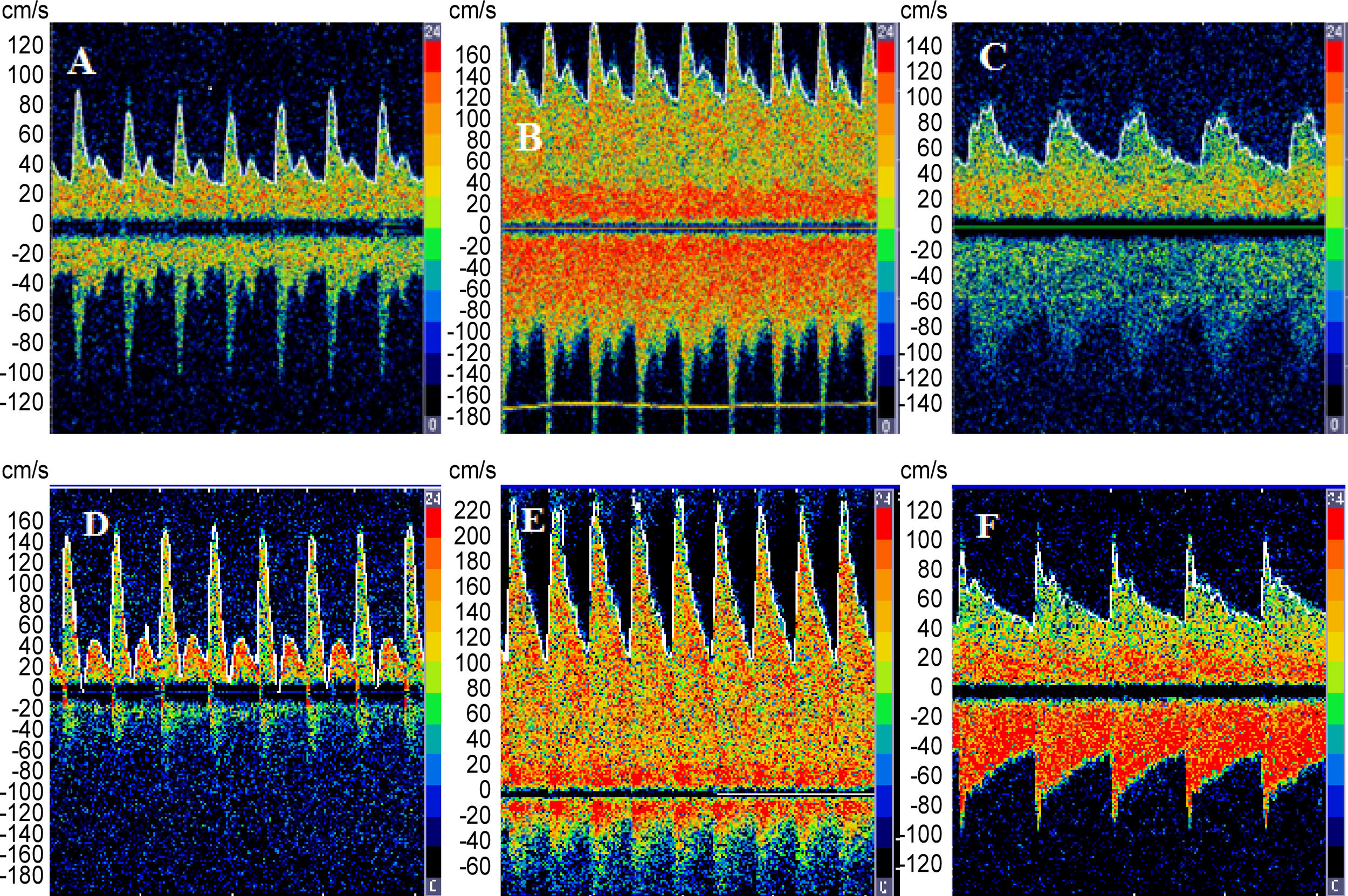
We report the case of a 47-year-old woman diagnosed with fulminant hepatic failure (FHF). Over a two-week period, the patient had jaundice and Grade 4 encephalopathy associated with coagulopathy and renal failure. On admission, the patient underwent endotracheal intubation and mechanical ventilation, fulfilling the clinical criteria for liver transplantation. The patient underwent urgent liver transplantation and was monitored before and after transplantation by transcranial Doppler (TCD). Norepinephrine was infused to increase the mean arterial blood pressure by approximately 20mmHg in order to calculate static cerebral autoregulation (CA) according to Tiecks et al. [1] Values>0.6 indicate preserved CA. In the case reported, cerebral blood flow (CBF) velocity showed lower values prior to transplant, high values immediately post-transplant, and a tendency to normalize within 48–72h (Fig. 1). Loss of CA was demonstrated before transplant; CA recovery was seen on the 3rd day after transplant. Unexpectedly, deterioration of CA was noted on the 5th day after transplantation, coinciding with hepatic artery thrombosis. The patient underwent liver retransplantation, and restoration of CA occurred 48h later (Table 1).
Performance of systemic and haemodynamic parameters (CBFV and CA) before and after transplantation (tx) liver in FHF.
| Before tx | 1st day after tx | 3rd Day after tx | 5th Day after tx | 7th day after tx and 2nd day after new liver tx | |
|---|---|---|---|---|---|
| ABP (mmHg) | 108 | 102 | 120 | 107 | 106 |
| Heart rate | 119 | 95 | 73 | 69 | 93 |
| PCO2 (mmHg) | 45 | 34 | 36.7 | 36 | 36.5 |
| Haemoglobin (g/dl) | 11.8 | 10.8 | 10.7 | 12.1 | 10.8 |
| Temperature (C) | 37 | 36 | 36.5 | 36.1 | 37 |
| CBFV MCA maximum (cm/s) | 45 | 145 | 66 | 161 | 63 |
| CA index MCA maximum (cm/s) | 0.25 | 0.33 | 0.92 | 0.30 | 0.70 |
ABP: arterial blood pressure; pCO2: partial pressure of carbon dioxide; CBFV: cerebral blood flow velocity; CA: cerebral autoregulation; MCA: middle cerebral artery.
Cerebral autoregulation refers to the inherent cerebrovascular physiological mechanisms that keep CBF relatively constant despite wide variation in arterial blood pressure levels. These mechanisms act to protect the brain from the harmful effects of oligaemia and hyperaemia due to decreased and increased perfusion pressure, respectively [2–3]. Loss of CA in FHF can be explained by the dysfunction of cerebral arterioles, leading to vasodilation, associated with metabolic derangement and toxic substances released from the necrotic liver [4], but the actual pathophysiological mechanism remains unknown. Both loss of CA and hyperaemia have been considered important factors for the worsening of hepatic encephalopathy, brain swelling, and unfavourable outcomes. The present case raises the possibility that CBF dynamics and CA capacity can be restored shortly after recovery of liver function [4]. TCD can be a useful method for real-time monitoring of FHF patients in terms of CBF velocity and CA. Improvement of CA can be directly associated with recovery of liver function; for this reason, CA may be a marker of liver function, as suggested by this case. Future studies should determine the dynamic behaviour of CA. Such evaluation can help elucidate the pathophysiology of FHF and enable better therapeutic management of patients.