Percutaneous liver biopsy (LB) is the gold standard method for evaluation and management of patients with liver disease. The purpose of this study was to characterize pediatric patients undergoing LB at British Columbia Children's Hospital, and to determine the rate and timing of complications following the procedure.
Material and methodsThe medical records of all pediat-ric patients who underwent LB during a six-year retrospective study were reviewed to collect demographic and procedure-related data.
Results223 LBs were performed, and 179 of these biopsies were percutaneous or transjugular. Elevated liver enzymes and cholestasis together accounted for almost 70% of the indications for LB, and the histological analysis of liver tissue yielded a specific diagnosis in 89 % of the cases. There were no deaths and no major complications related to LB. The most frequent minor complication was pain (59% of LBs) and the other complications were bleeding-related and classified as minor. The vast majority of complications (88%) were recognized within 8 h of the LB.
ConclusionsLB is a valuable and safe procedure in pediatric patients with a low rate of complications. Pediatric patients can be discharged home safely should no complications occur within the first 8-12 h after the procedure.
Percutaneous liver biopsy (LB) has long been considered the gold standard method for evaluation and management of patients with liver disease.1 First described in Germany in1883 by Paul Ehrlich2,3 the technique became widely accepted in adults after Menghini reported, in 1958, a more simplified and safer technique using his needle.4,5 In pediat-ric practice, percutaneous LB was first reported in the late 1950s and has since been regarded as useful and safe.6,7
Many institutions now choose to perform the percutaneous LB under real-time sonographic guidance in an effort to decrease the complication rate and to achieve targeted sampling.1,8 A transjugular (TJ) LB is selected for those patients who have severe coagulopathy, ascites, massive obesity or a vascular lesion,9 but this approach is technically difficult in small infants. Recently, the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) defined “minor” complications of LB in children to include: pain; subcapsular bleeding that does not require transfusion or prolonged hospitalization, infection, minor bile leak or haemobilia, and arteriov-enous fistula. “Major” LB complications include: bleeding or haemobilia requiring transfusion, need for surgery or intensive care management, pneumothorax or haemotho-rax, and death.10
The purpose of this single centered retrospective study was to characterize pediatric patients undergoing liver biopsy at British Columbia Children's Hospital (BCCH), and to determine the rate and timing of minor and major complications following the procedure.
Material and MethodsThe study was conducted on all patients aged 0-18 years old identified by the Anatomical Pathology Department at BCCH who underwent liver biopsy between January 2006 to December 2012. The medical records of the patients were reviewed and demographic data including the date of birth, gender, weight, height and body mass index, were recorded. Procedure-related data that were collected included patient age at LB, indication for LB, method of LB, division requisitioning the LB, final diagnosis, inpatient/ outpatient status at time of referral for LB, pre-biopsy complete blood count (CBC) and INR, post-biopsy hemoglobin, duration of hospital stay post biopsy, type of complications and their temporal association with the LB. At BCCH the post-LB protocol generally involves an overnight hospital stay with measurement of CBC 4 h post-procedure. Post-biopsy hemorrhage was defined as a drop in hemoglobin (Hb) of more than 20 g/L from the pre-biopsy value. Descriptive statistics and standard student T tests were applied for data analysis. The study was approved by the University of British Columbia Institutional Review Board.
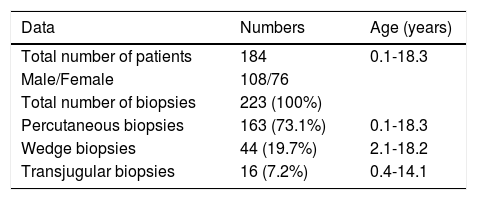
RESULTSA total of 184 patients (108 males) underwent 223 LB, of which 163 (73.1%) were percutaneous, 16 (7.2%) were transjugular (TJ) and 44 (19.7%) were surgical wedge biopsies. The surgical wedge biopsies were excluded from all further analysis (Table 1).
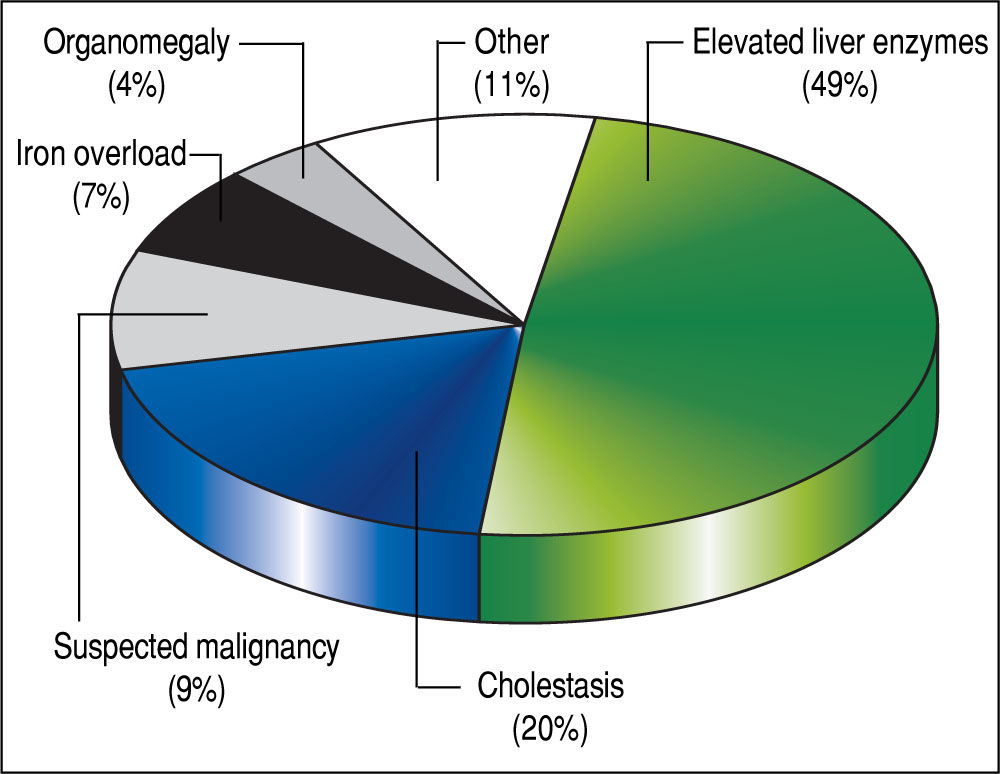
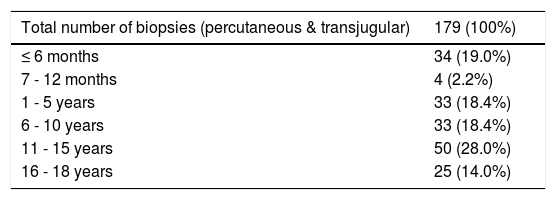
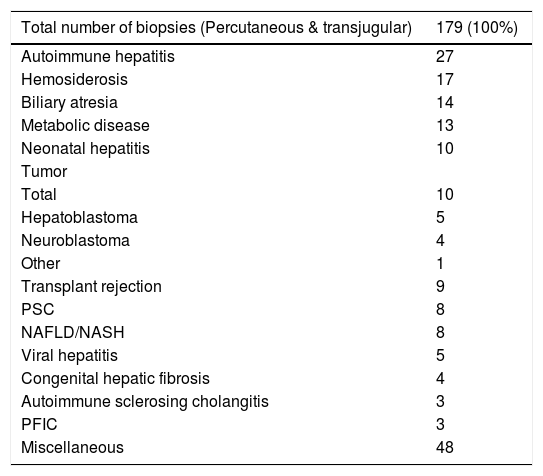
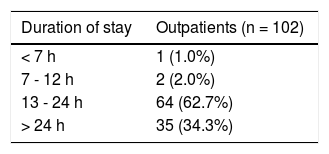
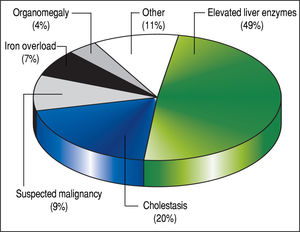
The 179 percutaneous and TJ biopsies were performed on 158 patients (91 males) at a median age of 8.5 years (range 0.1 to 18.3 years). Among these patients, 12 underwent the procedure twice, three patients underwent three biopsies and one patient had four procedures. Of the 179 LB, 30% were in the 11-15 year old age group and almost 20% of the cases were less than 6 months of age (Table 2). The leading indication for the LB was elevated liver enzymes (49%) (Figure 1). All infants less than 6 months of age had LB to assess for neonatal cholestasis. Autoimmune hepatitis was diagnosed in 27 (15%), and biliary atresia in 14 (8%) of liver biopsies respectively (Table 3). A majority of the LBs were requested by the Gastroenter-ology (151 biopsies, 84%) and Haematology/Oncology services (24 biopsies, 13%). Of the LBs, 102 (57%) were requested in outpatients, 75 (42%) requested for inpatients and 2 (1.1%) for intensive care unit patients. The mean and median duration of hospital stay after LB for the outpatient group was 23 h (range 6.5 - 33 hrs) (Table 4).
Liver biopsy diagnosis.
| Total number of biopsies (Percutaneous & transjugular) | 179 (100%) |
|---|---|
| Autoimmune hepatitis | 27 |
| Hemosiderosis | 17 |
| Biliary atresia | 14 |
| Metabolic disease | 13 |
| Neonatal hepatitis | 10 |
| Tumor | |
| Total | 10 |
| Hepatoblastoma | 5 |
| Neuroblastoma | 4 |
| Other | 1 |
| Transplant rejection | 9 |
| PSC | 8 |
| NAFLD/NASH | 8 |
| Viral hepatitis | 5 |
| Congenital hepatic fibrosis | 4 |
| Autoimmune sclerosing cholangitis | 3 |
| PFIC | 3 |
| Miscellaneous | 48 |
PSC: primary sclerosing cholangitis. NAFLD: Non-Alcoholic Fatty Liver Disease. NASH: Non-Alcoholic Steatohepatitis. PFIC: progressive familial intrahepatic cholestasis.
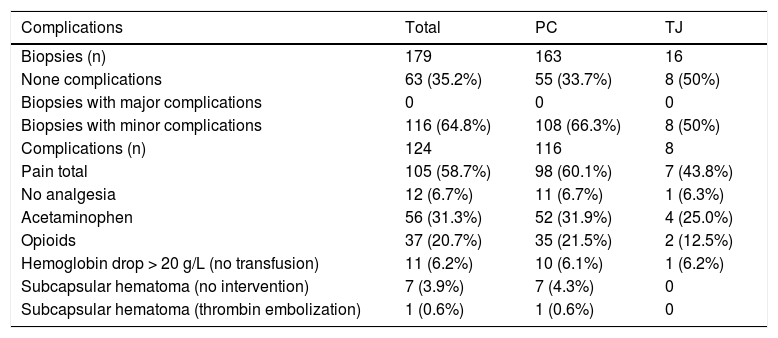
There were no major complications and no mortality. Minor complications occurred in 116 biopsies (65%), with some procedures having more than one complication totaling 124 complications post LB. The most frequent minor complication was pain (59%). Of these, 7% did not require any analgesia. Acetaminophen was administered after 56 LB procedures (31%) and opiate analgesics were required after 37 (21%) biopsies (Table 5). Among children younger than one year of age, pain responsive to oral analgesics occurred in 74% of patients. In children 8 years of age or older, pain was reported in 53% of the cases. Eleven patients (6%) had a drop in Hb of more than 20 g/L post-biopsy but none required transfusion. Pre-biopsy Hb levels for these 11 patients ranged from 107 g/L to 167 g/L, platelet count 69,000/μL to 565,000/μL, and INR 0.93 to 1.24 respectively. In 7 biopsies (4%) a small hematoma developed at the biopsy site immediately post-procedure and local compression was applied. One patient required thrombin embolization of a capsular hematoma by U/S in the immediate post-biopsy period. Among the 8 patients with hematoma, the pre-biopsy Hb level ranged from 100 g/L to 135 g/L, platelets 75,000/μL to 147,000/μL, and INR 0.97 to 1.23.There was no statistical difference in the minor complications among the percutaneous LB (66%) compared with transjugular LB (50%) groups (Table 5).
Complications by type of biopsy.
| Complications | Total | PC | TJ |
|---|---|---|---|
| Biopsies (n) | 179 | 163 | 16 |
| None complications | 63 (35.2%) | 55 (33.7%) | 8 (50%) |
| Biopsies with major complications | 0 | 0 | 0 |
| Biopsies with minor complications | 116 (64.8%) | 108 (66.3%) | 8 (50%) |
| Complications (n) | 124 | 116 | 8 |
| Pain total | 105 (58.7%) | 98 (60.1%) | 7 (43.8%) |
| No analgesia | 12 (6.7%) | 11 (6.7%) | 1 (6.3%) |
| Acetaminophen | 56 (31.3%) | 52 (31.9%) | 4 (25.0%) |
| Opioids | 37 (20.7%) | 35 (21.5%) | 2 (12.5%) |
| Hemoglobin drop > 20 g/L (no transfusion) | 11 (6.2%) | 10 (6.1%) | 1 (6.2%) |
| Subcapsular hematoma (no intervention) | 7 (3.9%) | 7 (4.3%) | 0 |
| Subcapsular hematoma (thrombin embolization) | 1 (0.6%) | 1 (0.6%) | 0 |
PC: percutaneous. TJ: transjugular.
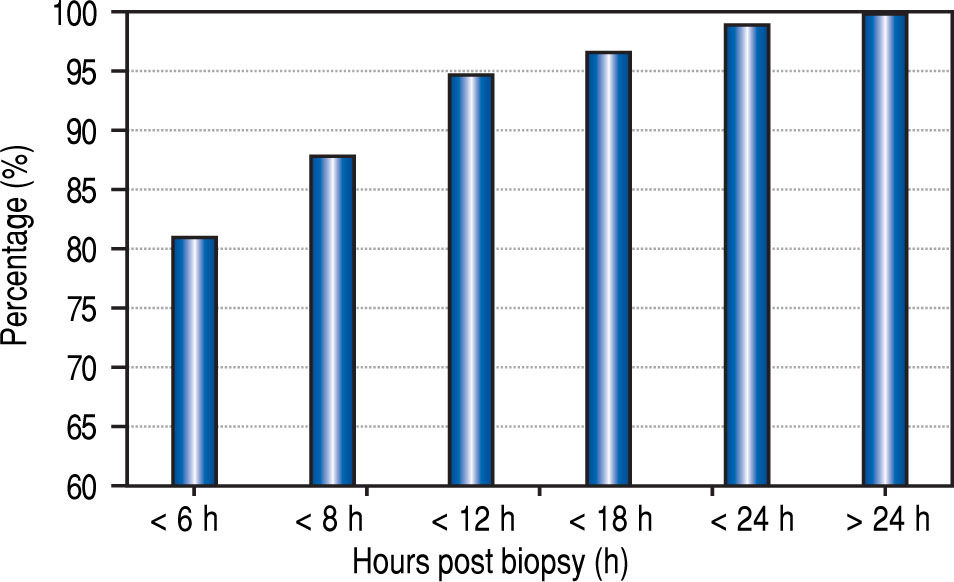
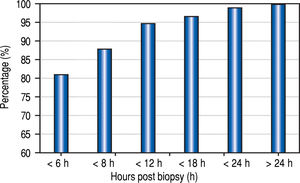
The median time from the LB to recognition of any complication was 1.8 h, (mean 3.5 h; range 0-42 h). Eighty-one percent of the complications were recognized within 6 h of biopsy, 88% within 8 h, and 95% within 12 h (Figure 2).
DiscussionLB is a well-established and reliable procedure for the assessment of liver disease in both children and adults.8,10 Several studies in adult patients with abnormal liver function tests have demonstrated the utility of LB for identifying a specific diagnosis and for determining the grade of fibrosis.11,12 In this study of pediatric patients, elevated liver enzymes and cholestasis together accounted for almost 70% of the indications for LB, and the histological analysis of liver tissue yielded a specific diagnosis in 89% of the cases. In particular, the LB was an especially valuable tool to help establish a diagnosis in young infants less than six months of age with conjugated hyperbilirubinemia. Biliary atresia accounted for 36% of the diagnoses in these cases of neonatal cholestasis. Overall in our series, autoimmune hepatitis was the leading diagnosis among the 179 LBs.
There were no deaths and no major complications related to LB. Pain accounted for 90% of the complications reported in this pediatric series. This minor complication, recognized in 59% of the patients, was most often managed with acetaminophen alone. Transient pain has been noted in 20-36 % of pediatric patients after LB.1,13 While pain has been recognized in as many as 85% of adults post LB,14 we believe the lower rate of this minor complication in children may be related to level of anesthesia delivered to children at the time of LB compared with adults. In very young infants post LB, pain symptoms may be over-reported because of the difficulty in differentiating this symptom from the general irritability often seen in hospitalized infants.
Many pediatric LB studies do not consider pain in the analysis of complications. In our study the overall incidence of complications excluding pain was 10.6%, a rate in keeping with other reports.15,16 All of these events were bleeding-related and classified as minor. Similar to other series, most of our cases of bleeding were managed conservatively.1 Some studies have suggested that young infants are at slightly higher risk for bleeding.15,17,18 Among the eight cases with hematoma in our study, four were under 4 months of age, but we could not assess age as a risk factor for bleeding complications due to our small sample size.
Among the 16 TJ biopsies (7% of LB) in this series, there were no major complications. Others have reported higher complication rates in both children and adults undergoing TJ.19
While ESPGHAN has recommended that the platelet count should be 60 x 109/L or higher, and the INR should be 1.5 or lower prior to a percutaneous LB,10 there is a lack of correlation between these parameters and bleeding risk.20 Of the patients with bleeding complication in our study, none had low pre-procedural platelet counts or elevations in the INR beyond the parameters recommended by ESPGHAN.
The optimal duration of observation after LB is another source of controversy. In 1989, the American Gastroen-terological Association published a consensus statement recommending a 6 h observation post LB in adult patients having no risk factors.21 Recently, Firpi, et al. have found that adult patients can be safely discharged after a one hour observation period.22,23 Similar to the policies in our centre, other pediatric centres keep patients in hospital for overnight observation post LB.24 However, the need for this lengthy duration of monitoring has been challenged. In a study of 597 children, Govender determined that almost all LB complications could be identified within the first 4 h after the procedure, and if no complications were encountered, patients could be discharged home safely.16 In another study, Matos observed children for at least 6 h post LB and, if there were no complications, the patients could be safely leave the hospital on the day of the procedure.25 Our data, although of limited sample size and retrospective, support these studies, and demonstrate that the vast majority of complications (88%) in pediatric patients can be recognized within 8 h of the LB. These results regarding the timing of discharge post LB have important health care cost implications.
In conclusion, our study confirms that LB has diagnostic value in pediatric patients and demonstrates that LB is a safe procedure with a low rate of complications. The majority of complications were minor, with pain being the most common. There were no deaths and no major complications post LB in this six-year retrospective single centre pediatric study. Our findings further support the notion that pediatric patients do not require a 24 h overnight hospital observation period post LB and instead can be discharged home safely should no complications occur within the first 8 h and certainly by 12 h after the procedure. Current practice guidelines for the monitoring of pediatric patients post LB deserve re-evaluation.
Abbreviations- •
BCCH: BC Children's Hospital.
- •
CBC: complete blood count.
- •
ESPGHAN: European Society for Pediatric Gastro-enterology, Hepatology and Nutrition.
- •
Hb: hemoglobin.
- •
INR: international normalized ratio.
- •
LB: liver biopsy.
- •
TJ: transjugular.
Jennifer Liang was supported by a Canadian Liver Foundation summer research studentship.