healthy and sham), 8 and 30 days post-BDL. Observed bands of T-bet (62 kDa), IFN-γ (25 kDa), GATA-3(50 kDa), IL-4 (18 kDa), ROR-γ (63 kDa), IL-17(25 kDa), and β-actin (43 kDa). B. Data are shown as densitometric ratios with respect to the β-actin value. Results were expressed as the mean±SD. Five rats per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
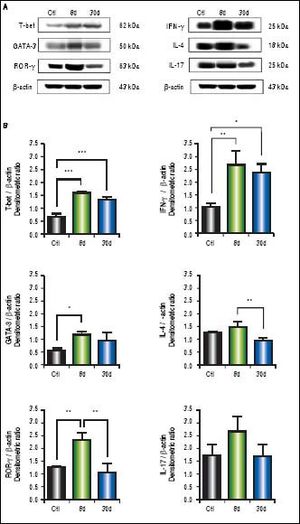
In the western blot analysis of colon extracts, T-bet (p < 0.001) and IFN-γ (p < 0.01) levels predominated at 8 and 30 days after BDL, whereas IL-4 and IL-17 exhibited no significant differences when compared to the control groups. GATA-3 (p < 0.05) and ROR-γ (p < 0.01) levels were significantly increased at eight days post-BDL, but to a lesser extent than T-bet and IFN-γ (Figure 5B).
Expression of T-bet, IFN-γ, GATA-3, IL-4, ROR-γ, and IL-17 in colon by Western blotting. A. Representative blot of control (healthy and sham), 8 and 30 days post-BDL. Observed bands of T-bet (62 kDa), IFN-γ (25 kDa), GATA-3 (50 kDa), IL-4 (18 kDa), ROR-γ (63 kDa), IL-17 (25 kDa), and β-actin (43 kDa). B. Data are shown as densitometric ratios with respect to the β-actin value. Results were expressed as the mean±SD. Five rats per group. *p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001.
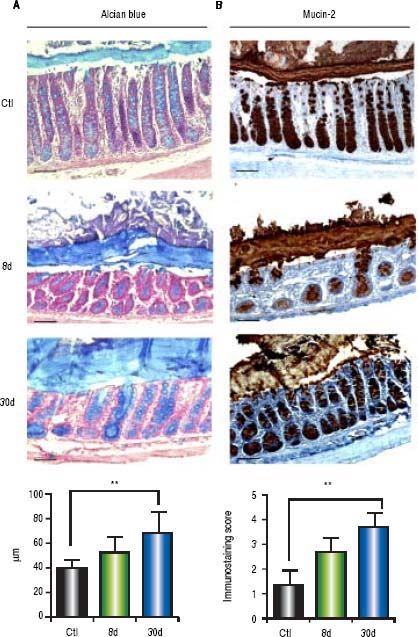
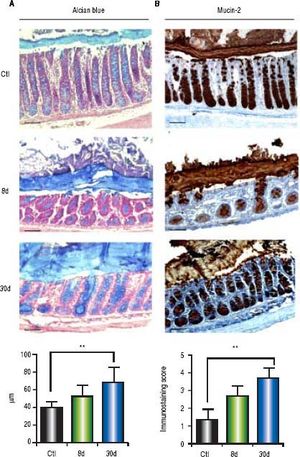
Alcian blue analysis showed significantly increased colonic mucus layer thickness at 8 days (p < 0.001) and 30 days (p < 0.001) post-BDL with respect to the control groups (Figure 6A). Similarly, colonic mucin-2 expression was notably increased during the entire process of liver damage by BDL. 100% of goblet cells were observed to be positive for mucin-2 immunostaining. However, the intensity of the staining was increased in parallel with the liver damage (Figure 6B). Significant differences were found only between the control and the 30 days post-BDL groups (p < 0.001).
Colonic mucus layer thickness by alcian blue and mucin-2 immunohistochemistry. A. Representative images of colon sections of control (healthy and sham), 8 and 30 days post-BDL with alcian blue staining and mucus layer thickness anaysis. B. Representative images of colon sections of control (healthy and sham), 8 and 30 days post-BDL with mucin-2 immunohistochemistry and intensity analysis. Bars represent 100 μm. Results were expressed as the mean±SD. Five rats per group. * p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001.
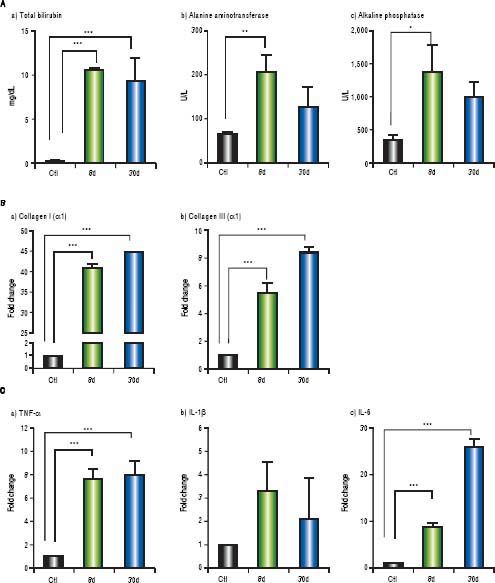
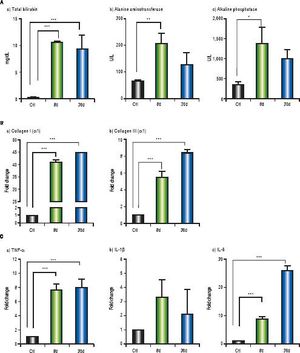
Markers of liver damage increase significantly: total bilirubin (p < 0.001), alanine aminotransferase (p < 0.01) and alkaline phosphatase (p < 0.05) from BDL groups (Figure 7A). Additionally, enhanced gene expression of the classical fibrogenic collagen I (α1) and collagen III ( α1) was observed during cholestasis (p < 0.001) (Figure 7B). On the other hand, TNF-α and IL-6 increased significantly at 8 and 30 days post-BDL (p < 0.001). IL-1β did not show any significant difference (Figure 7C).
Hepatic biochemicl tests, fibrosis and inflammation-associated gene expression. A. Biochemici tests: tota bilirubin (mg/dL), alanine aminotransferase (U/L) and alkaline phosphatase (IU/L) in control, 8 and 30 days post-BDL. B. Collagen I (α1) and collagen III (α1) relative expression in liver by 2-ΔΔCTanalysis. C. TNF-α, IL-1βand IL-6 relative expression in liver by 2-ΔΔCTanalysis. β-2 microglobulin was used as reference gene. Results were expressed as mean±SD. Five rats per group *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
BT has been postulated as the angular mechanism in the pathogenesis of spontaneous bacterial infections in liver cirrhosis. Our results show that BT to the MLNs and liver was evident at 8 days post-BDL. Interestingly, we note that in the group of sham rats, MLNs also had positive bacterial loads, while the liver remained bacteria free. This point is consistent with Yorihiko Ogata, et al., 2003 who also found positive bacterial cultures in the MLNs of sham rats in the BDL model.16 A possible trigger for this BT could be surgical stress,17 which may have influenced our results. Regarding to MALDI-TOF analysis, it showed that Escherichia coli predominates in MLNs of BDL animals, whereas Staphylococcus chromogenes in sham rats. Indeed, both bacteria are pathogens, but they have a different virulence grade. Thus, a possible explanation for the substantial difference between both groups is that apparently sham animals might have the ability to contain and avoid bacterial migration to liver, in contrast to BDL group. It is possible that BT control is dictated by the immunogenicity and immuno-evasion mechanisms of the different microorganisms, as well as alterations on systemic and local immune response. Gram negative bacteria such as Escherichia coli, Klebsiella pneumonia and other enterobacteria, have been postulated to be the most significant in the MLN translocation during alcoholic and viral liver damage.18 Nonetheless, in cholestatic processes it is poorly known.
In parallel, we observed a dramatic intestinal bacterial overgrowth associated with the genera Escherichia/Shigella during the entire period of liver damage (8 and 30 days). Interestingly, these species are the main cause of infections in patients with liver cirrhosis.19
The etiology of bacterial overgrowth in liver cirrhosis is multifactorial.20 Our results suggest that the decrease in the bile acid flow promotes intestinal bacteria overgrowth of the genera Escherichia/Shigella, which are related to the BT and hepatic damage. An important additional finding of our histopathological analysis of the intestines from the BDL groups was the central lacteal cavity dilatation. This phenomenon has been linked to higher rates of BT in MLNs and endotoxemia.16,21
The local over-expression of IFN-γ may lead to inflammatory chronic processes and damage of the intestinal epithelium.22 Our results show a significant increase of IFN-γ in the small intestine during the entire process of hepatic injury in our model. However, we observed that the expression of T-bet decreased in the 30 day post-BDL group. These results suggest that perhaps the central cell source of IFN-γ might be other cells of the immune system, such as NK cells, γδ T cells or memory CD8+ T cells, which produce IFN-γ in the presence of the transcription factor EOMES.23–25
IFN-γ increased was more evident in the colon. It is important to mention that this study is the first to evaluate the expression of intestinal IFN-γ in the experimental model of BDL. These results suggest that the over-expression of IFN-γ might, at least initially, lead to the restoration of intestinal homeostasis. Additionally, the presence of IFN-γ might lead to decreased stability of the intestinal epithelia.
Previous studies by Li, et al., 2007, show that the stimulus of IFN-γ and TNF-α in epithelial cells induces alterations in the lipid composition of the tight junction membrane microdomains.26 Nonetheless, further studies evaluating tight junction proteins in our experimental model would assist in better understanding the mechanisms of intestinal barrier protection during liver damage.
Previous studies show that IL-4 has a potent STAT6-dependent effect on ion flux through the cells of the intestinal epithelium, mucosal permeability and mucin production.6,8 Our results show that the liver damage, both in early and late phases, produced an increase in levels of GATA-3 and IL-4 in the small intestine. This increase could be attributed to an anti-inflammatory response, that is, the system’s attempt to try to counter the high levels of IFN-γ in the local microenvironment.
On the other hand, our results show a significant increase in the levels of ROR-γ and IL-17 in the small intestine. This may impact in the dysfunction of the intestinal barrier. Jingjing Meng, et al., 2015, reported in a model of sepsis induced by of CLP (Cecum Ligation and Puncture) that over-expression of intestinal IL-17A induces alterations in the organization of the tight junction proteins, and promoted the dissemination of bacteria.27 In our experimental model, IL-17 may have a dual effect: to induce the elimination of pathogens and, on the other hand, to drive alterations in the integrity of the intestinal epithelium. Without doubt, future studies will be required in order to elucidate the role of IL-17 in the intestinal homeostasis.
The mucins are proteins with a large number of O-gly-cosylations.28 Our results show a significant increase in the depth of the mucus layer related with an increase in mucin-2, in parallel with liver damage. These data agree with those reported in mouse models and in patients with chronic alcohol-induced liver damage.29,30 However, in the BDL model, mucin-2 has not been previously evaluated.
In this context, considering the chronic inflammation in our experimental model, the resulting increased thickness of the intestinal mucus layer does not prevent BT. We must consider that it is probable that there are alterations in the glycosylation of mucin-2, thus potentiating the penetration of bacteria across the intestinal barrier and the perpetuation of liver damage.
Interestingly, in the experimental models of CCl4 and NASH, the liver damage correlates with BT and pro-inflammatory cytokine production.31,32 Here, in our BDL model, we have shown a hepatic overexpression of TNF-α and IL-6. Given that these cytokines can be produced after stimulation of the TLR4/LPS pathway, we consider that this pathway might be determinant during the hepatic damage process. Importantly, systemic LPS is remarkably high after 24 h of duct ligation in the BDL model.12 TNF-α has an important role in the perpetuation of HSC activation and ECM synthesis.33 Despite the fact that IL-6 has a controversial role during the hepatic fibrogenesis, this cytokine is a pivotal mediator of ductular reactions in humans with biliary fibrosis;34 moreover, IL-6 produced by Kupffer cells promotes survival and proliferation of HSC, contributing to the exacerbation of the liver fibrosis.35 Thus, in our model, we believe that the proinflammatory process might be tightly associated to the overexpression of collagen I and collagen III at 8 and 30 days after bile duct ligation, which in turn might exacerbate and/or sustain the hepatic damage.
Collectively, our data suggest that the intestinal inflammation and high thickness mucus layer can facilitate BT and perpetuate liver damage in the BDL model.
Financial SupportThis work was supported by a grant from Universidad de Guadalajara (REC/747/2016). The authors declare that there is no conflict of interest.
Abbreviations- •
BDL: bile duct ligation.
- •
BT: bacterial translocation.
- •
MLNs: mesenteric lymph nodes.
The authors are indebted to Dr. Annie Riera Leal for her technical assistance in immunohistochemistry assays.