Biliary secretion in health and disease is reviewed. The powerful techniques of molecular biology have enabled cloning of the transporters involved in biliary secretion and the enterohepatic circulation of bile acids. This, in turn has permitted elucidation of their function as well as their regulation by nuclear receptors. Bile acid secretion is required for efficient lipid absorption, and bile acids also possess powerful direct and indirect antimicrobial functions in the small intestine. The enterohepatic circulation results from efficient ileal absorption, and is highly regulated at two sites. In the hepatocyte, biosynthesis of bile acids is regulated in negative feedback manner by the nuclear receptor FXR as well as by cytokines and by a peptide (FGF-19) liberated by bile acids from the ileal enterocyte. In the ileal enterocyte, bile acid reclamation is regulated in negative feedback manner by FXR and other nuclear receptors. The bile salt export pump (BSEP) mediates uphill canalicular bile acid secretion. Inborn defects in its function cause intrahepatic cholestasis in infants; inhibition of its function by drugs causes hepatotoxicity. Bile acid therapy is based on correction of bile acid deficiency by supplemental bile acids or displacement in which a noncytotoxic bile acid (ursodeoxycholic acid, ursodiol, UDCA) is administered and dilutes out the endogenous cytotoxic bile acids. Administration of primary bile acids may be lifesaving in inborn defects of bile acid biosynthesis. A synthetic bile acid, norUDCA is absorbed by the biliary ductules after secretion and cures the peribiliary fibrosis occurring in the MDR2-/- mouse which lacks biliary phospholipid.
In this brief review, emphasis will be on current concepts of biliary secretion and excretion as perceived by the author. Alterations in disease and therapeutic approaches to correcting defects will be summarized. The emphasis will be on human physiology and pathophysiology, but studies in experimental animals will be considered when they elucidate events in man. Two review articles in the Handbook of Physiology, the first1 being an overview of bile secretion and the second2 being an overview of the enterohepatic circulation of bile acids serve as a summary of understanding about twenty years ago. More recent reviews of the enterohepatic circulation of bile acids are available.3,4 Other reviews deal with nuclear receptors for which bile acids are the ligands;3 reviews of the canalicular transporter for bile acids6,7 and overall hepatocyte transport of organic anions are also available.8-11
Bile as a digestive secretion: Bile, a detergent-rich fluid secreted by the liver into the intestinal tract is present in all vertebrates, so far as is known. The gallbladder is a reservoir between the liver and the small intestine, in which bile is stored, concentrated, and discharged during digestion. The gallbladder is present in most vertebrates, including early vertebrates such as the coelacanth. [The gallbladder is lacking in ancient mammals (manatee, hyrax, elephant, rhinoceros, and horse), in some pigeon species, and in a few mammals such as certain even-toed ungulates (musk and other deer, the giraffe, some rodents, as well as whales and porpoises]. I have argued that the presence of a gallbladder indicates a recycling pool of bile salt, if this view is correct, then the genes for development of the gallbladder arose at about the same time as the genes for bile salt synthesis. Nonetheless, a recycling pool of bile acids occurs in species that lack a gallbladder as well as in the cholecystectomized person. Thus the presence of a gallbladder indicates the presence of a bile acid pool but the absence of a gallbladder does not exclude a recycling bile acid pool.
Powerful new tools of molecular and cell biologyGreat advances in understanding canalicular transport have been made in the past two decades. The most important advance has been the identification (cloning) of the genes encoding the proteins mediating transport by the sinusoidal canalicular membrane of the hepatocyte.8-12 Cloning of a gene in turn permits studies of its regulation by defining the elements of its promoter site (reviewed in 13). In addition, knowledge of gene structure permits the development of animals in which the gene is ablated (knocked out) or over-expressed (so called transgenic animals). It also permits tissue specific expansion or deletion of a gene. This technique is valuable when the genetic knockout is fatal in the embryonic stage. One can also perform site directed mutagenesis to find which parts of the gene are essential for transcribing a functional molecule.
In the past few years, the presence of small, «interfering» RNA molecules that destroy complementary messenger RNA and thereby prevent protein translation has been identified.14 Thus, it is possible to knock down a specific gene product in a cell using synthetic interference RNA. All of these tools have led to an enormous understanding of the transport proteins of the hepatocyte.
Another conceptual advance was the recognition that hepatocytes are polarized, and this led in time, to methods15 permitting separation of basolateral domains (the membrane of the hepatocyte facing the sinusoid) and of apical domains (canalicular membranes).
The ability to transfect cells has led to the creation of what may be considered artificial hepatocytes in which either a basolateral (sinusoidal) or apical (canalicular) transporter or both are transfected. Such cells mediate vectorial transport and are invaluable to define unequivocally which substrates are actually transported by individual transporters.16,17
Two new techniques are rapidly changing the whole concept of genetic expression. The first is microarray analysis (c.f. 18). In this technique changes in RNA are measured by hybridizing a cell’s RNA (converted to DNA for the experiment) to a library of DNA molecules. The technique can be used to measure which mRNA molecules are up regulated or down regulated, in response to a given perturbation of a cell in culture. For example, the effect of adding a bile acid at several concentrations can be assessed. This technique permits changes in thousands of RNA molecules to be assessed in a relatively short time. Of course, results have to be confirmed by the polymerase chain reaction, a technique that is routine in every laboratory today.
Microarray analysis only provides information on changes in RNA molecules and such do not always correlate with protein content as proteins may be degraded at differing rates once synthesized. To assess the protein content of the cell, specific antibodies are required. Alternatively, the technique of proteome analysis19 is an alternative; this new method is undergoing rapid development. In this technique proteins are fractionated by charge and/or molecular weight and then quantified by the combination of enzymatic cleavage, mass spectrometry and complex computer programs. Examples of proteome analysis are an early study of cholangiocyte proteins20 and a more recent study of the proteome of the outer membrane of yeast mitochondria21 It is not impossible to envision a complete proteomic analysis of the canalicular membrane in the next decade.
The last technique that has proved to be extremely powerful for understanding events in biliary secretion is improved imaging using confocal laser microscopy. Antibodies (either polyclonal or monoclonal) are generated to a given transport protein and tagged with a fluorescent dye. The protein can then now be visualized together with an antibody for a protein or organelle whose location is known. One then visualizes the first fluorescent dye, then the second, then both (so called merge) and thereby identifies the precise cellular localization of the protein in question.
Two other imaging techniques should be noted briefly. The first are dyes (usually fluorescent) that are used as indicators to measure the intracellular concentrations of signaling molecules such as Ca 2+ and ATP. The second is the generation of DNA constructs in which a gene for a fluorescent protein or luciferase is added to the gene for a desired protein. When such fusion genes are transfected into cells, it is possible to visualize the protein in question by its fluorescence or luminescence, enabling its movement in the cell to be traced using videomicroscopy. Using videomi-croscopy, one can watch the actual movement of such tagged proteins in real time, for example, as they move from the Golgi to the canalicular membrane.22
Using these techniques, there has been a great expansion in our knowledge of cholangiocytes, the epithelial cells of the biliary ductules. As noted above, the proteins of these cells have been determined by 2 dimensional electrophoresis and mass spectrometry,20 and differences in function between small and large cholangiocytes have been elucidated23 Indeed, it is fair to say that the last decades have witnessed an explosion in cholangiocyte biology and pathobiology.
Thus the evolution of these enormously powerful technique in genetics, and cell and molecular biology have resulted in a vast increase in our understanding of the events of biliary secretion.
Bile as a digestive secretionLuminal events: Bile is a key digestive secretion, and thus joins saliva, gastric and pancreatic juice as a functional secretion enabling the highly efficient assimilation of dietary foodstuffs. The only important constituents of bile so far as digestion is concerned are its bile salts. Bile salts have multiple functions in the organism, and current concepts of bile acid (salt) function are summarized in Table I.
Functions of bile acids currently recognized
| • Whole organism |
| • Elimination of cholesterol |
| • Hepatocyte |
| • Insertion of canalicular bile acid and phospholipid transporters |
| • Induction of bile flow and biliary lipid secretion |
| • Promotion of mitosis during hepatic regeneration |
| • Regulation of gene expression via nuclear receptors |
| • Biliary tract |
| • Lumen |
| • Solubilization of cholesterol |
| • Micellar trapping of cholephilic xenobiotics |
| • Antimicrobial effects |
| • Cholangiocytes |
| • Stimulation of bicarbonate secretion via CFTR and AE2 |
| • Promotion of proliferation when bile duct obstructed |
| • Small intestine |
| • Lumen |
| • Solubilization of dietary lipids, especially fat soluble vitamins |
| • Solubilization of lipophilic drugs |
| • Antimicrobial effects |
| • Ileal enterocyte |
| • Regulation of gene expression via nuclear receptors |
| • Secretion of antimicrobial factors (FXR mediated) |
| • Secretion of FGF-19, a peptide regulating bile acid biosynthesis |
| • Large intestine |
| • Colonic enterocyte |
| • Modulation of electrolyte absorption and secretion |
| • Muscle layer |
| • Promotion of propulsive motility |
| • Brown adipose tissue |
| • Stimulation of thermogenesis by thyroid hormone |
Bile salts may be defined as water soluble, amphipathic end products of cholesterol metabolism formed by conjugating a bile acid to taurine or glycine (or a bile alcohol to sulfate). The anions of such conjugated bile salts are impermeable to cell membranes and have a remarkable ability to dissolve biliary phosphatidylcho-line, as well as the products generated by the action of pancreatic lipases and esterases on dietary esterified lipid [fatty acids (partly ionized) and 2-monoacyl glycerol (monoglyceride)]. Esterified lipid in the diet of carnivores and fish consists mostly of triglyceride although a small proportion of waxes (long chain fatty acids esterified to long chain alcohols) may also be present in dietary lipids. Herbivores must be able to hydrolyze the great variety of plant lipids by their pancreatic lipases and esterases.
The final product of such lipolysis is mostly fatty acid. Long chain fatty acids are poorly soluble at small intestinal pH because of the formation of acid soaps (one molecule of ionized fatty acid and one molecule of protonated (non-ionized fatty acid) that have extremely low solubility at the slightly acidic pH of jejunal content.4 However, the partly ionized fatty acids readily form mixed micelles with bile salts. Such micellar solubilization increases the amount of fatty acid present in the aqueous phase by about a thousand fold. The micelle diffuses more slowly than single molecules because of its size. Because diffusion rates are related to volume, which is a cube root of the molecular weight, the mixed micelle, despite its having a molecular weight at least 200 times that of a fatty acid monomer, nonetheless diffuses at about one seventh the rate of individual molecules. Therefore micellar solubilization increases the rate of diffusion to the cell membrane by a factor of at least a hundred. Fatty acids that are water soluble do not require solubilization by bile acids for absorption. This includes fatty acids with a chain length ≤ 14 carbon atoms as well as most unsaturated fatty acids.
The mixed micelle has a hydrocarbon core composed of the hydrocarbon chains of the fatty acids. This hydrocarbon can in turn dissolve other dietary lipids such as fat soluble vitamins. Solubilization of fat soluble vitamins thereby enabling their efficient intestinal absorption is probably the most important function of mixed micelles. However, little is known about the role of mixed micelles in fat soluble vitamin absorption in non-mammalian species such as reptiles.
To form mixed micelles, bile salts must be present at a concentration at which they form mixed micelles. High conjugated bile acid concentrations are present in the small intestine for several reasons. First, because of concentration in the gallbladder, bile salts enter the small intestine at very high concentrations. Second, because they are present solely as anions at small intestinal pH (because they are strong acids), they are membrane impermeable. Finally, in humans and presumably in other mammals dilution of small intestinal content during digestion is not great.
How does one check for the presence of micelles? One can add to a sample of any body fluid a dye that is lipid soluble but water-insoluble.5 The solubilization of such a dye (Orange OT is used by chemists), but the principle is not different than staining fat droplets in a histological section with Sudan III. The current view is that micelles are present in bile and small intestinal content and nowhere else in the body.
The micelle is believed to be spherical based on biophysical studies. The bile salts are wedge shaped molecules. They put their hydrophobic back between the heads of the fatty acids and push them apart, converting a bilayer arrangement to a spherical arrangement. The molecular arrangement in the mixed micelle was proposed on the basis of complex small angle neutron scattering techniques led by Rex Hjelm and his colleagues at the Los Alamos National Laboratory.6 The spherical micelle differs from the original drum shaped micelle proposed by Small in which the a bilayer of fatty acid was surrounded by a one molecule thick bilayer of bile acid molecules.
Both the bile salts and the fatty acids remain in any given micelle for only some milliseconds. They move out from the micelle and exchange with fatty acids and bile salt molecules present as monomers in the surrounding aqueous phase. The mixed micelle is a flickering cluster. Uptake at the cell membrane is believed to be both passive (high capacity, low affinity) and carrier-mediated (low capacity, high affinity). Passive flip flop of fatty acids (in protonated, uncharged form) across a lipid bilayers is bidirectional, the direction being determined by the concentration gradient. At least one fatty acid transporter is also a coenzyme A synthetase.27 Linking a fatty acid to Coenzyme A in thio-ester linkage prevents back diffusion.
The presence of a micellar phase during digestion in man is well established by sampling small intestinal content and isolating the micellar phase by ultracentrifugation28 or ultrafiltration,29 and similar studies have been performed in large animals such as the dog and the cow. However, as noted, experimental isolation of a micellar phase has been performed in relatively few other vertebrates.
Bile salts might also play a role in protein digestion. Bile salts will adsorb to any hydrophobic domains of dietary proteins, and this might in turn promote protein denaturation, rendering proteins more susceptible to digestion by the proteolytic enzymes.
Bile salts solubilize biliary lipids (phosphatidyl-choline and cholesterol) as well as dietary lipids. Biliary phospholipid – about 6 grams/day in the adult – serves to emulsify dietary triglyceride, but is rapidly hydrolyzed to lysophosphatidylcholine and fatty acid. Lysophosphatidylcholine is water soluble and presumably partitions between the aqueous phase and the mixed micelles. It is rapidly absorbed. Biliary cholesterol is solubilized in mixed micelles and mixes with dietary cholesterol.
Cholesterol absorption varies between individuals and ranges widely. In the past, changing cholesterol absorption was not considered very important as increasing dietary cholesterol (c.f. 30) or blocking cholesterol absorption by feeding high dose plant sterols31 had only small effects on levels of plasma or biliary cholesterol. The reason for the lack of influence of dietary cholesterol on biliary or plasma lipids was related to homeostatic control by negative feedback of cholesterol synthesis by the hepatocyte. When less cholesterol reached the hepatocyte, cholesterol biosynthesis increased; when more cholesterol reached the hepatocyte, cholesterol biosynthesis decreased.
However, in the past few years, cholesterol absorption has become a topic of intense scrutiny. This view has been modified strikingly in the past few years, as NPC1L1, a cholesterol transport protein present in the apical membrane of the enterocyte was identified, and as a new drug, ezetimibe, was shown to block cholesterol uptake by this transporter.32 Moreover, combining ezetimibe with a statin (which inhibits cholesterol synthesis) was shown to greatly enhance the hypocholesterolemic effect of the statin.33
The mixed micelles also solubilize plant sterols such as sitosterol. In the past, these molecules were considered to be poorly absorbed because of their different molecular shape. With the identification of an ATP-energized cholesterol (and plant sterol) efflux pump34 formed by two half transporters (ABC5 and ABC8), it became clear that plant sterols were absorbed (presumably by the cholesterol importer). However, as plant sterols in contrast to cholesterol underwent little esterification, they are not incorporated into chylomicrons and effluxed back into the lumen via ABC5/ABC8. In the past, it had been assumed erroneously that plant sterols did not enter the enterocyte.
An extremely rare disease, sitosterolemia, was shown to be caused by defects in ABC5 and/or ABC8,35,36 and is characterized by the accumulation of plant sterols in the body evidenced by sterol-rich xanthomata. The laboratory group of Gerald Salen has just reported that feeding ezetimibe to such a patient caused a dramatic fall in plasma levels of plant sterols and regression of xanthomata.37
The ultimate fate of fatty acid in the enterocyte is re-esterification to form triglyceride, and packaging of the triglyceride droplets into chylomicrons. Details of intracellular processing is beyond the scope of this review, but reviews are available.
It is reasonable to speculate that bile salts play a general role in keeping the absorptive surface of the small intestine clean. Bile salts should adsorb to food residues, giving them a negative charge, thereby precluding their aggregation. However, this has not been tested experimentally. Cells at the tip of the villus are undergoing continuous apoptosis followed by shedding. Cellular lipids will be solubilized by bile salts and delivered to more caudal enterocytes.
Besides forming mixed micelles with dietary lipids and their lipolysis products, and cleaning the small intestinal surface, conjugated bile acids also have potent antimicrobial effects in the small intestine.38 Conjugated bile acids thus join defensins and IgA as luminal molecules inhibiting the growth of bacteria.
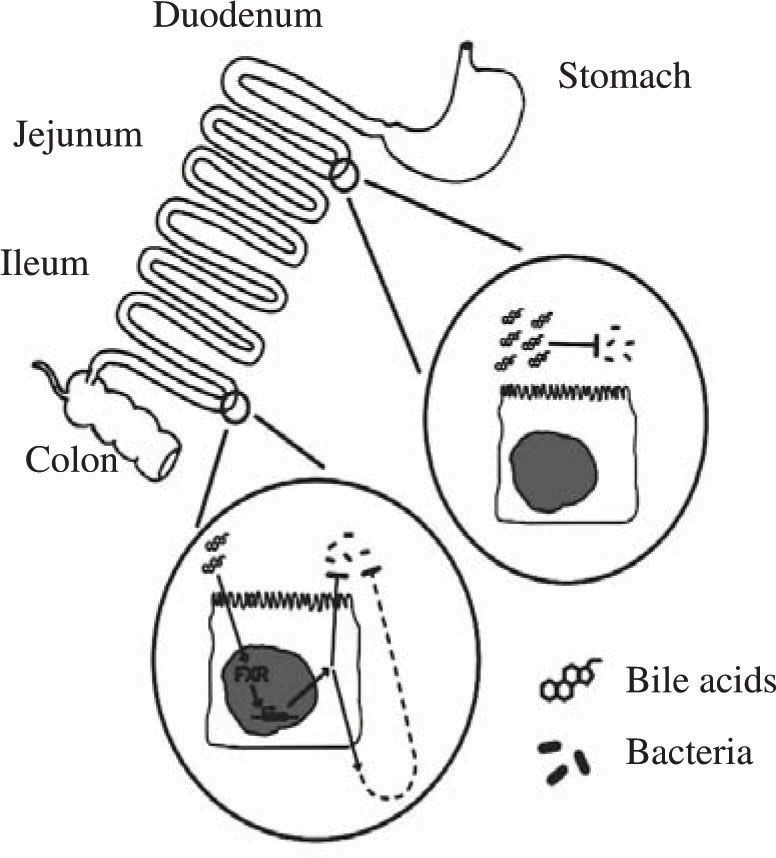
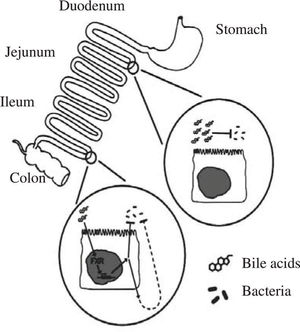
The evidence for a potent antimicrobial effect of conjugated bile acids is based on a number of reports, mostly in the surgical literature, showing that bile duct ligation leads to bacterial proliferation in the small intestine. Extending this work was a study by Lorenzo-Zuniga et al39 who performed studies in rats with carbon tetrachloride induced cirrhosis, a condition in which bile acid secretion is decreased, leading to lower intraluminal concentrations of bile acids. As a consequence, bacterial overgrowth in the small intestine and bacterial translocation to intestinal lymph nodes occurs. Oral administration of two conjugated bile acids (cholylglycine or cholylsarcosine) abolished bacterial overgrowth, decreased endotoxemia, and increased survival. Inagaki et al40 extended this work by testing whether administration of an agonist of the nuclear receptor FXR (known to have bile acids as its major ligand) would have an effect on the bacterial overgrowth occurring in the bile duct ligated mouse. They found that administration of GW4064, an FXR agonist synthesized by Glaxo Smith Kline, led to a remarkable fall in bacterial proliferation in the small intestine. As these animals had bile duct ligation, the effect of this FXR agonist must indicate a second indirect effect by which bile acids exert an antimicrobial effect. Figure 1 summarizes the antimicrobial effects of bile acids in the small intestine.
Schematic depiction of the antimicrobial effects of bile acids in the small intestinal lumen. Bile acids, possibly aided by fatty acids, have a direct antimicrobial effect on luminal bacteria. Bile acids also have an indirect effect on luminal bacteria, mediated by the nuclear receptor FXR; the mechanism of this effect has not been clarified. Taken from reference 38.
Intraluminal deficiency of bile acids. A conjugated bile acid deficiency occurs when the enterohepatic circulation is obstructed, diverted, or when intestinal conservation of bile acids is impaired because of ileal dysfunction. In patients with a short bowel syndrome, severe bile acid malabsorption occurs because most patients with this condition have lost their ileum. The therapy currently practiced is to enrich dietary triglyceride in medium chain triglyceride, as medium chain fatty acids are water soluble and do not require micellar solubilization for absorption. Moreover they are absorbed extremely rapidly as they are absorbed both transcellularly and paracellularly. Fat soluble vitamins are given parenterally.
Patients with short bowel syndrome have both a loss of intestinal absorptive surface as well as defective micellar solubilization. The feeding of conjugated bile acids can correct the defect in fat digestion. A conjugated bile acid analogue, cholylsarcosine, was synthesized, found the physicochemical properties of the natural conjugates of cholic acid41 and shown to be resistant to bacterial degradation (deconjugation and dehydroxylation) in animals42 and man.43 Addition of cholylsarcosine to the diet increased triglyceride absorption in dogs with bile acid malabsorption induced by ileal resection.44 In patients with short bowel syndrome cholylsarcosine administration increased triglyceride absorption and induced weight gain.44,46 However, cholylsarcosine is investigational and few patients have been treated to date. Pharmaceutical companies have shown little interest in cholylsarcosine because there is no patent protection and the perceived market is small. Cholylsarcosine can cause gastric irritation, but if an enteric coating is used, the compound must be rapidly released in the duodenum, as this is a major site of fat absorption, and small intestinal transit is often very rapid in patients with short bowel syndrome. Such a formulation of cholylsarcosine has been reported.47
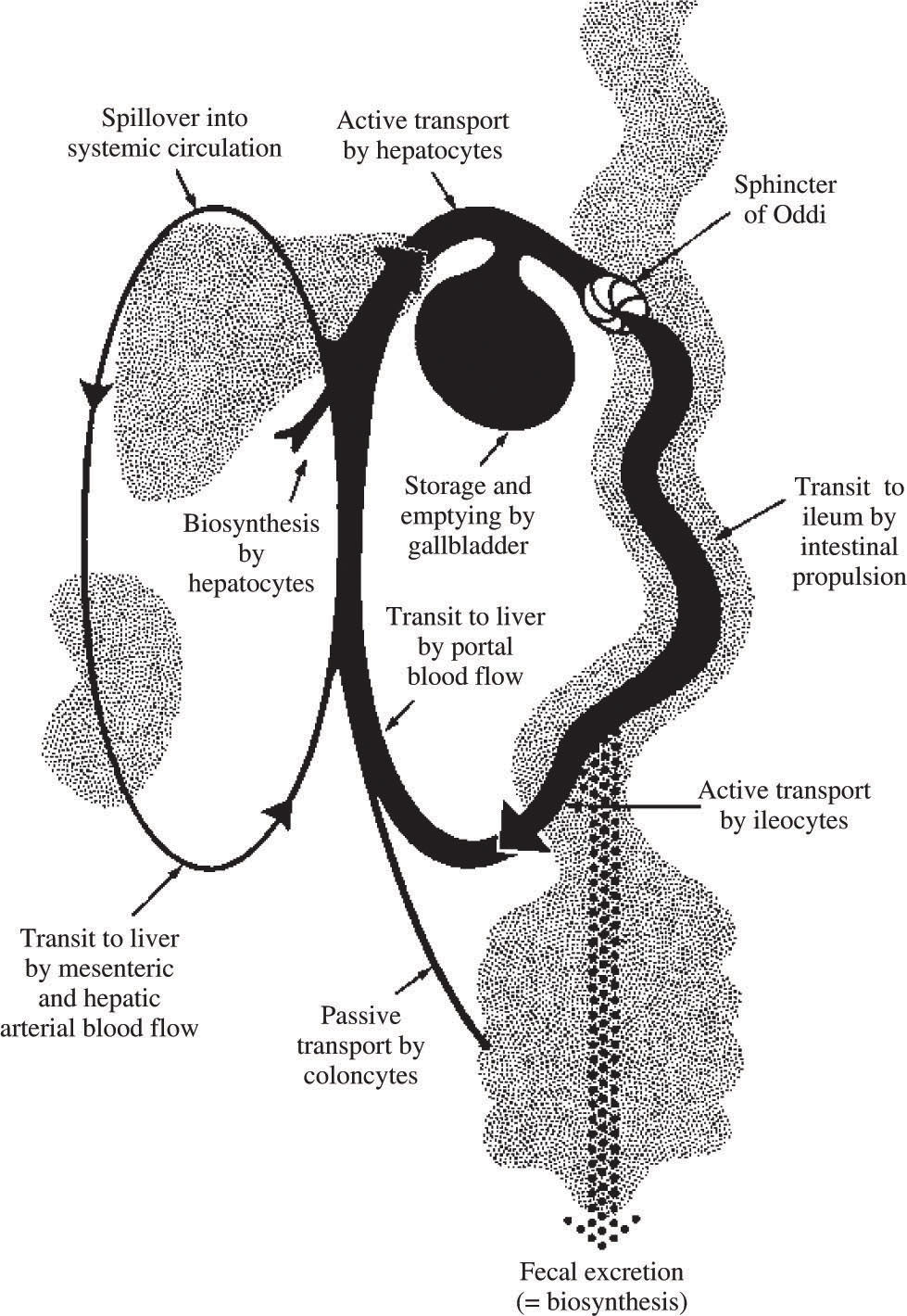
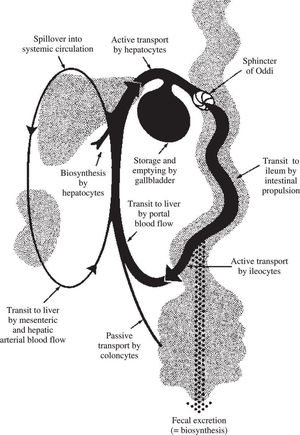
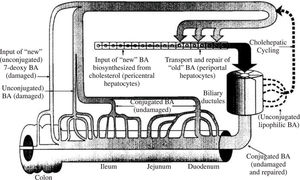
Bile acid recycling: the enterohepatic circulationIn health, daily bile acid secretion when measured by an indicator dilution technique is 30-50 mmoles day.48 Daily synthesis averages about 1 mmole/day. The ability to secrete more bile acid than is synthesized results from a recycling bile acid pool. Development of a bile acid pool results from efficient intestinal conservation mediated by in large part by the ileal conjugated bile acid transport system. Schematic views of the enterohepatic circulation of bile acids are shown in Figures 2 and Figure 3.
Schematic depiction of the enterohepatic circulation and metabolism of bile acids. Normally, bile acids are efficiently conjugated (amidated) with glycine or taurine, and cholehepatic shunting is small. The ileal apical bile acid transporter is present on the apical membrane of cholangiocytes, so a modest amount of cholehepatic cycling of conjugated bile acids does occur. Bile acid synthesis is balanced by fecal loss.
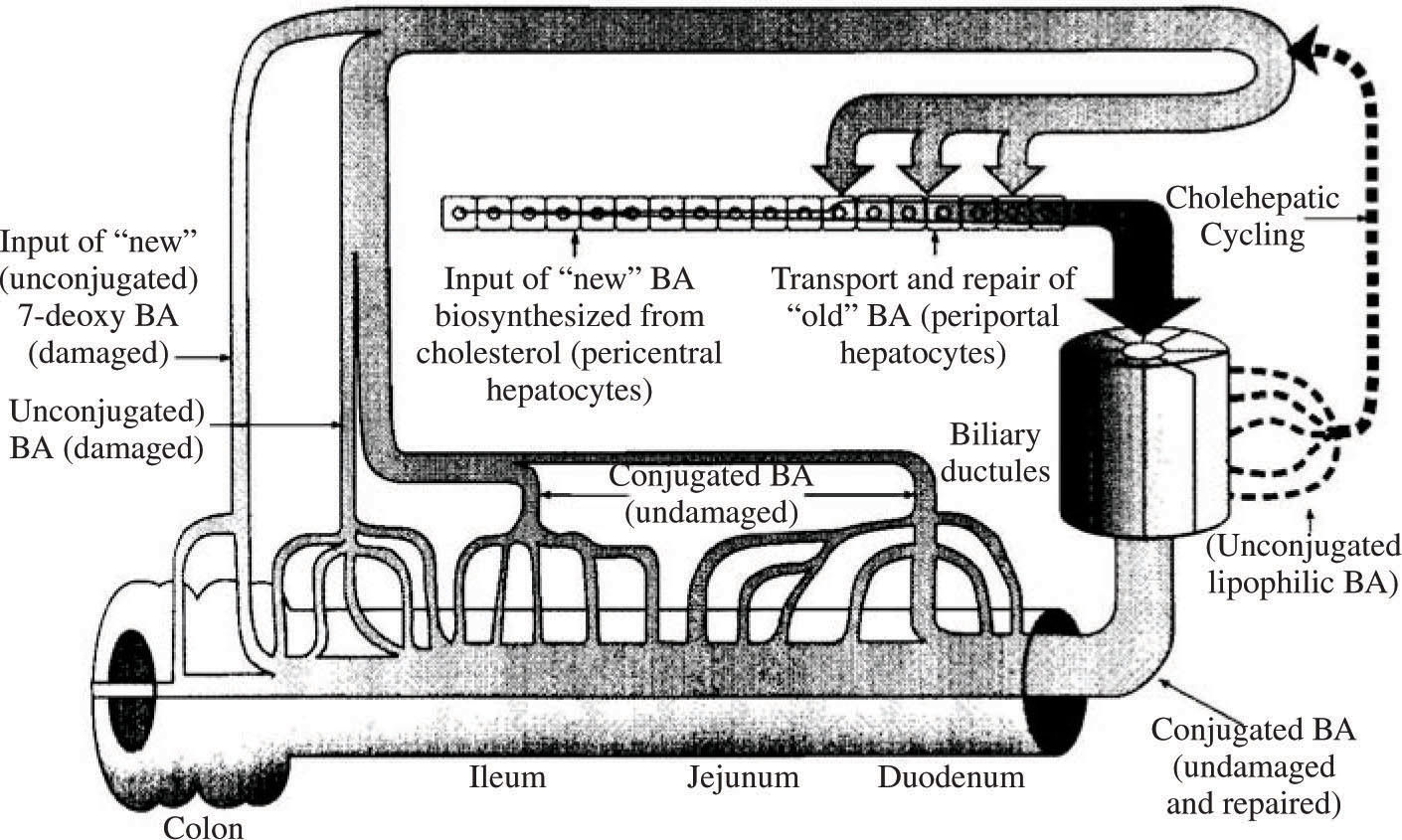
The enterohepatic circulation is regulated at two sites. The first is regulation of biosynthesis from cholesterol, which is mediated in negative feedback manner by several mechanisms. First, bile acids in the hepatocyte activate a heterodimeric nuclear receptor (RXR-FXR) whose activation induce the synthesis of a protein named shp. 5Shp together with activators binds to the promoter site of cyp7A1, (cholesterol 7α-hydroxylase) which is the rate limiting enzyme in bile acid biosynthesis. Binding of shp down regulates bile acid biosynthesis. Second, there is a shp independent pathway for down regulation, activated by inflammatory cytokines.49 Finally, FGF-19, a newly identified protein that is released from the ileal enterocyte by bile acids, also down regulates bile acid biosynthesis.50,51 Ileal absorption of bile acids is also regulated in a negative feedback manner by bile acids, as discussed below.
Bile acid uptake across the apical membrane of the ileal enterocyte is mediated by a sodium dependent conjugated bile acid cotransporter [apical sodium dependent bile acid transporter (ASBT) that has been found in every vertebrate in which it is sought.3 In humans, it is expressed weakly in cholangiocytes, in the gallbladder, in the renal tubule and in the placenta.3 A sodium independent bile salt transporter (oatp3) is present throughout the small intestine and may promote limited bile salt absorption.52 There is little information on the contribution of this transporter to bile salt conservation in vertebrates. Paracellular absorption of bile acids is believed to be negligible as the bile salt molecule is too large to pass via the tight junctions between small intestinal epithelial cells. Whether subjects with increased intestinal permeability absorb bile acids via a paracellular route is not known. Were this to occur, the intraluminal concentration of bile acids might fall, leading to bacterial proliferation. Were bacterial proliferation to damage the paracellular junctions, a vicious cycle might ensue.
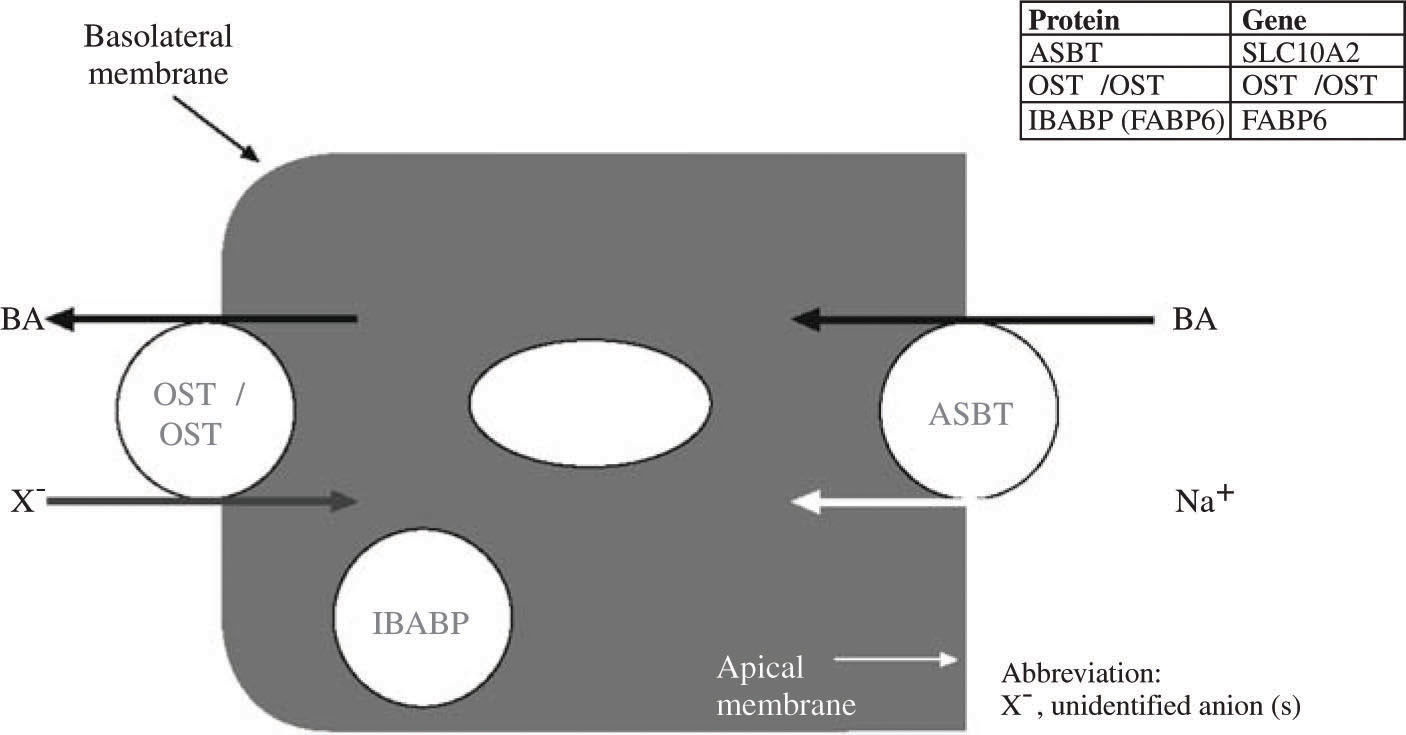
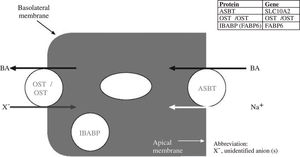
A bile acid binding protein in the ileal enterocyte plays an as yet undefined role in promoting vectorial transport. Exit from the ileal enterocyte is mediated by a heterodimeric bile salt transporter composed of two sub-units OSTalpha and OSTbeta.53,54Figure 4 shows a schematic view of vectorial transport of bile acids through the ileal enterocyte.
Schematic illustration of the bile acid transport by the ileal enterocyte. During active absorption, the ileal bile acid binding protein (IBA-BP) may translocate to the nucleus. When IBAPB is ablated, bile acid absorption still occurs, so the protein is not required for active bile acid absorption. Abbreviations of the protein and gene are given in the insert.
Ileal transport in man and the mouse appears to be also regulated in a negative feedback manner – thus bile acid feeding down regulates bile acid transport, and bile acid sequestrant feeding upregulates bile acid transport.3 Up-regulation may involve recruitment of more orad epithelial cells rather than enhanced transport by individual ileal enterocytes. Details of regulation of the ileal apical sodium dependent transporter are being clarified.
Bile acids are transported to the liver in portal venous blood are efficiently extracted despite being highly albumin-bound. The extent to which bile acids are bound to albumin depends on bile acid structure: trihydroxy bile acids are bound much less completely (70%) than dihydroxy bile acids (99%). Uptake is dependent on bile acid structure and is greater for trihydroxy bile acids than di-hydroxy bile acids, and for a given steroid moiety, is greater for conjugated bile acids than unconjugated bile acids. Fractional extraction of bile acids remains constant despite varying bile acid loads to the liver. Therefore bile acid extraction is said to be «blood flow limited».
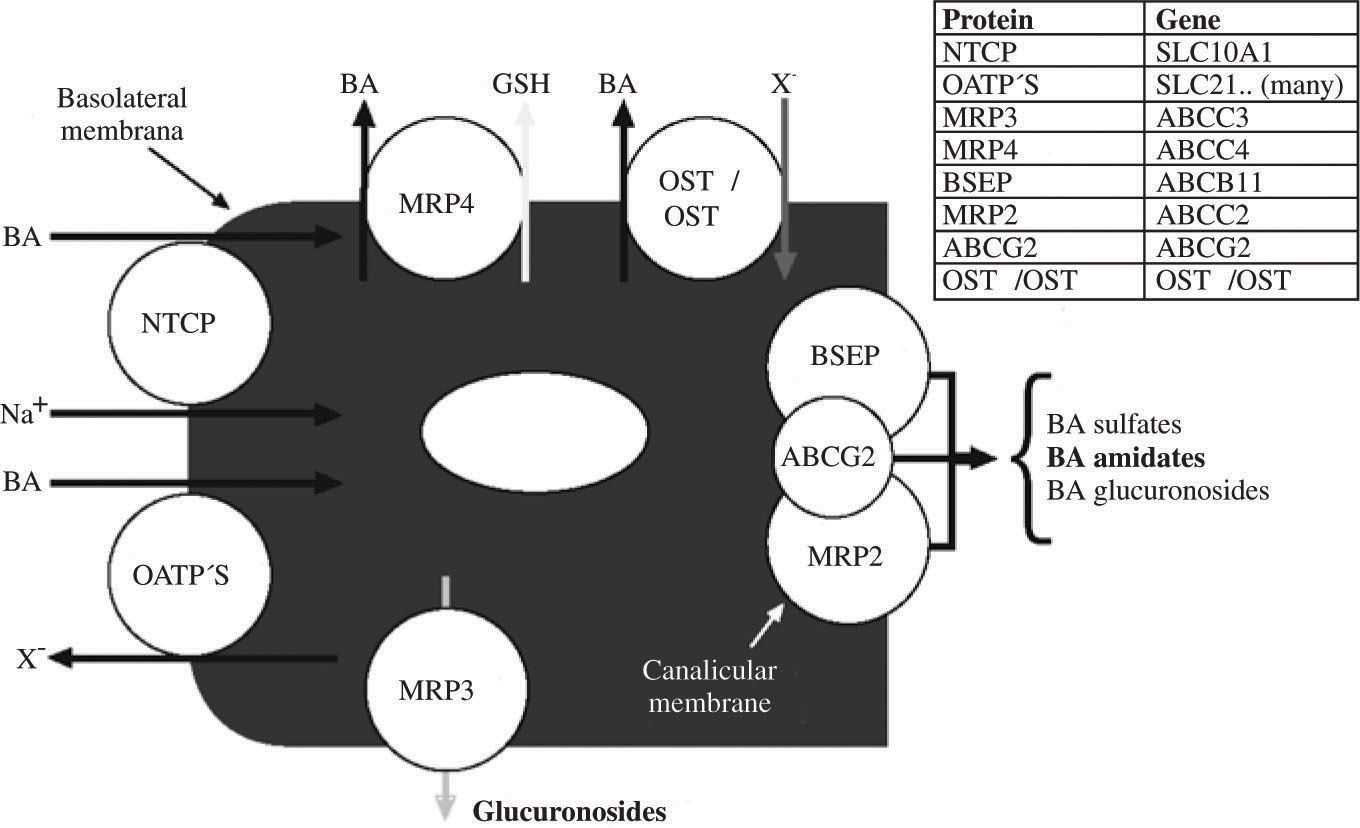
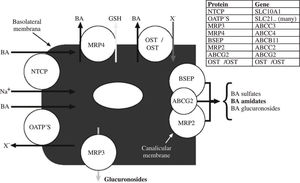
Vectorial transport by the hepatocyte involves uptake at the basolateral membrane and active secretion across the canalicular membrane. Basolateral uptake is mediated by both sodium dependent and sodium independent transporters. Although there are continuing efforts to define the substrate specificity and role of the many sinusoidal membrane uptake proteins,8-11 It is still not clear which transporters other than the sodium dependent transporter are involved in bile acid uptake. Figure 5 illustrates schematically the major transporters involved in vectorial transport of bile acids through the hepatocyte.
Schematic illustration of bile acid transport by the hepatocyte. The substrate specificity of the three canalicular transporters is not yet perfectly defined and varies between species. ABCG2 is also known as the breast cancer related gene. Normally, the proportion of bile acid sulfates or bile acid glucuronosides (glucuronides) in bile is quite low. MRP4 promotes the cotransport of conjugated bile acids and reduced glutathione. MRP3 is thought to be involved in efflux of glucuronosides. OSTα/OSTβ upregulate markedly in cholestatic liver disease and pump bile acids out of the hepatocyte into sinusoidal blood. Abbreviations of the protein and gene are given in the insert.
Secretion across the canalicular membrane involves predominantly the canalicular bile salt export pump (BSEP), which is energized by hydrolysis of ATP. Since its cloning in 1998,55 BSEP has been studied in considerable detail.6,7 The human BSEP transports both conjugated and unconjugated bile acids as well as sulfated litho-holyl conjugates. About 50 mutations have been identified in infants born with «primary familial intrahepatic cholestasis» (type 2).56 Defects in BSEP may involve failure of mRNA to be formed, biosynthesis of a non-functional transporter, or biosynthesis of a transporter that is formed but not delivered to the canalicular membrane. When infants are born with non-functioning BSEP, they develop hepatocyte necrosis and liver fibrosis, leading ultimately to liver failure. Liver transplantation is required and is life-saving.
BSEP transports not only bile acids, but also a variety of drugs, including several statins.57,58 Inhibition of this transporter by drugs can cause hepatotoxicity,57,59 and in vitro screening techniques are being developed that will permit elucidation of interaction of drugs (and their metabolites) with BSEP.60
The major canalicular transporter for organic anions other than bile acids is MRP2. It transports many drug metabolites and bilirubin glucuronides. In animals, MRP2 mediates canalicular secretion of bile acid sulfates. However, in man, BSEP appears to mediate the canalicular secretion of sulfated (and amidated) derivatives of lithocholic acid.61 Such transport should be important in patients with cholestatic liver disease who ingest ursodiol, as ursodiol is converted to lithocholic acid by colonic bacteria.
Bile as an excretory fluidOverview: The major excretory constituents of bile are bile acids – end products of cholesterol metabolism – and bile pigments (conjugated bilirubin and/or biliver-din – end products of heme metabolism, and polyvalent metal cations. A survey of biliary lipid composition in over 100 vertebrates showed that in many vertebrates, only bile acids are present in appreciable proportions, i.e. in many vertebrates the phospholipid/bile acid and cholesterol/bile acid ratio is extremely low.62
Human bile is lipid rich containing a high ratio of phospholipid to bile acid (0.3) and a high ratio of cholesterol to bile acid (0.07). Man appears to differ from all other animals characterized to date in eliminating cholesterol from the body to a greater extent as cholesterol than by conversion to bile acids. Balance studies suggest that perhaps 60% of cholesterol is eliminated from the body as cholesterol, the remainder being eliminated as bile acids.63 In contrast, in most animals, the virtual absence of cholesterol in bile suggests that most cholesterol is eliminated from the body by biotransformation to bile acids.62
Biliary excretion permits the organism to eliminate substrates that cannot be eliminated via renal excretion. Besides bile acids, biliary excretion involves the excretion of organic molecules and heavy metal cations that are highly protein bound.64,65 Thus bile serves as the elimination route for plant sterols, lipophilic drug metabolites, and heavy metals. Because none of such molecules have an appreciable enterohepatic circulation, their concentration in bile is quite low.
The digestive function of bile acids has already been summarized, and bile acid transport into bile by BSEP was discussed above.
Biliary phospholipid secretion. The major phospholipid of mammalian bile is phosphatidylcholine (PC) which is not believed to have any important digestive function. This is because phospholipids are present in dietary lipids, and in addition, the enterocyte can biosynthesize PC which is a key part of the lipid surface layer of chylomicrons. Phospholipid serves to solubilize cholesterol in bile, as it forms the lipid core of the mixed micelles present in bile that can in turn solubilize cholesterol and other biliary lipids. The secretion of PC into bile is mediated by a phospholipid flip-pase termed MDR2 in mice and MDR3 in man.66
To date, the only canalicular protein involved in biliary phospholipid secretion is MDR2.66 This transporter can be shown in vitro to flip PC molecules across membranes, but it has been proposed that the flippase also contributes to the generation of hemivesicles containing mostly PC, as these are present by electron microscopy in wild type mice, but not in MDR2 knockout mice.67 Bile acids convert the hemivesicles into mixed micelles by adsorbing to the lipid bilayer and at a sufficiently high bile acid/phospholipid ratio destroying the bilayer stability.
The luminal side of the canalicular membrane must be highly resistant to the solubilizing action of bile acids. Sphingomyelin which is a membrane stabilizer is present in the luminal face of the canalicular membrane but is present in mammalian bile in only trace amounts. Phosphatidylserine is believed to be flipped back from the luminal face to the cellular face by FIC1. Primary familial intrahepatic cholestasis type I involves mutations in a gene called FIC1. One view of the protein encoded for by this gene is that it promotes the flipflop of phosphati-dylserine from the biliary face of the canalicular membrane to the cytosolic face. Defective removal of this phospholipid from the biliary face of the canalicular membrane results canalicular membrane fragility. As a result phosphatidylserine as well as canalicular proteins are released into bile when bile flow is induced by infusing bile acids.68
It is generally assumed that the PC molecules that enter bile at the canaliculus remain in the mixed micelle and are not absorbed by the biliary ductules. The validity of this assumption is not known. In the gallbladder, the phospholipid/bile salt ratio decreases as bile is concentrated, suggesting that some phospholipid is absorbed by the gallbladder epithelium.69
Knockout of this gene in mice results in the absence of biliary phospholipid and causes the development of a peribiliary fibrosis.66 Thus biliary PC serves to protect the biliary ductules from the membrane solubilizing action of bile acids. The monomeric activity of bile acids is higher in the absence of phospholipids, and the increased monomeric activity of bile acids has been considered the causal agent for biliary ductule injury, Nonetheless, the increase in the monomeric bile acid concentration is modest, if findings in model systems apply to the in vivo situation.70 When phospholipid is absent from bile, the cholesterol concentration is also greatly reduced. Some work on other organs suggests71 that the combination of PC and cholesterol in bile may render membranes resistant to bile acids. If this speculation is correct, the bile duct injury observed in the MDR-/- mouse might result from the decreased concentration of both phospholipid and cholesterol.
The phenotype of decreased MDR function in man has been reported to be calculous biliary disease,72 presumably because of defective micellar solubilization of cholesterol; mutations in MDR have also been reported in cholestasis of pregnancy.73 Biliary lipid analyses have not been reported. Our laboratory reported a group of patients with gallbladder inflammation despite the absence of gallstones, a disease named chronic acalculous cholecystitis.74 Biliary lipid analyses showed dilute bile with a low PC/bile acid ratio. This observation has not been confirmed. In these patients, the decreased phospholipid could result from rather than cause the mucosal inflammation. There would appear need for additional work to characterize the phenotype of MDR3 deficiency in man.
In the National Cooperative Gallstone study performed in the United States, phospholipid/bile acid ratios showed a normal distribution, and patients with low phospholipid/bile acid ratios were not identified as having a distinct phenotype.75 It is rational to treat MDR3 deficiency patients with ursodeoxycholic acid (UDCA), but no controlled studies have examined efficacy.
The MDR2 knockout mouse develops a striking peribiliary fibrosis. This can be treated successfully by administering norursodeoxycholic acid, the C23 (C24-nor) homologue of UDCA;76 such a molecule has an isobutanoic acid side chain and thereby differs from UDCA which has an isopentanoic acid side chain. Nor UDCA is secreted intact into bile and reabsorbed in the biliary ductules to undergo cholehepatic shunting in animals77 and apparently in man.78 In MDR2 knockout mice, there is a striking improvement in fibrosis, a decrease in leukocyte infiltration and ductular proliferation, and a marked increase in the activity of detoxifying enzymes such as sulfotransferase. UDCA has a much weaker effect. Whether nor UDCA will prove to have any clinical value in man is quite uncertain. In contrast to other natural bile acids, it has considerable renal excretion, especially of its ester glucuronide, its major metabolite. Although the peribiliary fibrosis of the MDR2 knockout mouse resembles primary sclerosing cholangitis, biliary phosphor lipid secretion is quite normal in this disease, so it remains quite uncertain that nor UDCA will have a therapeutic effect in sclerosing cholangitis.
Biliary cholesterol secretion: Cholesterol is secreted into bile largely by the heterodimeric transporter ABC5/ABC8.79,80 The protein is considered to be a cholesterol flippase, flipping cholesterol molecules from the cytosolic face of the canalicular membrane to the luminal face.81 Over expression of this transporter results in increased biliary cholesterol secretion.82 Knockout of this transporter does not fully eliminate biliary cholesterol secretion, indicating that either other cholesterol transporters are present in the canaliculus, or that post canalicular mechanisms for cholesterol entry into bile exist, or both.
Cholesterol is absorbed in the biliary tract in some species, based on the observation that at least in the dog, the cholesterol/bile acid ratio increases markedly when ezetimibe is given. Ezetimibe is secreted into bile as a glucuronide that maintains pharmacodynamic activity.83 Cholesterol absorption in the biliary tract is inhibited and biliary cholesterol increases markedly. As yet, whether such vigorous cholesterol absorption by cholangiocytes that occurs in the dog, is also present in other species is not known. Cholesterol can exit the cholangiocyte by basolateral ATP5/ATP8. During gallbladder storage, the proportion of cholesterol in bile falls, indicating that cholesterol is absorbed by the healthy gallbladder.84 Chronic absorption of cholesterol from supersaturated bile leads to oxidative stress that in turn causes impaired gallbladder motility, and this promotes cholesterol gallstone formation.85,86
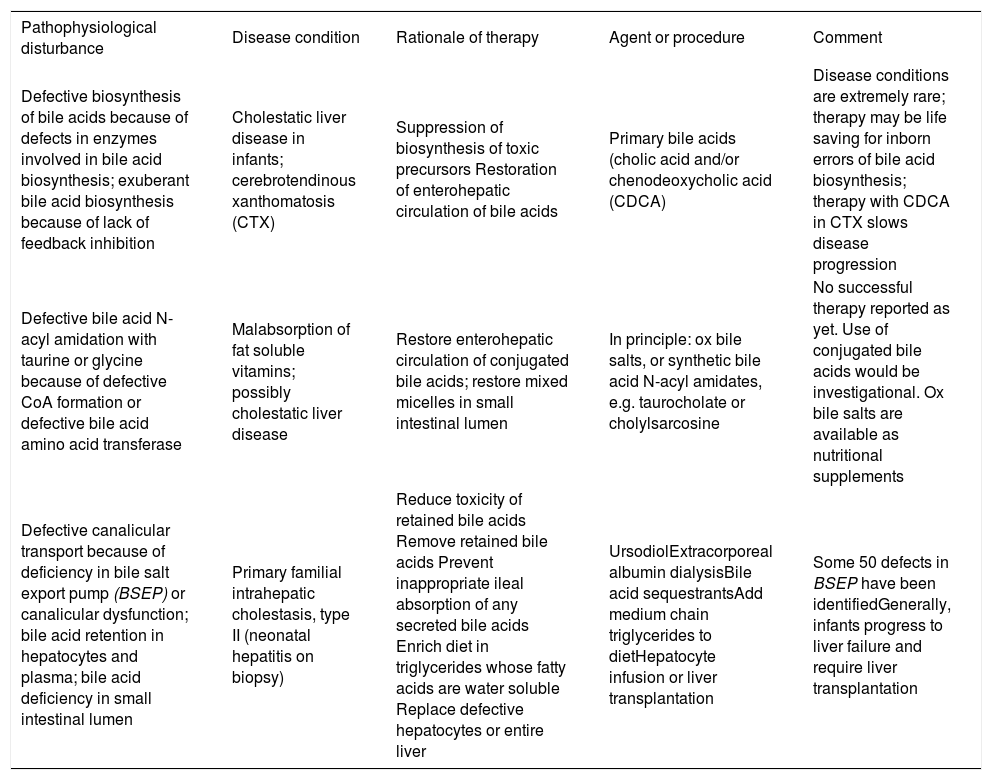
Bile acid therapy: Bile acid therapy is still used infrequently in liver and intestinal disease, with the single exception of ursodiol that is used by most physicians for a variety of uncommon hepatobiliary diseases in which cholestasis occurs. An overview of the rationale of bile acid therapy is given in Table II and Table III.
Rationale of bile acid therapy: liver defects.
| Pathophysiological disturbance | Disease condition | Rationale of therapy | Agent or procedure | Comment |
|---|---|---|---|---|
| Defective biosynthesis of bile acids because of defects in enzymes involved in bile acid biosynthesis; exuberant bile acid biosynthesis because of lack of feedback inhibition | Cholestatic liver disease in infants; cerebrotendinous xanthomatosis (CTX) | Suppression of biosynthesis of toxic precursors Restoration of enterohepatic circulation of bile acids | Primary bile acids (cholic acid and/or chenodeoxycholic acid (CDCA) | Disease conditions are extremely rare; therapy may be life saving for inborn errors of bile acid biosynthesis; therapy with CDCA in CTX slows disease progression |
| Defective bile acid N-acyl amidation with taurine or glycine because of defective CoA formation or defective bile acid amino acid transferase | Malabsorption of fat soluble vitamins; possibly cholestatic liver disease | Restore enterohepatic circulation of conjugated bile acids; restore mixed micelles in small intestinal lumen | In principle: ox bile salts, or synthetic bile acid N-acyl amidates, e.g. taurocholate or cholylsarcosine | No successful therapy reported as yet. Use of conjugated bile acids would be investigational. Ox bile salts are available as nutritional supplements |
| Defective canalicular transport because of deficiency in bile salt export pump (BSEP) or canalicular dysfunction; bile acid retention in hepatocytes and plasma; bile acid deficiency in small intestinal lumen | Primary familial intrahepatic cholestasis, type II (neonatal hepatitis on biopsy) | Reduce toxicity of retained bile acids Remove retained bile acids Prevent inappropriate ileal absorption of any secreted bile acids Enrich diet in triglycerides whose fatty acids are water soluble Replace defective hepatocytes or entire liver | UrsodiolExtracorporeal albumin dialysisBile acid sequestrantsAdd medium chain triglycerides to dietHepatocyte infusion or liver transplantation | Some 50 defects in BSEP have been identifiedGenerally, infants progress to liver failure and require liver transplantation |
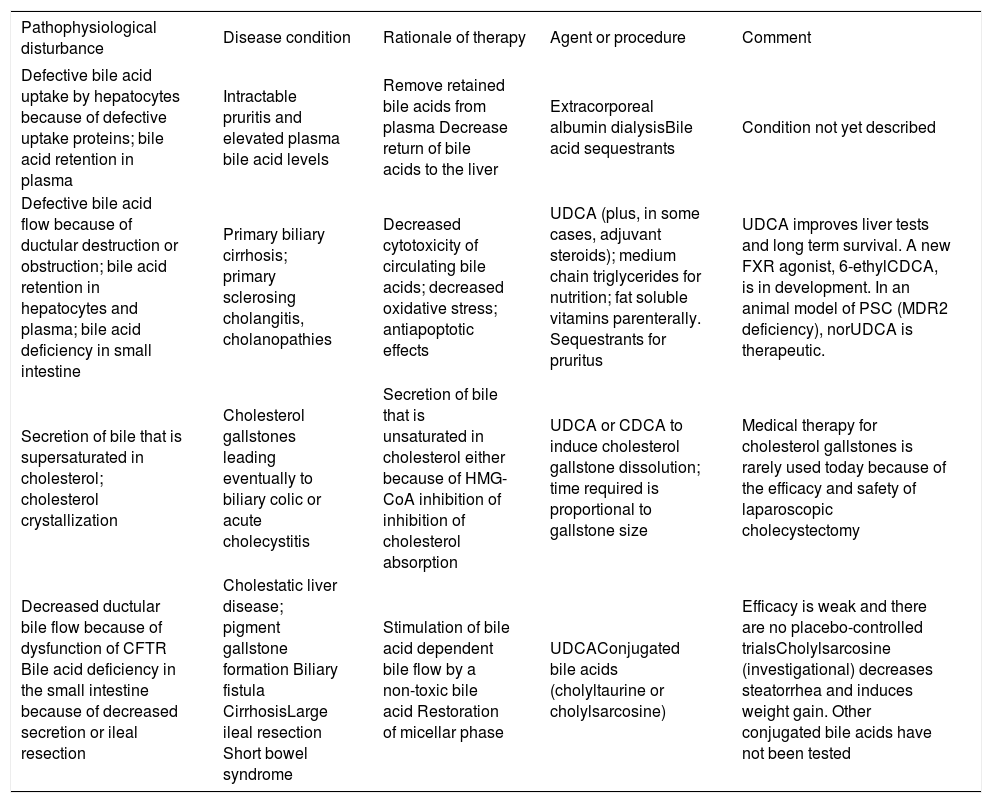
Rationale of bile acid therapy: liver disease (continued) and intestinal disease.
| Pathophysiological disturbance | Disease condition | Rationale of therapy | Agent or procedure | Comment |
|---|---|---|---|---|
| Defective bile acid uptake by hepatocytes because of defective uptake proteins; bile acid retention in plasma | Intractable pruritis and elevated plasma bile acid levels | Remove retained bile acids from plasma Decrease return of bile acids to the liver | Extracorporeal albumin dialysisBile acid sequestrants | Condition not yet described |
| Defective bile acid flow because of ductular destruction or obstruction; bile acid retention in hepatocytes and plasma; bile acid deficiency in small intestine | Primary biliary cirrhosis; primary sclerosing cholangitis, cholanopathies | Decreased cytotoxicity of circulating bile acids; decreased oxidative stress; antiapoptotic effects | UDCA (plus, in some cases, adjuvant steroids); medium chain triglycerides for nutrition; fat soluble vitamins parenterally. Sequestrants for pruritus | UDCA improves liver tests and long term survival. A new FXR agonist, 6-ethylCDCA, is in development. In an animal model of PSC (MDR2 deficiency), norUDCA is therapeutic. |
| Secretion of bile that is supersaturated in cholesterol; cholesterol crystallization | Cholesterol gallstones leading eventually to biliary colic or acute cholecystitis | Secretion of bile that is unsaturated in cholesterol either because of HMG-CoA inhibition of inhibition of cholesterol absorption | UDCA or CDCA to induce cholesterol gallstone dissolution; time required is proportional to gallstone size | Medical therapy for cholesterol gallstones is rarely used today because of the efficacy and safety of laparoscopic cholecystectomy |
| Decreased ductular bile flow because of dysfunction of CFTR Bile acid deficiency in the small intestine because of decreased secretion or ileal resection | Cholestatic liver disease; pigment gallstone formation Biliary fistula CirrhosisLarge ileal resection Short bowel syndrome | Stimulation of bile acid dependent bile flow by a non-toxic bile acid Restoration of micellar phase | UDCAConjugated bile acids (cholyltaurine or cholylsarcosine) | Efficacy is weak and there are no placebo-controlled trialsCholylsarcosine (investigational) decreases steatorrhea and induces weight gain. Other conjugated bile acids have not been tested |
At present, there are four rationales for bile acid therapy. Bile acid replacement is when bile acids are administered to replace a deficiency state. Such occurs in inborn errors of bile acid biosynthesis or bile acid conjugation, as well as in conditions when there is a deficiency in the small intestine because of ileal dysfunction. This occurs in short bowel syndrome, as the ileum has usually been resected in such patients. Bile acid deficiency in the small intestine also occurs in cholestatic liver disease, but usually bile acid secretion into the small intestine is sufficient to permit adequate lipid absorption in the adult, and it has been considered dangerous to administer exogenous bile acids other than ursodiol when endogenous bile acids are already.
Bile acid displacement occurs when the composition of the circulating bile acids is changed by exogenous bile acid administration, but there is relatively little change in bile acid secretion. Bile acid displacement is the rationale when ursodiol is used in cholestatic liver diseases. The mechanism of action of ursodiol is complex and involves replacement of endogenous cytotoxic bile acids [chenodeoxycholic (CDCA) and DCA) by UDCA a non-cytotoxic bile acid; the mechanism is likely to be competition for active ileal transport. UDCA also has anti apoptotic effects, and anti-oxidative injury effects. It may also reduce endoplasmic reticulum «stress» and appears to also have anti-inflammatory effects. UDCA is also used to lower the cholesterol proportion in bile and thereby induce gallstone dissolution. UDCA is amidated with glycine or taurine in the liver, and the resulting UDCA conjugates, as any natural conjugated bile acid, induce bile acid dependent bile flow. In cystic fibrosis, ductular bile flow is decreased because of non-functioning of the CFTR chloride channel. Increased canalicular bile flow induced by UDCA administration is thought to benefit children with cystic fibrosis by reducing the likelihood of developing chronic liver disease.
A third rationale for bile acid therapy may be termed FXR activation. Bile acids are ligands for the nuclear receptor, FXR, which regulates many hepatocyte activities. A new FXR agonist, 6-ethyl-CDCA has been synthesized and shown to have antifibrotic effects and anticholestatic effects in experimental models in the mouse. The effect of this FXR agonist could involve not only FXR activation in hepatocytes but also. The compound is undergoing early clinical studies with hopes of eventual marketing.
A fourth rationale for bile acid therapy is ductular targeting, using a bile acid that is secreted in part in unconjugated form by BSEP and whose unconjugated form is membrane permeable, resulting in absorption in the biliary ductules. As noted above, an example of such a bile acid is nor UDCA, which causes a marked diminution in the peribiliary fibrosis occurring in the MDR-/- knockout mouse that is unable to secrete phosphatidylcholine into bile. Nor UDCA is an investigational compound, and as yet there is no proof of principle that the compound will have useful efficacy in man.
Epilogue: The inability to sample canalicular bile means that details of biliary lipid secretion are often deduced or inferred; nonetheless, great progress has been made in understanding canalicular bile secretion. The explanation for the increased cholesterol phosphor lipid ratio (or cholesterol/bile acid ratio) present in cholesterol gallstone patients is being actively pursued at a genetic level.87-89 Much needs to be done to clarify the clinical phenotype of decreased MDR3 function in man. Nonetheless, identification of the major biliary lipid transporters and elucidation of their regulation has been a remarkable achievement. Progress in this area is likely to be great in the coming decades.