Hepatocellular carcinoma (HCC) is the most common primary tumor of the liver and is the fifth most common cancer in the world; its incidence has been increasing in recent years. Extrahepatic spread is present at the time of diagnosis in only about 5 to 15% of patients. Skeletal metastasis of HCC occurs less frequently compared with other cancers and is considered a rare primary form of presentation. We report two cases of unsuspected HCC presenting with multiple bone lesions as the initial presentation. The first patient was a 76-year-old man with symptoms of fatigue and back pain. The PET-CT revealed the hypercaptant bone lesions and a liver lesion. The pathology report showed that the metastases were positive for the hepatic marker HEPAR-1, indicating that they had originated from the HCC. The second patient was a 56-year-old man. He presented to the emergency department for right shoulder pain and weakness of the entire right arm with no history of trauma. During hospitalization, the patient became quadriplegic. MRI revealed osseous blastic lesions in the cervical vertebrae and right shoulder. A CT-guided biopsy was performed in the cervical lesion and showed poorly differentiated carcinoma. Immunohistochemistry staining was positive for HEPAR-1. In conclusion, this cases show an unusual presentation of HCC with skeletal metastasis.
Hepatocellular carcinoma (HCC) is the most common primary tumor of the liver and is the fifth most common cancer in the world; its incidence has been increasing in recent years. HCC usually develops in the setting of chronic liver disease, particularly in patients with chronic hepatitis B or hepatitis C virus infection.1 The diagnosis of HCC can be difficult and often requires the use of one or more imaging modalities.2 Ideally, tumors should be detected when about 2 cm in size so that all treatment options can be offered. However, HCC is frequently diagnosed late in its course because of the absence of symptoms. As a result, many patients have untreatable disease when first diagnosed. The median survival following diagnosis is 6 to 20 months. Large tumor size, vascular invasion, poor functional status, and nodal metastases are all associated with a poor outcome.3
HCC should be suspected in patients with previously compensated cirrhosis evolving with complications such as ascites, encephalopathy, jaundice, or variceal bleeding.4 These complications are often associated with extension of the tumor into the hepatic or portal veins, or are secondary to arteriovenous shunting induced by the tumor.5
Extrahepatic spread is present at the time of diagnosis in only about 5 to 15% of patients. Extrahepatic metastases are more common in patients with advanced-stage primary tumors (> 5 cm and large vessel vascular invasion). Extrahepatic recurrence is uncommon after locoregional therapy (5 to 24%).6 The most common sites of distant metastasis are lung (49%), intra-abdominal organs (24%), and, rarely, skeletal muscle.7 Skeletal metastasis of HCC occurs less frequently compared with other cancers and is considered a rare primary form of presentation.8 Bone metastases from HCC commonly appear as expansive soft tissue masses with bone destruction. Fifty percent of patients with bone metastasis have poorly controlled pain. Radiation therapy is considered the standard care for management of painful bony metastases.9 We report two cases of unsuspected HCC presenting with multiple bone lesions as the initial presentation.
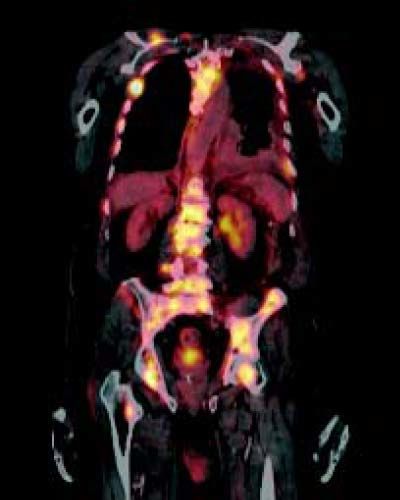
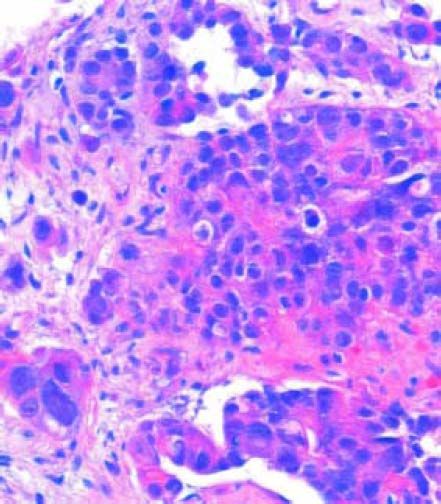
Case ReportCase 1The first patient was a 76-year-old man without underlying hepatitis B or C, who was a nonsmoker and not a heavy drinker. He had a medical history of hypertension and major depressive disorder, both of which had received adequate medical treatment. He presented to our hospital with symptoms of fatigue and back pain. Clinical examination revealed no further information. Patients body mass index was 21 kg/m2. The patient’s laboratory studies showed: hemoglobin (Hb) concentration, 10.5 g/ dL; creatinine serum concentration, 1.66 mg/dL; ALT, 21 U/L; AST, 81 U/L; alkaline phosphatase (ALP), 1,559 lU/L; gamma-glutamyl transferase (GGT), 166 lU/L; and lactate dehydrogenase (LDH), 923 lU/L. Because of low hemoglobin levels gastric endoscopy was performed and no signs of bleeding or portal hypertension (variceal disease) were seen. Because of the alterations in the hepatic panel, noncontrast CT was ordered. The CT demonstrated bilateral pleural effusion, a small liver, and osseous blastic and lytic lesions in every vertebral body, the seventh left rib, fifth right rib, sacrum, and ilium. A normal prostate-specific antigen (PSA) concentration of 0.8 ng/mL and no alteration in protein electrophoresis were found. Because of these findings, an 18FDG PET-CT was ordered. The PET-CT revealed the hypercaptant bone lesions described earlier and a liver lesion measuring 5 x 4 cm that was not seen in the noncontrast CT (Figures 1 and 2). Because we could not identify whether the lesion had originated in the liver or gall bladder, a CT-guided biopsy of the pubic bone was performed. The pathology report showed that the metastases were positive for the hepatic marker HEPAR-1, indicating that they had originated from the HCC (Figure 3). The lesions were negative for TTF-1, PSA, synaptophysin and chromogranin. Serum AFP concentration was 7,724 µg/L.
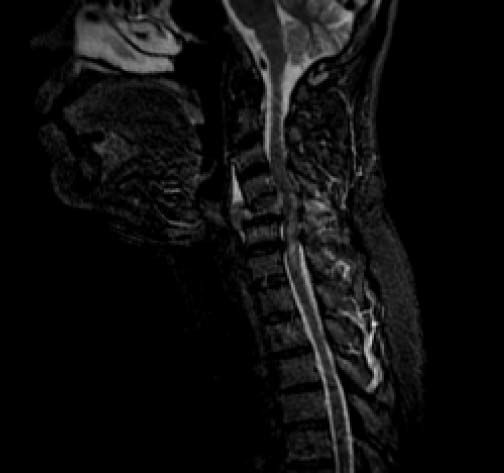
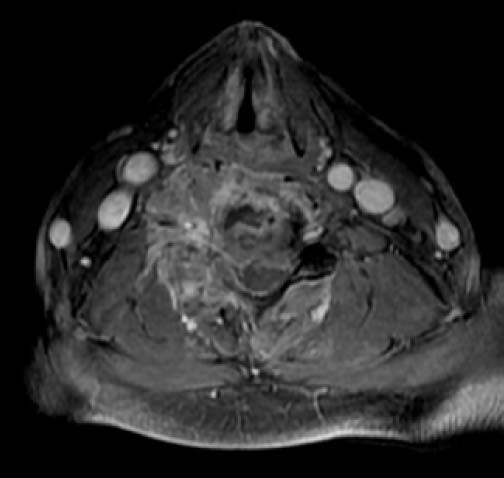
The second patient was a 56-year-old man. He was a nonsmoker and had consumed beer since the age of 17 years, and binge drinking at least once a week. He presented to the emergency department for right shoulder pain and weakness of the entire right arm with no history of trauma. Physical exploration identified the “stigmata” of chronic liver disease: sclera icterus, gynecomastia, and palmar erythema. Initially, Parsonage-Turner syndrome was diagnosed. Laboratory values were Hb concentration, 11 g/dL; BUN, 28.2 mg/dL; creatinine, 1.47 mg/dL; ALT, 89 U/L; AST, 181 U/L; ALP, 1,300 IU/L; GGT, 200 IU/L; and LDH, 700 IU/L. During hospitalization, the patient became quadriplegic. MRI revealed osseous blastic lesions in the cervical vertebrae and right shoulder (Figures 4 and 5). A CT-guided biopsy was performed in the cervical lesion and showed poorly differentiated carcinoma. Immunohistochemistry staining was positive for HEPAR-1 and negative for TTF-1, PSA, synaptophysin and chromogranin. These findings were compatible with metastatic HCC (Figure 6). Serum AFP concentration was 5,234 µg/L. Abdominal US showed two liver lesions, a 2 x 2 cm lesion in the left lobe and a 4.5 x 4.5 cm lesion in the 6th segment, both lesions were heterogeneous primary hyperechoic and showed vascularity within the lesion. An abdominal CT confirmed the presence of both lesions with classical HCC enhacement. A hepatitis virus panel was negative.
Although much less common in Western Europe and North America, HCC still accounts for 1 to 2% of all malignant tumors. HCC occurs frequently in sub-Saharan Africa and in Asia. Hepatitis B and C viruses, dietary aflatoxin B1, and cirrhosis from any cause are the common risk factors in that order of importance. Several lines of evidence, including molecular biology and animal studies, support these etiological linkages.10 HCC survival depends on stage at diagnosis. AJCC/UICC staging system have shown to povide the best stratification of prognosis, in patients with HCC undergoing liver transplantation, with 5 year survival rate of:11
- •
55% for stage I.
- •
37% for stage II.
- •
16% for stage III.
- •
< 7% for stage IV.
Improved HCC survival rates because of recent therapeutic advancements may have increased the frequency of remote metastasis. Metastatic spread to the bones occurs in 13 to 16% of HCC patients and has been described thoroughly. The most common sites of skeletal involvement are, in descending order, the vertebrae, pelvis, ribs, skull, humerus, and sternum. Much less is known about the initial clinical presentation of unsuspected HCC associated with bone metastasis. The literature documents a modest number of isolated reports, most of which involve the vertebrae. Most reported cases of sacral/ lumbosacral metastasis are accompanied by either multiple metastatic spread elsewhere in the body or previously known HCC.12
The presence of bone metastasis have not been associated with absence of cirrhosis, in a consecutive series of 395 patients with pathologically verified hepatocellular carcinoma, 20 patients (5%) had bone metastasis at initial presentation. The age, sex, hepatitis B surface antigen seropositivity, alpha-fetoprotein level, and frequency of associated cirrhosis were not statistically different from those in patients without initial bone metastasis.13
Skeletal metastasis appears to be unique among various hematogenous metastasis of HCC because it can occur before clinical manifestations of liver disease and is usually symptomatic, as in the present cases. The pulmonary and systemic circulation is thought to be the main route of metastasis to the skeletal system.14 Because the clinical importance of diagnosing bone metastasis from HCC has increased with improved HCC patient prognosis, and because radiation therapy can palliate symptoms, radiologists should take care not to miss base metastasis at an early stage when the initial symptoms appear. Although HCC should be included in the differential diagnosis of osteolytic, hypervascular lesions, bone metastasis from renal cell carcinoma, thyroid gland cancer, parotid gland cancer, and pheochromocytoma may show similar imaging findings.15
The diagnosis of metastatic hepatocellular carcinoma is suspected from the examined sections stained with hematoxylin and eosin for the morphological characteristics of the tumor. Although these neoplasms correspond to poorly differentiated carcinomas, they are focally trabecular with an acinar pattern. The nuclei have nuclear inclusions in the cytoplasm and pigment compatible with brown bile is observed. With these microscopic findings and positivity for Hepar-1, the histopathological diagnosis of hepatocellular carcinoma is made. Hepar-1 (human hepatocyte paraffin-1) is an antigen that reflects hepatocyte differentiation in approximately 90% of hepatocellular carcinomas and to be considered positive, the staining must be granular and cytoplasmic. It must be noted that in the study of any tumor, immunohistochemistry is nonspecific. Immunohistochemistry is used to supplement the morphological study of neoplasms.16
In conclusion, this cases show an unusual presentation of HCC with skeletal metastases. A plausible explanation for this rarity is that both the lymphangitic and hematogenous spread of HCC is closely associated with tumor staging. Thus, a more advanced hepatic tumor should unveil itself with local symptoms and lead to the diagnosis of HCC before the symptoms of metastatic spread are present.