Prohibitin (PHB) 1 is involved in multiple regulatory pathways in liver disease to protect hepatocytes, and its function is associated with subcellular localization. PHB1 located in the nucleus, cytoplasm and the mitochondrial inner membrane has anti-oxidative stress and anti-inflammatory effects in hepatitis and cirrhosis, which can protect liver cells from damage caused by inflammatory factors and reactive oxygen species (ROS) stimulation. The low expression of PHB1 located in the nucleus of liver cancer cells inhibits the proliferation and metastasis of liver cancer; thus, PHB1 exhibits the function of a tumor suppressor gene. Understanding the mechanisms of PHB1 in liver diseases may be useful for further research on the disease and may provide new ideas for the development of targeted therapeutic drugs in the future. Therefore, this review puts forward an overview of the role of PHB1 and its protective mechanism in liver diseases.
The prohibitin (PHB) family is composed of two members, PHB1 and PHB2, which are evolutionarily conserved and widely distributed in biological cells such as bacteria, plants, and animals. Among them, PHB1 is an intracellular protein. PHB1 was once a kind of cDNA isolated from the differential hybridization of normal and regenerated liver tissue RNA from rats. It is called a prohibitin protein because it can prevent cell metastasis. PHB1 inhibits the proliferation of tumor cells by delaying the cell cycle from Phase G1 to S. The human PHB1 gene, located on chromosome 17q21, contains seven exons. The first and second exon constitutes a 5′ untranslated region (UTR), and the seventh exon has a 3' UTR of 700bp. Studies have shown that 3' UTR gene mutation is associated with the development of cancer [1].
PHB1 is distributed in various cell compartments, mainly located in the mitochondria and nucleus, while a small amount exists in the cell membrane and cytoplasm. Therefore, PHB1 is involved in a variety of essential cellular regulatory mechanisms, including the cell proliferation, differentiation, and oxidative stress. In most cases, PHB1 can not only inhibit breast cancer cells in estrogen receptor (ER)-positive breast cancer by inducing androgen receptor (AR) expression, but also interact with oncogenes to inhibit the proliferation of human osteosarcoma cells [2,3]. However occasionally, PHB1 could promote the proliferation and invasion of gallbladder cancer cells [4]. The controversial might be related to the subcellular localization of PHB1 in different cells, making it difficult to unify the function of PHB1 in different kinds of diseases.
Research on liver diseases demonstrates that although PHB1 has different distribution in various compartments of hepatocytes, it protects liver cells generally. PHB1 interacts with nuclear erythroid 2 p45-related factor (NRF) 2, C-MYC, Histone deacetylase (HDAC) 4 and other genes in different cell regions. It can protect the liver from damage caused by reactive oxygen species (ROS) and inflammatory factors, as well as inhibit the proliferation and metastasis of liver cancer cells. This article reviews the specific protective effects of PHB1 in liver disease.
Hepatitis is caused by a variety of pathogenic factors such as viruses, alcohol, drugs, autoimmune diseases, and so on. In particular, viral hepatitis infection is a worldwide epidemic and the leading cause of chronic hepatitis. Simultaneously, as standards of living are increasing, so too are overindulgences in fatty foods and alcohol, leading to a consistent increase of patients with steatohepatitis and alcoholic hepatitis. Cholestasis and increased production of ROS-caused by long-term inflammatory infiltration of the liver [5]. Therefore, it is particularly important to study the anti-oxidative stress and anti-inflammatory mechanism of hepatocytes.
2PHB1 in hepatitis2.1Nuclear PHB1 protects liver cells from oxidative stressMultiple lines of evidence indicate that PHB1 is closely linked to other liver diseases, including hepatitis, liver fibrosis and cirrhosis. In hepatitis, PHB1 protects the liver from oxidants and inflammatory effects.
PHB1 expression is low in cholestasis-induced liver injury. PHB1 localized in the hepatocyte nucleus interacts with NRF2 and C-MYC to regulate the cell oxidative stress process [6]. NRF2 is a transcription factor with a basic leucine zipper structure and is the master regulator of cellular redox reactions. The Keap1-Nrf2-ARE signaling pathway plays an important role in cholestasis-induced liver injury, and Nrf2 knockout (KO) mice treated with lithochoic acid (LCA) showed more severe multifocal hepatic necrosis with cholangitis [7,8]. The biotinylated antioxidant reaction element (ARE) fragment in pull-down followed by proteomics has showed that NRF2 and PHB1 expression were down-regulated and C-MYC up-regulated after LCA treatment [9]. The expression of the biphasic enzyme in the binding reaction of the second phase of the liver is mainly achieved by the Keap1-NRF2-ARE signaling pathway, and the NRF2 enters the nucleus to regulate the ARE-mediated redox II. The expression of the phase metabolic enzyme gene further exerts an antioxidant effect. PHB1 is similar to ARE as an antioxidant interacting with NRF2, which activates the transcription of antioxidant protein genes and exerts antioxidant effects [6]. In contrast, the proto-oncogene C-MYC inhibits the expression of PHB1 and NRF2 by activating miR-27 in cholestasis-induced liver injury [9]. Consequently, the interaction between PHB1 and NRF2 is inhibited, resulting in uncontrolled oxidative stress and further damage to liver cells. However, some report emphasized that the concentration of ROS and the regulation of p53 are involved in the antioxidant action of NRF2 [10]. Moreover, p53 can change the effect on the cellular environment with hypoxia and excessive ROS, and thus play an important role in promoting cell survival according to the environment [11]. Within the nucleus, PHB1 interacts with multiple proteins to modulate the transcriptional activity of transcription factors p53. The relation between ROS concentration and genes such as PHB1, NRF2, and C-MYC in hepatocyte oxidative stress needs further research.
2.2The protective effect of PHB1 associated with STAT3Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in the world, and 10–20% of patients can develop non-alcoholic steatohepatitis (NASH). Long-term fatty infiltration, inflammatory response and oxidative stress caused by hepatocyte injury, necrosis and fibrosis tend to cause NASH to further develop into cirrhosis or liver cancer. The specific process by which NAFLD develops into NASH is unclear, and is currently thought to be the result of a variety of mechanisms, including oxidative stress, inflammatory factor release, and endoplasmic reticulum stress.
There is evidence that mitochondrial dysfunction in hepatocytes is an important cause of the induction of NASH and an important factor in the induction of early alcoholic hepatitis [12]. The down-regulation of PHB1 and STAT3 can be found by detecting the mitochondrial protein expression profile of NASH liver rats [13]. Low expression of PHB1 is also detected in the liver of NASH rats induced by methionine-choline deficiency, accompanied by oxidative stress and inflammatory factor release, including up-regulation of interleukin (IL)-6 and tumor necrosis factor (TNF)-α expression [14]. As a mitochondrial partner, PHB1 plays an important role in protection against oxidative stress and maintaining mitochondrial homeostasis [15,16]. At present, the cause of down-regulation of PHB1 expression in NASH has not been found, but PHB1 can be induced to transfer to mitochondria under the action of drugs [13]. PHB1 has this subcellular shuttle function, and the subcellular localization of PHB1 is also noteworthy during the development of NASH, which might provide evidence for disease progression.
In spite of the relationship between PHB1 and signal transducer and activator of transcription (STAT) 3 is not currently described in hepatocytes. However, PHB1 is regulated by STAT3 in myocardial cell mitochondria. Moreover, IL-6 activates STAT3 and increases the expression of PHB1 to exert anti-oxidative stress which is up-regulated in NASH. As a result, PHB1 interacts with STAT3 to protect cells from inflammatory factor TNF-α-induced ROS damage in mitochondria [17,18]. IL-6 appears to be associated with the expression level of PHB1 in hepatitis, and the role of IL-6 in the development of hepatitis is controversial. In alcoholic hepatitis, IL-6 exerts anti-oxidative stress to protect liver cells against ethanol-mediated ROS [19]. Noted in studies of hepatic ischemia–reperfusion injury, IL-6-activated STAT3 phosphorylation has protective effects on the liver [20]. Thus, in hepatocytes, both anti-oxidative stress and pro-inflammatory effects make the inflammatory factor IL-6 controversial, and the interaction of PHB1 with IL-6/STAT3 in hepatocyte mitochondria may also be associated with NASH development.
2.3PHB1 has anti-hepatitis effectsViral hepatitis infection is one of the important causes of chronic hepatitis. In the hepatocellular carcinoma cell line stably transfected with hepatitis B virus (HBV), the expression of PHB1 and p53 can be up-regulated after adding interferon (IFN)-α for chronic hepatitis B, which will demonstrate a dose-dependent increase [21]. PHB1 is able to bind to and enhance the transcriptional activity of p53, suggesting that IFN-α-induced PHB1 may activate p53 against HBV [22].
PHB1 in hepatocyte nucleus and liver mitochondria is mainly involved in anti-oxidative stress and anti-inflammatory action to protect hepatocytes. In contrast, PHB1 located in the plasma membrane is the only way for Hepatocytes infected by hepatitis C virus (HCV). The CRaf binding site of PHB1 is located near the C-terminal region and indicates that PHB1-CRaf interaction is a critical step for HCV infection [23]. An abnormal increase in oxidative stress is one of the characteristics of HCV infection. HCV generates excessive ROS and affects the steady state level of PHB1 in mitochondria, leading to an impaired mitochondrial oxidative respiratory chain [24]. PHB1 is characterized by multiple pathways distributed in different cell regions, so preventing PHB1 from targeting plasma membrane phosphorylation may be a method to prevent HCV invasion.
3PHB1 protects DNA from cirrhosis damageLong-term cholestasis can easily cause inflammation and collagen deposition, resulting in further damage to the liver and leading to liver fibrosis. Liver fibrosis can deteriorate and gradually develop into cirrhosis [25]. In primary bile acid cirrhosis, PHB1 expression levels were significantly reduced relative to a normal liver [26]. Liver-specific phb1 KO mice can spontaneously develop liver injury, liver fibrosis, and liver cancer [27]. Phb1 KO mice treated with bile-duct ligation (BDL) might show lower survival, suggesting that PHB1 may have a protective effect on liver cells in liver injury caused by cholestasis [28]. HDAC4 is closely related to the formation of hepatic fibrosis [29], and the expression of liver fibrosis markers is detected in biliary epithelial cells of phb1 KO mice after BDL treatment [26]. PHB1 appears to be a regulator of liver fibrosis. PHB1 located in the cytoplasm of the liver inhibits HDAC4 activation, prevents HDAC4 from entering the nucleus for transcriptional regulation, and leads to DNA damage. Meanwhile, the expression level of genes related to inflammation is reduced [29]. Silencing the expression of HDAC4, genes involved in inflammation can be regulated by HDAC4, and thus PHB1 may partially protect liver cells through anti-inflammatory effects in liver damage. Down-regulation of PHB1 in mitochondria increases ROS signaling and TNF-α-induced autophagy, and PHB1 heterozygous mice are more sensitive to liver damage and inflammatory invasion than wild-type mice [30,31]. All above suggest that there is a close relationship between PHB1 and inflammatory cytokines, and that it is involved in the regulation of liver fibrosis in the liver, which may be related to HDAC4. Further research is required to clarify the specific mechanism.
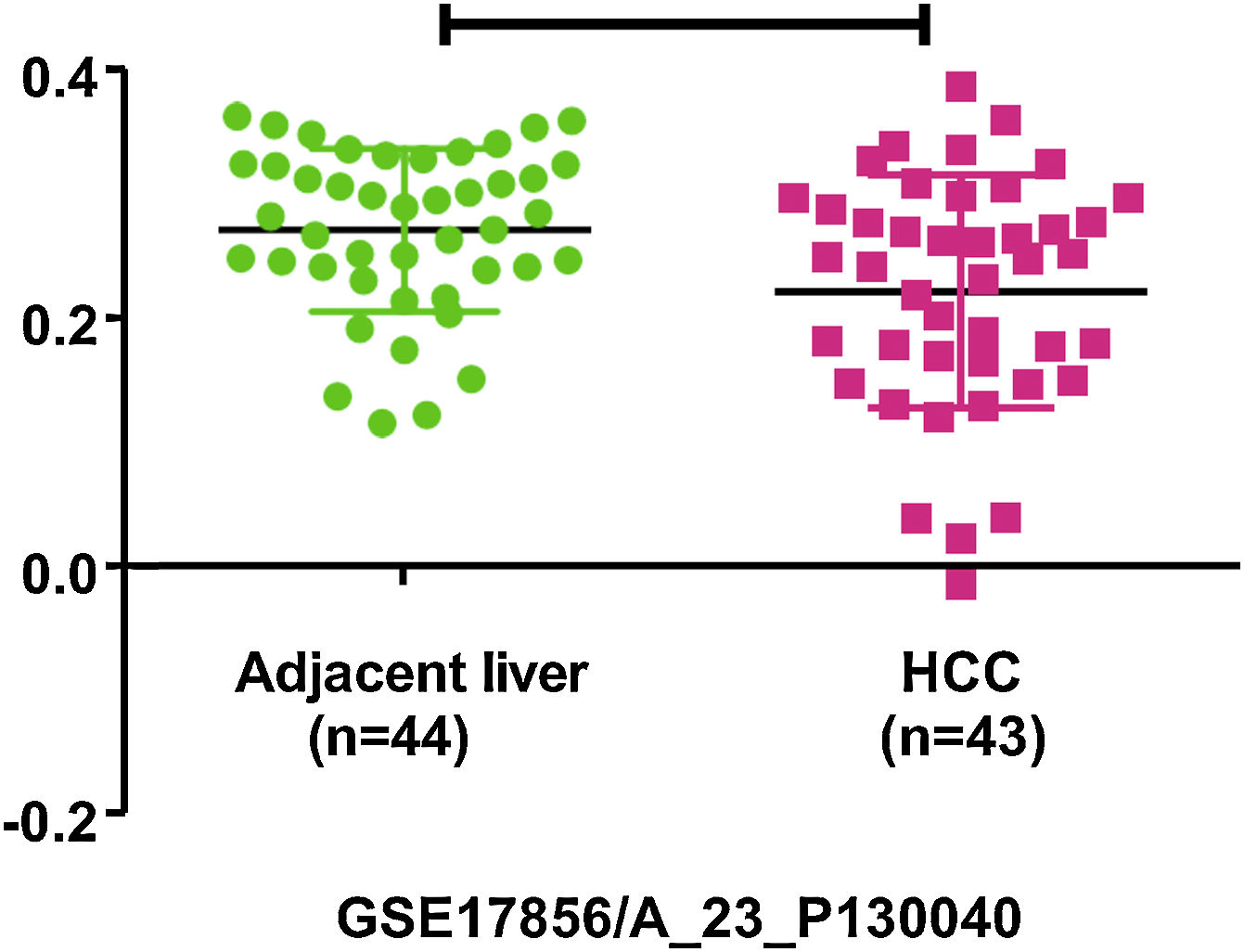
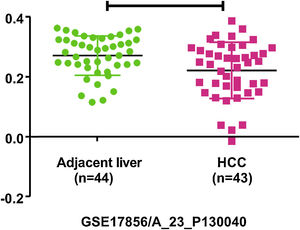
4PHB1 in liver cancer4.1PHB1 inhibits the cell proliferation and metastasis of liver cancerLiver cancer is one of the most malignant and fatal of all tumors. The persistent progression and malignant transformation of chronic liver inflammation can lead to liver cancer, including primary liver cancer and secondary liver cancer [32]. Primary liver cancer is divided into three types: hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA) and mixed liver cancer. The research found that PHB1 expression is significantly decreased in HCC and CCA cells, and overexpression of PHB1 can inhibit cell proliferation of HCC and CCA [6]. The phb1 KO mice can develop HCC or CCA, while liver-specific PHB1 heterozygous mice after left and median bile-duct ligation (LMBDL) can develop into CCA with obvious cancer metastasis [5]. Clinical data screening by the Gene Expression Omnibus (GEO) revealed that PHB1 was under-expressed in HCC (Fig. 1). GSE17856 HCC data were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). These all suggest that PHB1 may act as a tumor suppressor gene and affect the proliferation of liver cancer. Some studies have found that PHB1 interacts with some genes in the nucleus of HCC, thereby inhibiting the proliferation of HCC cells (Fig. 2).
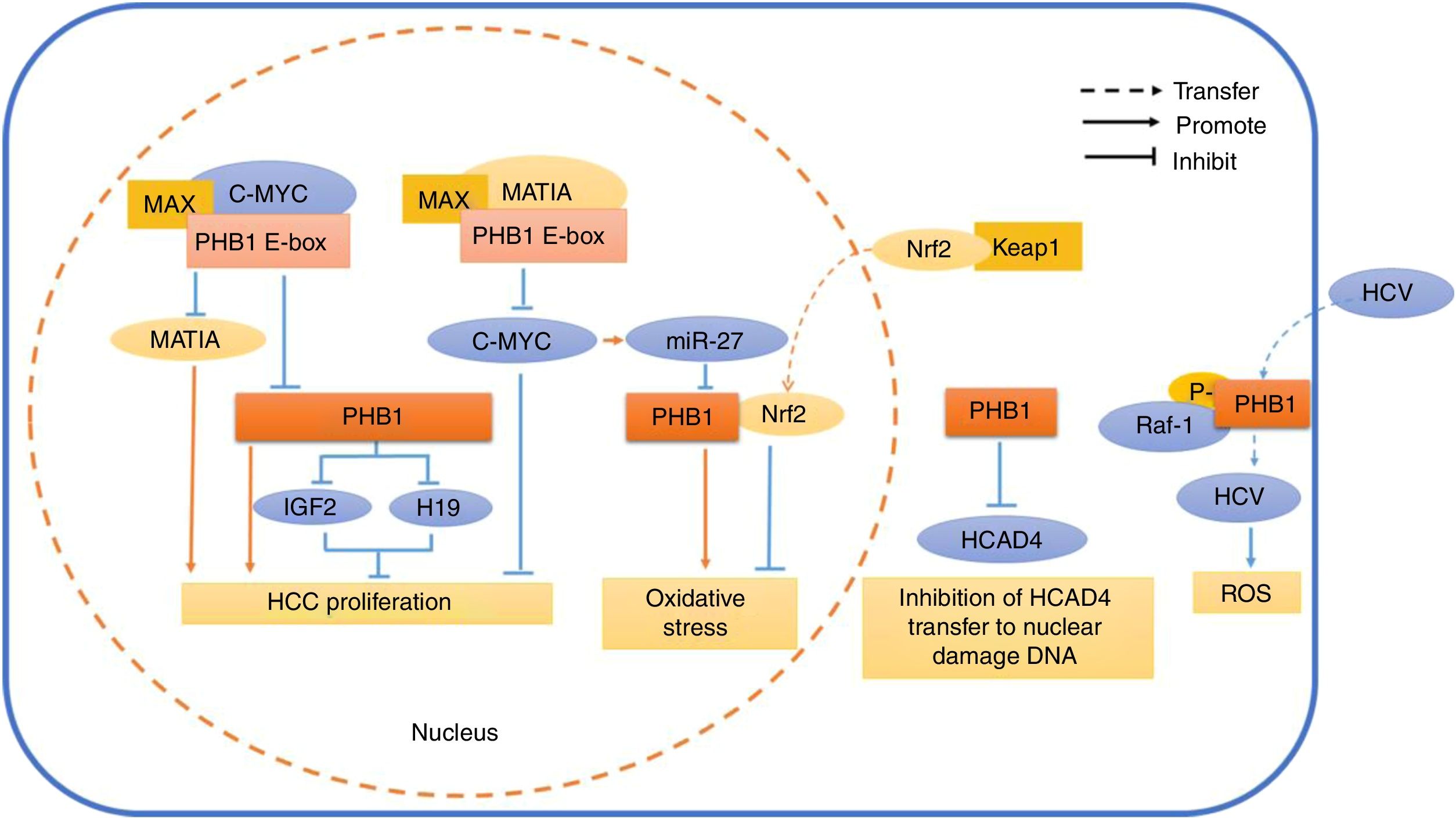
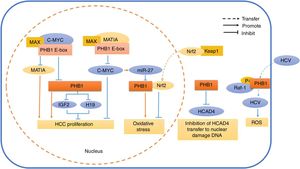
Schematic diagram of PHB1 mediating the related reaction of different cell compartments in hepatocytes. In the nucleus, PHB1 interacts with MAT1A and C-MYC by regulating the PHB1 E-box. At the same time, PHB1 inhibits the expression of the imprinting genes IGF2 and H19, thereby inhibiting the proliferation of HCC cells. Nrf2, which is transferred from the cytoplasm into the nucleus, interacts with PHB1 to inhibit oxidative stress in hepatocytes. However, the oncogene C-MYC-mediated miR-27 inhibits the expression of PHB1 and promotes oxidative stress. In the cytoplasm, PHB1 protects DNA from damage by preventing HCAD4 from transferring to the nucleus. In the cell membrane, the phosphorylated PHB1 interacts with Raf-1 to facilitate the transfer of HCV virus into the cell.
PHB1 located in the nucleus interacts with genes in liver cancer to inhibit the cell proliferation and metastasis. PHB1 interacts with MAT1A-encoded Methionine Adenosyltransferase (MAT) α1 to inhibit the growth of hepatoma cells [6]. MAT1A is low-expressed in HCC and CCA, and a decrease in MAT1A expression is found in animal models and human HCC associated with diseases and prognosis of greater severity [33]. MATIA activation inhibits the proliferation and metastasis of HCC and CCA. MATα1 is a tumor suppressor in CCA, and proto-oncogene C-MYC is highly expressed in HCC and CCA [34]. In HCC and CCA, PHB1 interacts with MAT1A, C-MYC, and v-Maf avian fascia fibrosarcoma Oncogene Homolog G (MAFG). These genes are mutually regulated through their binding to E-box elements. In the non-tumor tissues, PHB1 and MAT1A bind to the E-box promoter by forming a heterodimer with MAX, respectively. Thus, they inhibited the proliferation of liver cancer cells by inhibiting E-box activity and down-regulating C-MYC and MAFG and realized negative regulation. Conversely, the proto-oncogenes C-MYC and MAFG form a dimer with MAX to occupy the E-box element to down-regulate PHB1 and MAT1A in liver tumor tissues and promote the cell proliferation of liver cancer [6,33]. However, PHB1 inhibits the expression of C-MYC and is incompletely regulated by MAT1A [6]. The expression level of PHB1 is decreased in S-Adenosylmethionine (SAMe)-D cells (HCC primary cells isolated from Mat1a KO mice). Over-expression of PHB1 in SAMe-D cells also showed inhibition of C-MYC and MAFG, suggesting that PHB1 may have another pathway regulating the expression of proto-oncogenes [6].
4.3PHB1 interacts with IGF2 and H19The anti-proliferative effect of PHB1 is also associated with the H19 gene and Insulin-like growth factor (IGF) 2. H19 and IGF2 belong to a genetic imprinting group. A genetic imprint is a gene-dependent single allele expression in which the other allele is expressed very weakly or not at all. IGF2 belongs to the paternally imprinted gene and H19 belongs to the maternally imprinted gene [35]. The genetic imprinting is performed when the alleles from the father and the mother are transferred to the progeny so that the alleles with the parental imprint have different expression characteristics. The modification is usually DNA methylation modification, including histone acetylation, methylation, and other modifications [36]. Although imprinted genes constitute only a minority of the human genome, deletion and abnormal expression of imprinted genes are some of the most common genetic factors that cause tumors. H19 and IGF2 are highly expressed in HCC, while IGF2 promotes HCC invasion. IGF2 down-regulates the expression of PHB1 and CCCTC-binding transcription factor (CTCF) [37,38]. In normal hepatocytes, the binding of the transcription factor CTCF to the gene's imprinting control region (ICR) regulates the expression of both genes through the mechanism of DNA methylation [37]. The expression of the imprinted genes was up-regulated in phb1 KO mice, and the binding activity of CTCF to ICR was decreased. Studies have shown that PHB1 and CTCF act together to regulate the expression of the H19-IGF2 gene [38]. By silencing expression and over-expression of PHB1 or H19 in SAMe-D cells, researchers found that PHB1 inhibited the proliferation of HCC cells, partly because PHB1 inhibited the expression of H19. The inhibition of PHB1 expression by H19 in HCC cells may be associated with a highly conserved 3′ UTR of PHB1. The PHB1 3' UTR exerts an anti-cell-proliferation effect by blocking the G1/S phase of the cell cycle [39]. Studies have shown that the mutation of PHB1 3' UTR is likely to be related to the development of gastric cancer [40]. Therefore, the antiproliferative effect of PHB1 is due in part to its 3' UTR. PHB1 is the same in gastric cancer, and the targeting of miR-27 binds to PHB1 3' UTR, which inhibits the expression of PHB1 and promotes tumor cell proliferation [28]. This suggests that H19 may inhibit the expression of PHB1 in liver cancer through microRNA or directly acting on PHB1 3' UTR.
5SummaryPHB1 protects the liver through anti-oxidative stress, anti-inflammatory response, and anti-fibrosis in hepatitis and cirrhosis. In liver cancer cells, although PHB1 acts as a tumor suppressor to inhibit cancer cell proliferation and metastasis, the role of PHB1 is still controversial and related to the regulation of apoptosis by PHB1. In breast and colon cancer studies [39,41], PHB1 activates the p53-inducible gene (PIG) 3, a target gene that regulates apoptosis downstream of p53, and promotes apoptosis. In the study of colonic inflammation, PHB1 inhibits the levels of anti-apoptotic genes B-cell lymphoma (Bcl)-xL and Bcl-2 by inhibiting STAT3 activation [42], thereby promoting apoptosis. In the study of cholangiocarcinoma, PHB1 expression was reduced in CCA [43]. It is noteworthy that the expression of PHB1 and colocalization with p53 are transferred from the cytoplasm to the nucleus under the stimulation of the induced apoptosis agent. This suggests that the subcellular localization of PHB1 and the transcription factor p53, which regulates apoptosis, may be the key to PHB1 regulation of apoptosis in hepatoma cells. The specific mechanism between PHB1 and apoptosis of liver cancer cells has not been studied in depth. PHB1 can undergo cell-range transfer under the stimulation of drugs. Then, in hepatitis, cirrhosis and liver cancer caused by external factors such as chemical poisons and alcohol, is the subcellular localization of PHB1 during disease development metastasized? This calls for further exploration and may provide new ideas for the development of anti-hepatocarcinoma drugs and targeted drugs.AbbreviationsPHB
prohibitin
ROSreactive oxygen species
UTRuntranslated region
ERestrogen receptor
ARandrogen receptor
NRFnuclear erythroid 2 p45-related factor
HDAChistone deacetylase
KOknockout
LCAlithocholic acid
AREantioxidant reaction element
NAFLDnon-alcoholic fatty liver disease
NASHnon-alcoholic steatohepatitis
ILinterleukin
TNFtumor necrosis factor
STATsignal transducer and activator of transcription
HBVhepatitis B virus
IFNinterferon
HCVhepatitis C virus
BDLbile-duct ligation
HCChepatocellular carcinoma
CCAcholangiocarcinoma
LMBDLleft and median bile-duct ligation
GEOgene expression omnibus
MATmethionine adenosyltransferase
MAFGv-maf avian fascia fibrosarcoma oncogene homolog g
SAMes-adenosylmethionine
IGFinsulin-like growth factor
CTCFCCCTC-binding transcription factor
ICRimprinting control region
PIGp53-inducible gene
BclB-cell lymphoma
Financial disclosureNone.
Conflict of interestThe authors have no conflicts of interest to declare.
National Natural Science Foundation of China (81541163); High-level talent research start-up fund of University of South China (2017XQD24); Hunan Provincial Innovation projects of College Students (2019-100-2184, 2185, 2225); the College Students' Innovative Project of University of South China (2018XJXZ192, 197, 352, 353); Hunan Provincial Cooperative Innovation Center for Molecular Target New Drug Study (2016-429).