There are no conflicts of interest. All the authors have read and approved the submitted manuscript. The study complies with current ethical considerations.
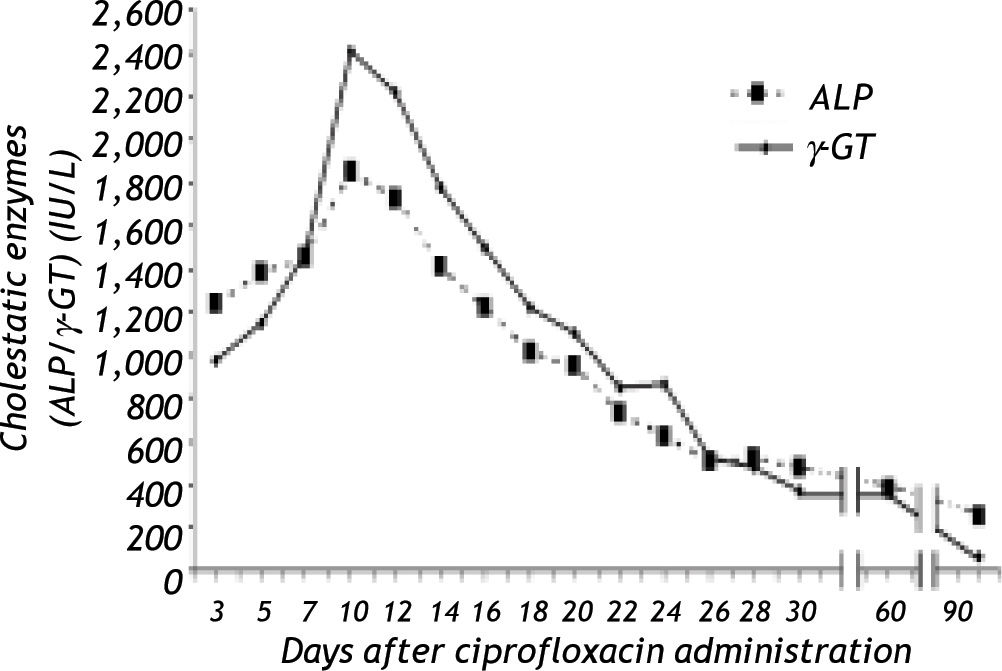
Dear EditorA 66 year-old male, farmer, was admitted to our Department with fever, nausea, vomiting and diarrhoea of 24 hours duration. His medical past and family history were unremarkable. He was heavy smoker (40 pack/year), but he denied any alcohol consumption during the last 2 years and he did not report any drug or herb administration for the last 6 months. He had no history of recent contact with jaundiced subjects or exposure to any possible source of infection with hepatitis viruses. Physical examination revealed a patient in general good health with increased bowel sounds, but with no other abnormal findings. His temperature was 38.2 °C, blood pressure 110/70 mmHg and pulse rate 70/min. Laboratory values showed: Ht 38.6%, Hb 12,7 g/dL, MCV 92 Fl, white blood count 1S,900/mm3 (neutrophils 77%, lymphocytes 20%), and normal platelet count (249.000/mm3). Erythrocyte sedimentation rate was 40mm/1sth, C-reactive protein was elevated (130 mg/L, normal < 10). Prothrombin time was 12.6 sec, total bilirubin 1.1 mg/dL (direct: 0.S mg/dL), aspartate aminotransferase (AST) 3S (normal < 40) IU/L, alanine aminotransferase (ALT) 38 (normal < 40) IU/L, alkaline phosphatase (ALP) 2S0 (normal < 280) IU/L, gamma-gluta-myltransferase (γ-GT) 63 (normal < 70) IU/L. Microscopic examination of the stools revealed the presence of numerous white cells, but the stool cultures performed at this time were later negative. The patient was commenced on ciprofloxacin 400 mg twice per day intravenously. Three days after the first dose of ciprofloxacin, the patient was afebrile with excellent clinical condition, but his liver function tests had been deteriorated (AST S20 IU/L, ALT S82 IU/L, ALP 1234 IU/L, γ-GT 977 IU/L, direct bilirubin: 1.48 mg/dL, INR:1.17). Ciprofloxacin was immediately discontinued and further evaluation was decided. Serum levels of IgG, IgA, IgM, ceruloplasmin, ferritin, thyroid test were within the normal levels. HBsAg, IgM anti-HBc, IgM anti-HAV, anti-HCV, tumour markers, autoantibodies (ANA, AMA, SMA, pANCA, cANCA, anti-LKM), and serological markers for Epstein-Barr virus, cytomegalovirus and herpes virus were negative. Serum HBV-DNA and HCV RNA were undetectable by qualitative polymerase chain reaction assays. Abdominal ultrasonography and magnetic resonance cholangio-pancreatography were normal. The patient refused to undergo to liver biopsy. His liver tests continued to rise (at 10th day after ciprofloxacin administration: AST SS0 IU/L, ALT S1S IU/L ALP 18S0 IU/L, γ-GT 2410) (Figure 1), but then they gradually improved. The patient was discharged IS days after his admission. His liver function tests returned to normal within 3 months and remained normal during the 10 months follow up period after discontinuation of ciprofloxacin.
The causality assessment of drug-induced liver damage relies on chronological and clinical criteria, while liver biopsy is not necessary in most cases, but it may be useful in order to show lesions suggestive of drug-induced liver injury.1 The above mentioned patient was admitted to our department with the diagnosis of gastroenteritis and his liver function tests were elevated 3 days after initiation of cipro-floxacin. Detailed clinical history and laboratory tests were negative for all common causes of acute hepatitis. The clinical course was uneventful, the liver enzymes returned to normal within 3 months after ciprofloxacin discontinuation and remained normal during the follow up period. Use of the Naranjo ADR Probability Scale2 indicated that hepatitis was probably due to ciprofloxacin. In the literature only 4 cases3,6 of ciprofloxacin-induced cholestatic hepatitis have been reported. Although, in only one of them,3 similar to our patient, significant elevation in cholestatic enzymes (ALP, γ-GT) was observed, our patient had unremarkable medical history and he was not under any other medication. Interestingly, acute liver injury may occur shortly6 (as in our case) or later during ciprofloxacin administration.3,5 In conclusions, in our patient, in contrast to the previous reports, no confounding factors (e. g. additional drugs, pathogens or environmental conditions) related with hepatitis were present, while the Naranjo algorithm yielded a probable association. Thus, this is the first case report, which provides clear evidence that ciprofloxacin, by itself and at therapeutic doses, can be a cause of acute cholestatic hepatitis.