Bile acids (BA), for decades considered only to have fat-emulsifying functions in the gut lumen, have recently emerged as novel car-dio-metabolic modulators. They have real endocrine effects, acting via multiple intracellular receptors in various organs and tissues. BA affect energy homeostasis through the modulation of glucose and lipid metabolism, predominantly by activating the nuclear far-nesoid X receptor (FXR), as well as the cytoplasmic membrane G protein-coupled BA receptor TGR5 in a variety of tissues; although numerous other intracellular targets of BA are also in play.The roles of BA in the pathogenesis of diabetes, obesity, metabolic syndrome, and cardiovascular diseases are seriously being considered, and BA and their derivatives seem to represent novel potential therapeutics to treat these diseases of civilization.
Bile acids (BA), which for decades were only considered to be involved in lipid digestion in the intestinal lumen and cholesterol solubilization in the bile, now appear to have versatile metabolic effects contributing significantly to energy homeostasis.1,2 By binding to multiple cy-toplasmic as well as nuclear receptors in various organs and tissues, they exert real endocrine functions.3 Based on these effects, BA are implicated in the pathogenesis of multiple metabolic diseases, including overweight and obesity, diabetes mellitus, as well as cardiovascular diseases. In fact, one of the first studies reporting these metabolic effects of BA had been published as early as 1997, in which the inhibitory action of cholic acid on a high-fat diet-induced hyperglycemia and obesity was described.4 Since then, our understanding on the effects of BA on energy homeostasis, as well as on glucose and lipid metabolism has increased tremendously.
Bile Acid MetabolismBA are amphiphilic molecules produced in a rather complicated biosynthetic pathway in the liver. As opposed to cholesterol, the substrate for BA synthesis, the major primary BA (cholic acid and chenodeoxycholic acid), are more hydrophilic, owing to the presence of additional hy-droxyl groups.5 These primary BA, after conjugation with glycine or taurine in the liver, are actively secreted, first into the bile and then into the small intestine to exert their primarily digestive functions. As precious molecules, their pool in the human body is maintained by an efficient enterohepatic circulation, preserving as much as 95% of the conjugated BA.5 This active transport in the distal ile-um is mediated via the ileal BA transporter (known under various abbreviations: IBAT/ASBT/ISBT/NTCP2)6 maintained under the control of the nuclear farnesoid X receptor (FXR), the intracellular BA sensor.7 Upon stimulation of FXR with BA, ileocytes secrete fibroblast growth factor 19 (FGF19), the major repressor of BA in the liver.8 Quite surprisingly, data from extensive rodent studies have demonstrated that FGF19 secreted from the ileum in response to feeding also has insulin-like functions; whereas, FGF21 (a counterpart to FGF19), secreted from the liver in response to prolonged fasting, has glucagon-like effects.9 In fact, while insulin/glucagon serve as immediately-acting fed-state and fasting-state hormones, FGFs19/21 can be considered as late-acting hormones.9 Interestingly, when administered in pharmacological doses, both FGF19 and FGF21 have insulin-sensitizing and hypolipidemic effects in animal models of both obesity and type 2 diabetes (T2DM).10,11 On the contrary, clinical studies are not as convincing, since most studies have shown a positive association of FGF21 concentrations with obesity and T2DM as well as increased CVD risk; this being explained as the result of some feedback mechanism.12 It is also important to note that gallbladder is an important source of FGF19.13 In addition, it is known that cholecystectomy is associated with increased insulin resistance14 as well as increased risk of non-alcoholic steatohepatitis (NASH).15 Impaired BA metabolism is likely to be an important pathogenic factor accounting for these observations.
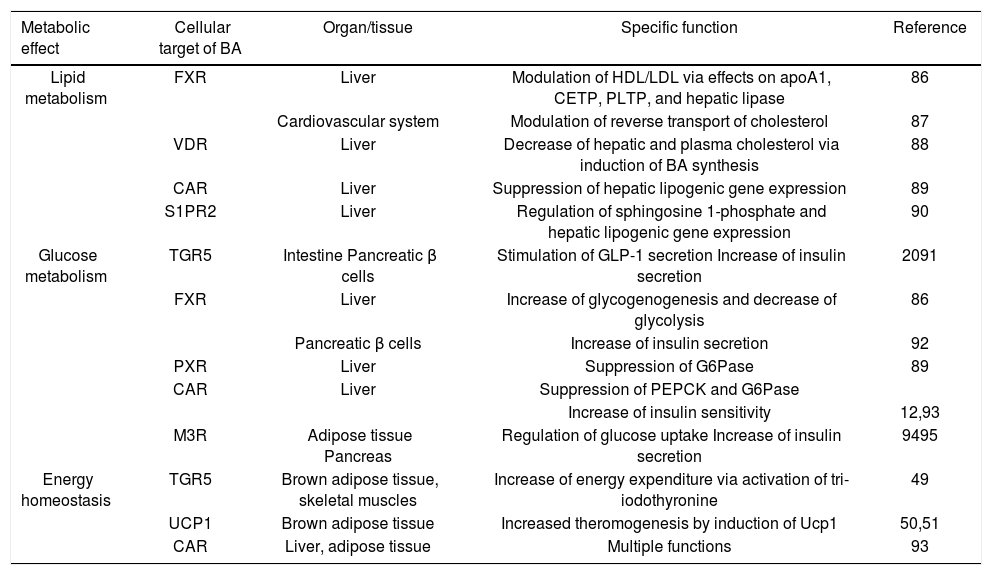
How Could Ba Be Metabolic Players?BA behave as real hormones, being produced in remote organs, and exerting their actions through activation of various membrane and nuclear receptors in specific target tis-sues16 (Table 1). BA nuclear receptors involve FXR, as mentioned above, together with the vitamin D receptor (VDR), and constitutive androstane receptor (CAR), preg-nane X receptor (PXR); the cytoplasmic membrane BA receptors include the G protein-coupled BA receptor TGR5, muscarinic receptors, sphingosine 1-phosphate receptor 2 (S1PR2),17 peroxisome proliferator-activated receptor-α (PPARα),18 and glucocorticoid receptor (GR).19 Thus, apart from the now well-known effects of BA on glucagon-like peptide 1 (GLP-1) secretion by enteroen-docrine small intestinal L cells,20 all of these additional, BA-nonspecific intracellular targets seem to play some role in the metabolism of glucose and lipids, and contribute to overall energy homeostasis (Table 1).
Bile acids as metabolic regulators.
| Metabolic effect | Cellular target of BA | Organ/tissue | Specific function | Reference |
|---|---|---|---|---|
| Lipid metabolism | FXR | Liver | Modulation of HDL/LDL via effects on apoA1, CETP, PLTP, and hepatic lipase | 86 |
| Cardiovascular system | Modulation of reverse transport of cholesterol | 87 | ||
| VDR | Liver | Decrease of hepatic and plasma cholesterol via induction of BA synthesis | 88 | |
| CAR | Liver | Suppression of hepatic lipogenic gene expression | 89 | |
| S1PR2 | Liver | Regulation of sphingosine 1-phosphate and hepatic lipogenic gene expression | 90 | |
| Glucose metabolism | TGR5 | Intestine Pancreatic β cells | Stimulation of GLP-1 secretion Increase of insulin secretion | 2091 |
| FXR | Liver | Increase of glycogenogenesis and decrease of glycolysis | 86 | |
| Pancreatic β cells | Increase of insulin secretion | 92 | ||
| PXR | Liver | Suppression of G6Pase | 89 | |
| CAR | Liver | Suppression of PEPCK and G6Pase | ||
| Increase of insulin sensitivity | 12,93 | |||
| M3R | Adipose tissue Pancreas | Regulation of glucose uptake Increase of insulin secretion | 9495 | |
| Energy homeostasis | TGR5 | Brown adipose tissue, skeletal muscles | Increase of energy expenditure via activation of tri-iodothyronine | 49 |
| UCP1 | Brown adipose tissue | Increased theromogenesis by induction of Ucp1 | 50,51 | |
| CAR | Liver, adipose tissue | Multiple functions | 93 |
In addition to these effects, BA were also demonstrated to affect intracellular targets posttranslationally. In fact, miR-26a was recently identified in mice as a novel downstream target of TGR5 activation in macrophages, acting through a JNK-dependent pathway.21 As shown in a mice study by Fu, et al., miR26a represents miRNA with pleio-tropic metabolic functions thus might be an additional BA-regulated anti-diabetic/anti-obesity intracellular tar-get.22 Interestingly, as can be implied from an in vitro study on human hepatocytes treated with chenodeoxycholic acid, BA seem to be potent regulators of a wide array of miRNAs, including those implicated in energy homeosta-sis.23
Interrelationship Among Ba, Intestinal Microbiome, and Metabolic DiseasesAn increasing accumulation of evidence suggests the importance of the gut microbiota in the development of obesity and metabolic diseases.24
Indeed, the gut microbiota of obese humans have a higher proportion of energy-harvesting Firmicutes bacteria, which is believed to increase the energy yield from the intestinal contents and accelerate fat accumulation in the human body.25 In fact, the ratio between the Firmicutes and Bacteroidetes phyla is important for short chain fatty acid production and is linked to obesity,26 but also to the production of secondary BA.27 Furthermore, microbial compositions have been shown to be fundamentally different between native Africans and African-Americans, with bu-tyrate-producing strains significantly more abundant in fecal samples from native Africans; while those producing secondary BA were much higher in African-Americans.28 Interestingly, an increase in sulfate-reducing bacteria, and a decrease in the butyrate producing species were detected in T2DM.29
Thus, it not surprising that gut microbiome (profoundly affecting BA metabolism) has a causal relationship with lifetime risk of cardiovascular disease.30 Indeed, increased serum concentrations of secondary BA were recently demonstrated in patients with chronic heart failure31 pointing to the atherogenic role of gut microbiome.
Ba as a Marker of Metabolic DiseasesSeveral recent lines of evidence suggest that BA not only affect the pathogenesis of metabolic diseases, but may serve as real markers of these conditions.
In fact, insulin resistance was positively associated with serum BA in both the nondiabetic and diabetic populations in a recent large Chinese study,32 although inconsistent results have been seen in previous human studies. In one study, total BA in the serum or plasma were unchanged in T2DM in comparison to controls,33 whereas in another study, BA were found to be increased in the diabetic population.34 Nevertheless, a positive correlation between total urinary BA excretion and HbA1c in diabetes patients was shown in an additional study,35 with additional supporting evidence conveyed by others.36-38 Indeed, the BA pool and fecal BA were elevated in diabetic patients with uncontrolled hyperglycemia,36 and insulin resistance was associated with increased BA in otherwise healthy subjects.37 Furthermore, higher circulating concentrations of BA were detected in obese patients with T2DM in a study by Vincent, et al.38
Mechanisms accounting for the association between glucose and BA metabolism have not been fully defined nor cleared up. Nevertheless, in a study with humanized CYP7A1-transgenic mice insulin was demonstrated to inhibit FoxO1 binding to the CYP7A1 gene promoter, resulting in induction of CYP7A1 gene expression and BA synthesis.35 Furthermore, glucose was reported to induce the CYP7A1 gene as well as activity (the rate limiting enzyme in a BA biosynthetic pathway).39 Interestingly, the bile salt hydrolase activity, inherent to specific microbial strains and deconjugating of BA in the intestinal lumen,40 is directly linked to obesity and cholesterol metabolism, as demonstrated in both animal as well as clinical studies.41,42
In line with the evidence on the positive association between BA and diabetes, discussed above, there is also a positive relationship between hypertension and BA levels observed in diabetic patients.32 Consistently, similar positive association of BA was reported also for NASH; with BA being importantly implicated in hepatic lipid metabo-lism.43,44 In addition, serum BA in obese subjects also var-ies,45,46 and respond to therapeutic intervention,47 although not all studies are consistent in their results.48
Hence, it is certain that we are only at the beginnings of our understanding to decide whether BA levels in the systemic circulation represent a feedback mechanism of disease manifestations, or if they are a true predisposing factor. Thus, future reverse causality studies are needed to fully understand this phenomenon.
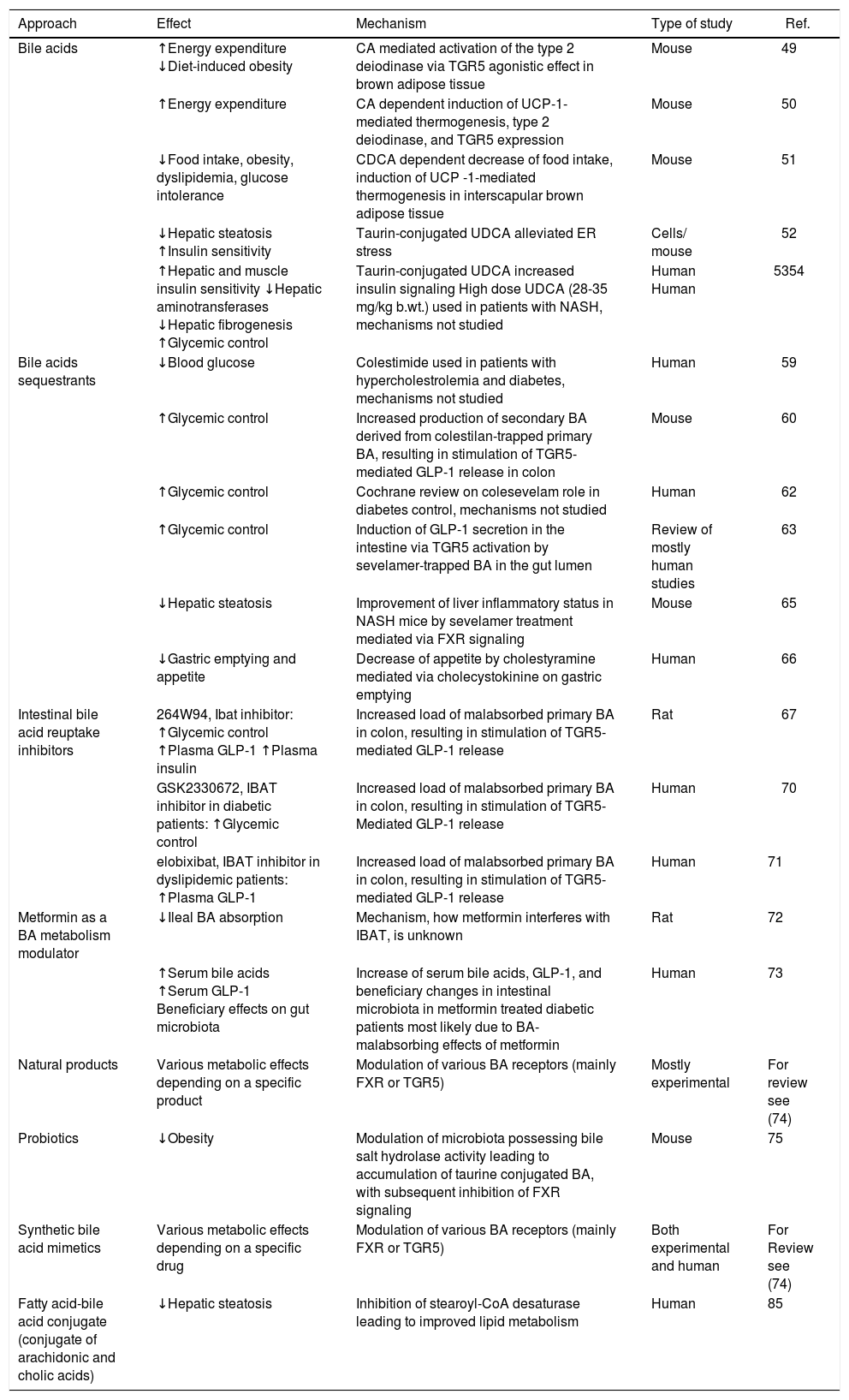
Ba as a Novel Therapeutics to Treat Metabolic DiseasesSeveral approaches to modulate BA metabolism with the aim to affect metabolic diseases have been proposed and already investigated in both experimental as well as clinical studies (Table 2).
BA as a novel therapeutics to treat metabolic diseases.
| Approach | Effect | Mechanism | Type of study | Ref. |
|---|---|---|---|---|
| Bile acids | ↑Energy expenditure ↓Diet-induced obesity | CA mediated activation of the type 2 deiodinase via TGR5 agonistic effect in brown adipose tissue | Mouse | 49 |
| ↑Energy expenditure | CA dependent induction of UCP-1-mediated thermogenesis, type 2 deiodinase, and TGR5 expression | Mouse | 50 | |
| ↓Food intake, obesity, dyslipidemia, glucose intolerance | CDCA dependent decrease of food intake, induction of UCP -1-mediated thermogenesis in interscapular brown adipose tissue | Mouse | 51 | |
| ↓Hepatic steatosis ↑Insulin sensitivity | Taurin-conjugated UDCA alleviated ER stress | Cells/ mouse | 52 | |
| ↑Hepatic and muscle insulin sensitivity ↓Hepatic aminotransferases ↓Hepatic fibrogenesis ↑Glycemic control | Taurin-conjugated UDCA increased insulin signaling High dose UDCA (28-35 mg/kg b.wt.) used in patients with NASH, mechanisms not studied | Human Human | 5354 | |
| Bile acids sequestrants | ↓Blood glucose | Colestimide used in patients with hypercholestrolemia and diabetes, mechanisms not studied | Human | 59 |
| ↑Glycemic control | Increased production of secondary BA derived from colestilan-trapped primary BA, resulting in stimulation of TGR5-mediated GLP-1 release in colon | Mouse | 60 | |
| ↑Glycemic control | Cochrane review on colesevelam role in diabetes control, mechanisms not studied | Human | 62 | |
| ↑Glycemic control | Induction of GLP-1 secretion in the intestine via TGR5 activation by sevelamer-trapped BA in the gut lumen | Review of mostly human studies | 63 | |
| ↓Hepatic steatosis | Improvement of liver inflammatory status in NASH mice by sevelamer treatment mediated via FXR signaling | Mouse | 65 | |
| ↓Gastric emptying and appetite | Decrease of appetite by cholestyramine mediated via cholecystokinine on gastric emptying | Human | 66 | |
| Intestinal bile acid reuptake inhibitors | 264W94, Ibat inhibitor: ↑Glycemic control ↑Plasma GLP-1 ↑Plasma insulin | Increased load of malabsorbed primary BA in colon, resulting in stimulation of TGR5-mediated GLP-1 release | Rat | 67 |
| GSK2330672, IBAT inhibitor in diabetic patients: ↑Glycemic control | Increased load of malabsorbed primary BA in colon, resulting in stimulation of TGR5-Mediated GLP-1 release | Human | 70 | |
| elobixibat, IBAT inhibitor in dyslipidemic patients: ↑Plasma GLP-1 | Increased load of malabsorbed primary BA in colon, resulting in stimulation of TGR5-mediated GLP-1 release | Human | 71 | |
| Metformin as a BA metabolism modulator | ↓Ileal BA absorption | Mechanism, how metformin interferes with IBAT, is unknown | Rat | 72 |
| ↑Serum bile acids ↑Serum GLP-1 Beneficiary effects on gut microbiota | Increase of serum bile acids, GLP-1, and beneficiary changes in intestinal microbiota in metformin treated diabetic patients most likely due to BA-malabsorbing effects of metformin | Human | 73 | |
| Natural products | Various metabolic effects depending on a specific product | Modulation of various BA receptors (mainly FXR or TGR5) | Mostly experimental | For review see (74) |
| Probiotics | ↓Obesity | Modulation of microbiota possessing bile salt hydrolase activity leading to accumulation of taurine conjugated BA, with subsequent inhibition of FXR signaling | Mouse | 75 |
| Synthetic bile acid mimetics | Various metabolic effects depending on a specific drug | Modulation of various BA receptors (mainly FXR or TGR5) | Both experimental and human | For Review see (74) |
| Fatty acid-bile acid conjugate (conjugate of arachidonic and cholic acids) | ↓Hepatic steatosis | Inhibition of stearoyl-CoA desaturase leading to improved lipid metabolism | Human | 85 |
The therapeutic potential of BA became apparent in an experimental mouse study when a diet enriched in chol-ic acid increased the energy expenditure and prevented diet-induced obesity by activation of the type 2 deiodi-nase via TGR5 agonistic effect in brown adipose tissue.49 Also, additional following studies were consistent with these data. A recent mechanistic study on Ucp1 deficient mice revealed the important role of uncoupling protein-1 in cholic acid-mediated effects upon energy expendi-ture,50 and the same effect, associated with reduction of obesity, dyslipidemia and glucose intolerance, was also reported in mice fed chenodeoxycholic acid.51 In addition, taurine-conjugated ursodeoxycholic acid (UDCA) reduced hepatic steatosis, and enhanced insulin sensitivity in both mice52 and humans.53 Simultaneously, a high-dose UDCA supplementation was demonstrated to ameliorate hepatic insulin resistance and improve glyc-emic control in patients with non-alcoholic steatohepa-titis,54 although another study did not confirm this finding.55 Surprisingly, also direct cardioprotective effects of UDCA mediated via modulation of ERK/Akt pathway were described in an experimental study by Hanafi, et al.,56 and anti-atherogenic effects of UDCA caused by the alleviation of endoplasmic reticulum stress were also recently reported.57
Ba SequestrantsIt is not only treatment with individual BA, but also BA depleting therapy that might have profound metabolic effects. In fact, administration of BA sequestrants was demonstrated to increase insulin sensitivity in human patients;58,59 an increased production of secondary BA derived from sequestrant-trapped primary BA, resulting in stimulation of TGR5-mediated GLP-1 release in colon, is believed to be one of the major mechanisms accounting for this effect, as shown in animal studies.60 Metabolic effects of BA sequestrants are thus mediated via BA acting in the intestinal lumen (in contrast to the effects of BA on peripheral tissues, when used as a systemic therapy). Based on these data colesevelam, a potent novel BA se-questrant, has been approved by the FDA for the treatment of T2DM - and advertised as the only drug to simultaneously decrease both serum LDL cholesterol and glucose concentrations.61,62 Thus, it is not surprising that other resins also act in a similar way, as has been demonstrated for sevelamer. Although primarily used as a phosphate binding resin in hemodialysis patients,63 this drug also has a potent BA binding capacity64 and blood glucose lowering effects.63 Interestingly, a recent experimental study by McGettigan, et al. demonstrated the potent therapeutic effects of sevelamer against non-alcoholic fatty liver disease (NAFLD) underscoring the importance of this ap-proach.65 And finally, based on a recent human study BA sequestrants have even been suggested to have an effect on appetite and weight control.66
Intestinal Ba Reuptake InhibitorsThe same mechanism also seems to be in play for IBAT/ASBT inhibitors. In fact, in their experimental study on Zucker diabetic fatty rats, Chen, et al. were able to demonstrate that oral administration of the potent ASBT inhibitor 264W94 significantly decreased HbA1c and glucose concentrations, prevented a drop of insulin levels, showed improvement on an oral glucose tolerance test, and increased plasma Glp-1, as well as insulin con-centrations.67 Further ASBT/IBAT inhibitors are now being searched for and studied experimentally for their possible metabolic effects.68,69
These promising experimental data have also recently been corroborated by two clinical studies. In a trial by Nunez, et al. on T2DM patients, BA intestinal re-uptake inhibitor GSK2330672 significantly improved glucose and lipid metabolism in these patients.70 Similar promising results were also obtained in a human study with elobixibat, another IBAT/ASBT inhibitor, which decreased LDL cholesterol and increased GLP-1 levels in patients with dyslipidemia.71 Mechanisms of metabolic action of intestinal BA reuptake inhibitors seem to be identical to those of BA sequestrants - i.e. increased load of malabsorbed primary BA in colon, resulting in stimulation of TGR5-medi-ated GLP-1 release.
Other Therapeutics Modulating Ba Metabolism in the Intestinal LumenInterestingly, the metabolic effects of several drugs routinely used in clinical medicine seem to be dependent (at least partially) on the modulation of BA metabolism. This is certainly true for metformin, whose hypoglycemic effect has been suggested can be accounted for by decreased intestinal BA absorption,72 as well as modulation of the gut microbiome.73
Natural ProductsSteroidal compounds, mimicking BA in their modulating effects on specific nuclear receptors, are widely distributed within the vegetal and marine realms. Recent high-throughput screening studies of traditional herbal medicines as well as marine organisms identified various novel nuclear receptor modulators closely related to structures of BA. These include plant FXR modulators (both agonists and antagonists), such as: guggulsterone, a compound derived from the tree Commipheora mukul, steroids from Ganoderma fruit, or stigmaterol from soy beans; as well as TGR5 activating compounds such as: oleanolic acid from the leaves of Oleaeuropea, or betulinic or ursolic acids.74 Interestingly, various potent steroidal compounds acting predominantly as FXR antagonists have recently been identified from marine sponges, and this list is certainly only in its early days.74
ProbioticsThe metabolic interaction between gut microbiome and the human organism host is indisputable. However quite surprisingly, some of these effects seems to be due to modulation of the intestinal metabolism of BA mediated by the intestinal bacteria. This was clearly shown in an experimental study by Li, et al., in which reduction of Lactobacilli, possessing bile salt hydrolase activity, led to accumulation of taurine conjugated β-muricholic acid, with subsequent inhibition of FXR signaling and decreased obesity.75 Thus, besides the known effects of probiotics on systemic cholesterol levels,76 it seems more than likely that probiotics might represent an important therapeutic/ preventive tool to also treat metabolic diseases, such as obesity, T2DM, and metabolic syndrome.77,78
Synthetic Ba MimeticsIn recent years, enormous progress has been achieved in medicinal chemistry in the field of synthetic compounds based on BA scaffolding. Virtually dozens of such molecules have been synthesized and tested for their biological effects, in particular the capabilities to modulate various nuclear receptors (mainly FXR and TGR5).74 Some of these have entered into various phases of clinical testing, and based on these data, in May 2016 FDA approved obeticholic acid (6α-ethyl CDCA, a FXR agonist) as an additive drug for UDCA non-responsive patients with primary biliary cholangitis (PBC) - truly the first drug for this indication in 20 last years.79 As both TGR5 and FXR are also functionally expressed in pancreatic β-cells, where they regulate insulin secretion (Table 1),80,81 FXR activation has been proposed as a promising therapeutic target for diabetic patients.82 Indeed, in a recent human trial, the treatment of patients suffering from NAFLD and T2DM with obeticholic acid (a potent FXR agonist) was demonstrated to increase insulin sensitivity;83 however, this was not confirmed in yet another study.84
Other BA-related drugs, such as INT-767 (a dual agonist of FXR and TGR5), and INT-777 (a selective activator of TGR5) are under investigation for their potential to treat diabetes and other metabolic diseases.
In addition to this effort, many other approaches to modify BA have recently been investigated, including manipulation of the UDCA scaffold, with promising results to perhaps be able to target metabolic diseases in the near future.74
Fatty Acid-Ba ConjugateThe conjugate of arachidonic and cholic acids (known as Aramchol), an inhibitor of stearoyl-CoA desaturase 1 (SCD-1), has been extensively studied in recent years for its anti-atherogenic and lipid-lowering properties. SCD-1 inhibition decreases hepatic fatty acid synthesis and enhances their β-oxidation, resulting in decreased hepatic storage of triacylglycerols and fatty acid esters. Indeed, in a small clinical study Aramchol has been shown to improve the status of patients with NASH, demonstrating a further possible use of BA in the clinical setting.85
ConclusionOur knowledge of the biological importance of BA has greatly changed during recent years - from considering BA as only lipid-solubilizing agents to realizing their endocrine functions significantly modulating both intermediary metabolism and energy homeostasis.
Nevertheless, we are still only at the beginning of our understanding how BA affect metabolic functions of a human body. This is mainly because of the following reasons. First, each BA interacts with more than one receptor, which on one hand accounts for a variety of their pathophysiological activities, but on the other hand disables to accurately identify the mechanism of action. Whereas some of the receptors are dedicated for BA binding, the others are promiscuous, binding a wide range of endogenous as well as exogenous ligands. Secondly, a human body contains a wide array of individual BA, each of them having different modulating activities against individual cellular receptors (which also differ in terms of their tissue expression). In addition, this aspect is complicated by the fact that most of our knowledge is derived from rodent studies, whose BA pool as well as spectrum is different from humans.
However, based on the novel data discussed above, BA are emerging as an important therapeutic tool, acting mainly via their specific cellular receptors, in particular FXR and TGR5. In addition, BA also seem to interact with the gut microbiome, and thus also exert their metabolic function indirectly. It is also important to note, that some beneficial effects of drugs being used for the treatment of metabolic syndrome and diabetes (as well as some other disorders) are likely to be mediated through their action on BA metabolism. And finally, the first novel BA-based drugs are being intensively investigated in clinical trials, and it is almost certain that they are going to significantly change our therapeutic armamentarium to treat these metabolic diseases.
Conflict of Interest StatementThe author declares that there are no financial nor commercial conflicts of interest.
AcknowledgementsThis work was supported by grants PROGRES Q25/ LF1 from the Czech Ministry of Education, and RVO-VFN64165/2017 from the Czech Ministry of Health.