Liver fibrosis assessment is a key issue in the evaluation of nonalcoholic fatty liver disease (NAFLD) patients. In the present study, we aimed to validate a noninvasive marker panel to assess significant and advanced fibrosis in these patients.
Method126 biopsy-proven NAFLD patients were included. NAFLD diagnosis was based on histological criteria. Fibrosis stages were determined according to NASH-Clinical Research Network criteria. Clinical and laboratorial data were collected during the interval of three months before or after liver biopsy. Histological fibrosis stages were classified as significant fibrosis (F2-F4) and advanced fibrosis (F3-F4). Five serum biomarkers [hyaluronic acid (HA), collagen type IV (cIV), procollagen type III (PC III), laminin (LN) and cholylglycine (CG)] were assessed by chemiluminescence immunoassays.
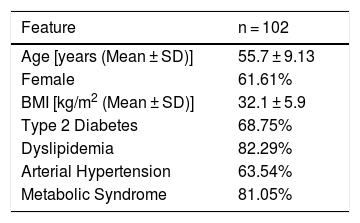
ResultsMost patients were female (61.61%), mean age: 55.7 ± 9.13 years old and mean BMI was 32.1 ± 5.9 kg/m2. Prevalence of diabetes, dyslipidemia, arterial hypertension, and metabolic syndrome was 68.75%, 82.29%, 63.54% and 81.05%, respectively. Patients with cIV above 30 ng/mL had a 5.57-times (IC: 1.86–16.69) the chance of having significant fibrosis and 7.61-times (IC: 2.27–25.54) the chance of having advanced fibrosis versus patients with values below 30 ng/mL. HA, PC III, LN and CG did not detect the presence of significant and advanced fibrosis. The AUROC of clV for detection of significant (0.718) and advanced fibrosis (0.791) was better than that of other serum biomarkers.
ConclusionType 4 collagen could predict the presence of significant and advanced fibrosis in NAFLD patients and it would be a useful tool in routine clinical practice.
Non-alcoholic fatty liver disease (NAFLD) is a pathological clinical condition that encompasses a large spectrum of diseases and a broad spectrum of manifestations, from isolated steatosis to non-alcoholic steatohepatitis (NASH), whose severity may vary according to the degree of fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [1]. Histologically, NAFLD can be differentiated into non-alcoholic fatty liver (NALF) or NASH according to the absence or presence of signs of hepatocellular damage, such as hepatocyte ballooning and necroinflammation, which are present in NASH [2,3]. In addition, it is necessary to exclude secondary causes of liver disease and significant alcohol consumption [4]. Currently, NAFLD is the most common cause of liver disease in the Western population [5], with an estimated prevalence ranging from 6.3 to 33% in the general population, depending upon the group studied and the diagnostic method used. However, the prevalence of NASH is lower, ranging from 3 to 5% in the general population [6].
Liver biopsy is still considered the gold standard for liver tissue evaluation in NAFLD, allowing for the ascertainment of parameters, such as steatosis, inflammation, ballooning and fibrosis and contributing to a precise NAFLD diagnosis and staging [7]. Of note, among NAFLD histologic features, the degree of liver fibrosis is the most closely associated with liver-related and all-cause mortality [8]. However, liver biopsy is an invasive procedure with associated risks, sometimes causing pain, hemorrhage, and even death, among other complications [9]. Issues regarding the quality of liver samples and interpretation of the results are also of concern. The quality of a liver biopsy is generally related to the size and number of portal spaces evaluated [10,11]. In addition, the results of liver histology evaluation can vary according to the subjective interpretation of pathologists [12]. Due to these limitations, noninvasive methods for liver fibrosis evaluation have been studied intensely and have improved in recent decades. These methods can be divided into three categories, namely, indirect markers, which can be assessed by routine clinical exams (e.g., aminotransferases and platelet count) [13], direct markers, which include serum levels of substances involved in the molecular pathogenesis of fibrosis, such as matrix metalloproteinases, hyaluronic acid, and cytokines [tumor necrosis factor-alpha (TNF-α) as well as transforming growth factor beta (TGF-β)] [13,14] and, non-invasive mechanical methods based on ultrasound such as transient elastography (TE or FibroScan®) and acoustic radiation force impulse (ARFI) technology, Virtual Touch® [15]. Analysis of the performance of these tests in different cohorts of patients provides information to estimate their usefulness in clinical practice. In the present study, we aimed to assess the usefulness of a new noninvasive marker panel [hyaluronic acid (HA), collagen type IV (cIV), procollagen type III (PC III), laminin (LN) and cholylglycine (CG)] for fibrosis detection in a cohort of biopsy-proven NAFLD patients.
2Patients and methodsWe conducted a cross-sectional study of adult patients (≥18 years old) with biopsy-proven NAFLD and included consecutive patients (n = 126) who attended NAFLD outpatient Unit at the University of São Paulo School of Medicine, São Paulo, Brazil. Diagnosis of NAFLD was based on histological criteria, and fibrosis stages were determined according to NASH-CRN criteria [16]. According to liver biopsy results, histological fibrosis stages were classified as significant fibrosis (F2-F4) or advanced fibrosis (F3-F4). The use of plasma or serum for biomarker analysis in the present study was approved by the Hospital das Clinicas Ethics Committee (294.198/ 2013).
2.1Clinical and laboratory assessmentClinical and laboratorial data were collected during the interval of three months before and three months after liver biopsy, and electronic medical records of patients undergoing liver biopsy were retrospectively studied. Patients with evidence of other liver diseases (autoimmune hepatitis, viral hepatitis, drug-induced liver injury, hemochromatosis, cholestatic liver disease, or Wilson’s disease) were excluded. In addition, subjects consuming excessive amounts of alcohol (alcohol intake >20 g/day for women; >30 g/day for men) at the time of the biopsy or in the past were excluded. The inclusion criterion was that the patient had biopsy-proven NAFLD.
Relevant clinical details, including sex, age, weight, and height, were obtained at the time of biopsy. Body mass index (BMI) was calculated by the formula weight (kg)/height (m2). Patients were identified as having Type 2 diabetes mellitus (T2DM) if they had been diagnosed according to the American Diabetes Association criteria [17] or if they were using an oral hypoglycemic drug or insulin. The presence of Metabolic syndrome (MetS) components was evaluated according to the National Cholesterol Education Program Adult Treatment Plan III (ATP III) guidelines [18].
2.2Histological analysisLiver biopsies were performed and conducted as per routine clinical care to investigate abnormal liver function tests [elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), or gamma-glutamyl transferase (GGT)] or to stage disease severity in patients with radiological evidence of fatty liver. Percutaneous liver biopsies were performed as per unit protocol at the site and were assessed by an experienced liver pathologist. Liver tissue was fixed in 4% formaldehyde, processed, and stained with hematoxylin-eosin and Masson trichrome for histological analysis. All specimens were scored by an experienced liver pathologist with expertise in NAFLD. Histological scoring was performed according to the NASH-Clinical Research Network criteria (CRN) [16]. NAFLD activity score was graded from 0 to 8, including scores for steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2). NASH was defined as steatosis with hepatocyte ballooning and inflammation ± fibrosis. Fibrosis was staged from F0 to F4.
2.3Serum biomarker analysisFive direct serum biomarkers that reflect the extracellular matrix (ECM) for the determination of liver fibrosis were assessed. Chemiluminescence immunoassays (Snibe Co., Ltd, Shenzhen, China) were used to detect the following five serum biomarkers: HA, cIV, PC III, LN and CG from fasting blood samples. Serum was separated by centrifugation and stored at −20 °C until it was assayed.
2.4Calculation of noninvasive fibrosis scoreWe calculated FIB-4 score [age (years) × AST (U/L) / platelets (109/L) x √ALT (U/L)] as a non-invasive fibrosis score and compared with cIV [19].
2.5Statistical analysisStatistical analysis was performed using SPSS Version 25 and Graph Pad Prism software. Continuous variables were expressed as mean ± standard deviation (SD). Qualitative data were presented as numbers with percentages. Normally distributed continuous variables were compared with Paired t test. Fisher’s exact and Chi-square with Yates' correction test was used for qualitative data. Overall diagnostic accuracy was evaluated by determining the area under the ROC curve (AUROC) and a comparison between the two ROC curves was made according to Hanley and McNeil method [20,21]. Two-tailed P values < 0.05 were considered statistically significant.
3Results3.1Clinical and laboratoryA total of 102 patients out of 126 NAFLD patients were evaluated (24 patients were excluded due to incomplete medical record data). Among the included patients, 61.61% were female with a mean age of 55.7 ± 9.13 years old. Mean BMI was 32.1 ± 5.9 kg/m2. Prevalence of T2DM, dyslipidemia, arterial hypertension and MetS was 68.75%, 82.29%, 63.54% and 81.05%, respectively (Table 1).
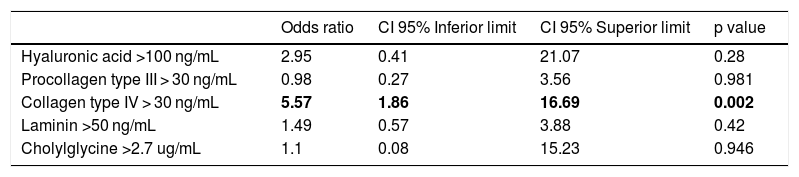
Table 2 presents a logistic regression model to evaluate the correlation of serum biomarkers with the presence of significant fibrosis (F2-F4). Adjusting for the other variables, patients with cIV above 30 ng/mL had a greater chance of having fibrosis F2-F4 when compared to cIV below 30 ng/mL (OR: 5.57; 95% CI: 1.86–16.69). On the other hand, the serum biomarkers HA, PC III, LN and CG were not statistically related to the presence of significant fibrosis (F2-F4) after adjustment for confounding variables.
Logistic regression model of serum biomarkers in NAFLD patients according to significant fibrosis (F2-F4).
| Odds ratio | CI 95% Inferior limit | CI 95% Superior limit | p value | |
|---|---|---|---|---|
| Hyaluronic acid >100 ng/mL | 2.95 | 0.41 | 21.07 | 0.28 |
| Procollagen type III > 30 ng/mL | 0.98 | 0.27 | 3.56 | 0.981 |
| Collagen type IV > 30 ng/mL | 5.57 | 1.86 | 16.69 | 0.002 |
| Laminin >50 ng/mL | 1.49 | 0.57 | 3.88 | 0.42 |
| Cholylglycine >2.7 ug/mL | 1.1 | 0.08 | 15.23 | 0.946 |
95% CI: 95% confidence interval.
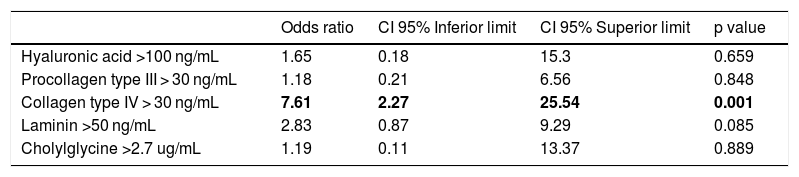
A logistic regression model was also used to address the correlation of serum biomarkers with the presence of advanced fibrosis F3-F4 (Table 3). Similarly to the results seen regarding cIV values and significant fibrosis, patients with cIV above 30 ng/mL had greater chance of having F3-F4 fibrosis when compared to cIV values below 30 ng/mL (OR: 7.61; 95 % CI:2.27–25.54), when adjusting for the other variables. The serum biomarkers HA, PC III, LN and CG were not statistically related to the presence of F3-F4 fibrosis after adjustment of variables.
Logistic regression model of serum biomarkers in NAFLD patients according to advanced fibrosis (F3-F4).
| Odds ratio | CI 95% Inferior limit | CI 95% Superior limit | p value | |
|---|---|---|---|---|
| Hyaluronic acid >100 ng/mL | 1.65 | 0.18 | 15.3 | 0.659 |
| Procollagen type III > 30 ng/mL | 1.18 | 0.21 | 6.56 | 0.848 |
| Collagen type IV > 30 ng/mL | 7.61 | 2.27 | 25.54 | 0.001 |
| Laminin >50 ng/mL | 2.83 | 0.87 | 9.29 | 0.085 |
| Cholylglycine >2.7 ug/mL | 1.19 | 0.11 | 13.37 | 0.889 |
95% CI: 95% confidence interval.
Although the serum biomarker LN (above 50 ng/mL) did not reach statistical significance at an assumed level of 0.05, it had a positive relationship (patients with LN above 50 ng/mL had 2.83-times the chance of having advanced fibrosis (F3-F4) with a P value of less than 0.10).
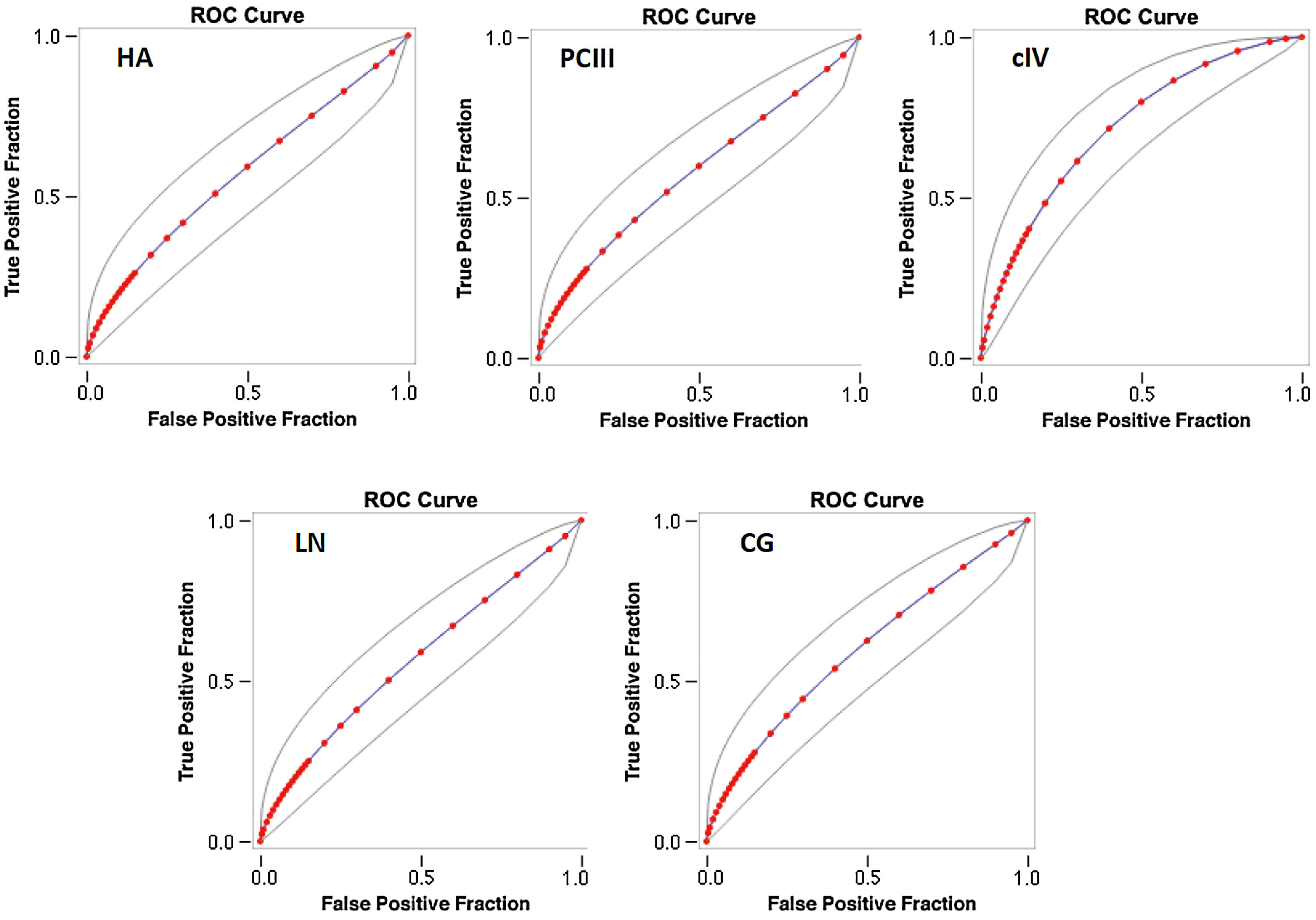
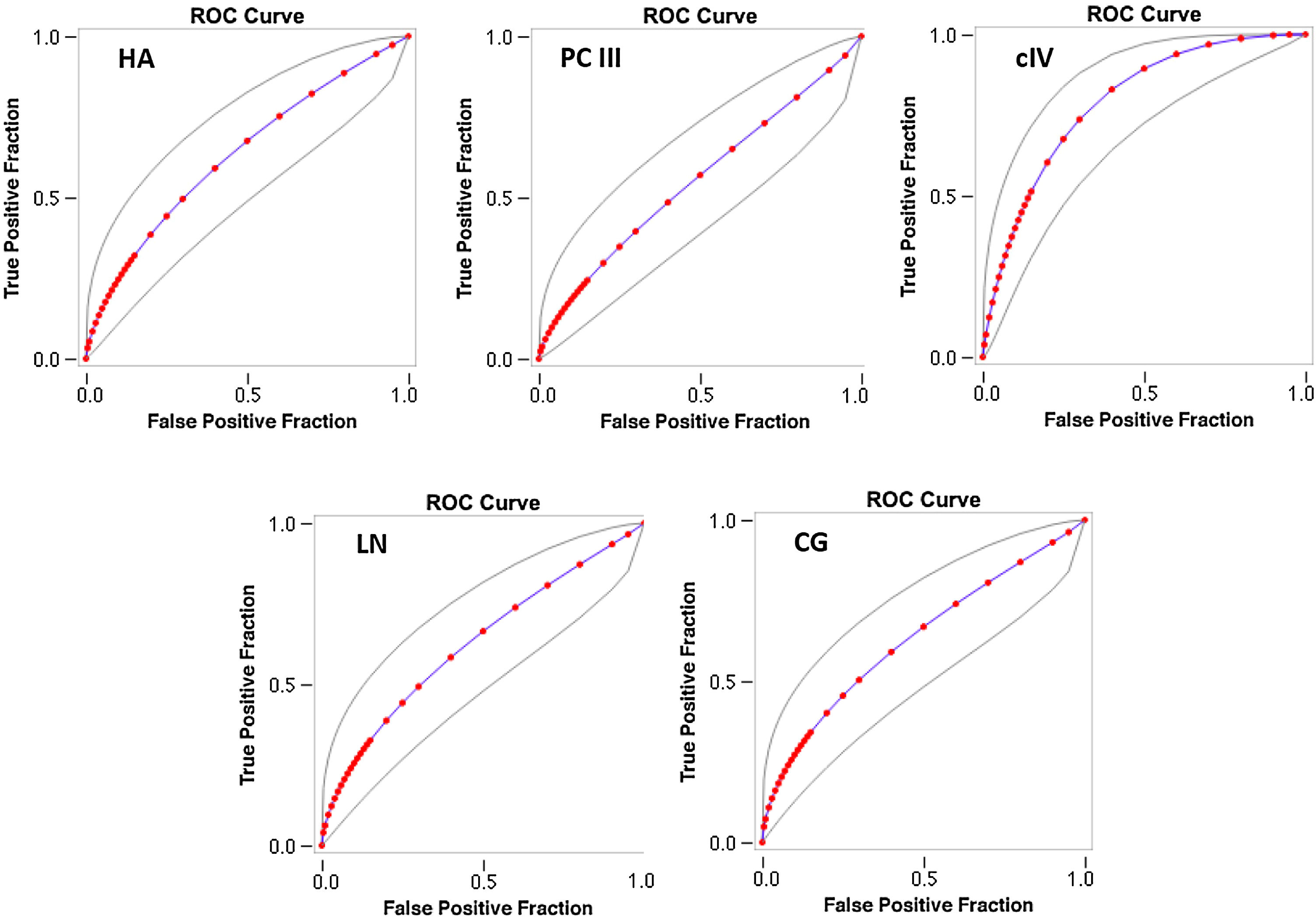
The AUROC of cIV for the detection of significant fibrosis was better (0.718) than that of other serum biomarkers (fitted ROC area: HA = 0.57, PC III = 0.576, LN = 0.567, CG = 0.594) (Fig. 1). In addition, the AUROC of cIV for the detection of advanced fibrosis was also better (0.791) than that of the other serum biomarkers (fitted ROC area: HA = 0.633, PC III = 0.553, LN = 0.627, CG = 0.632) (Fig. 2).
The AUROC curves were similar between cIV and FIB4 for significant fibrosis (FIB4 AUROC 0.778 (± 0.0526); cIV AUROC 0.754 (± 0.0546)) as well as for advanced fibrosis [FIB4 AUROC 0.831 (± 0.0656); cIV 0.863 (± 0.057)]. However, for significant fibrosis, cIV had sensitivity of 73.13% and specificity of 73.91%, while FIB4 had a sensitivity of 64.71% and 100% of specificity (p < 0.001 by McNemar's test). For advanced fibrosis, cIV had sensitivity of 92.54% and specificity of 47.83% versus 87.06% and 100% for FIB4, also statistically significant (p < 0.001 by McNemar's test).
4DiscussionIn the present study, we identified a simple serum biomarker type 4 collagen (cIV) that could predict the presence of significant fibrosis (F2-F4) in NAFLD patients and that could be a useful tool in routine clinical practice.
The estimated overall prevalence of NAFLD is 25-30% and may reach 40% in some areas of the United States [22–24]. This prevalence tends to increase, making NAFLD a large worldwide public health problem in the future, with an increase in the number of liver transplantations secondary to NASH. In addition, patients with NAFLD are at an increased risk of overall mortality, cardiovascular disease, infectious disease, cirrhosis, and HCC [25]. Most of these complications related to NAFLD are associated directly with fibrosis [8,26]. Therefore, predicting the degree of fibrosis in patients using low-cost tools may be important, as there is a great need for better biomarkers to predict significant (F2-F4) and advanced (F3-F4) fibrosis in the general population. Currently, noninvasive tests are suboptimal with regard to helping with fibrosis diagnosis and risk stratification and to being able to assist in the determination of liver biopsy indication [27]. Moreover, a problem in evaluating the performance of noninvasive tests is that the gold standard for diagnosis, liver biopsy, is also imperfect and may lead to a biased analysis [28].
In the present study, we evaluated the efficacy of low-cost serum fibrosis biomarkers in the prediction of not only advanced fibrosis but also significant fibrosis (F2-F4).In order to accomplish this, we evaluated the accuracy of five serum fibrosis biomarkers in NAFLD patients to predict significant fibrosis (F2-F4) and we confirmed that they could be measured in the routine laboratory setting to guide which patient would need liver biopsy.
Biopsy results have revealed that cIV could be a good biomarker for the diagnosis of significant fibrosis (F2-F4). Patients with cIV levels above 30 ng/mL had a 5.57-fold higher chance of having fibrosis F2-F4 than patients with cIV below 30 ng/mL, after adjusting for the other variables, while the HA, PC III, LN and CG biomarkers were not significantly related to the presence of fibrosis F2-F4 after adjustment of variables. These findings are particularly important because most noninvasive clinical and laboratory scores divide individuals into two large groups according to the absence or presence of advanced fibrosis. Intermediate grades, such as F2, are in the gray zone. The serum biomarker cIV was able to discriminate the stages of significant fibrosis. In addition, cIV was able to detect advanced fibrosis with accuracy similar to other noninvasive markers, such as FIB-4 and NAFLD fibrosis score. Recently, a Japanese study demonstrated, similar to our study, that cIV had an AUROC of 0.803 for discriminating NAFLD patients with significant fibrosis (F2-F4) and 0.830 for discriminating patients with advanced fibrosis (F3-F4) [29]. Another interesting Japanese study by Okanoue et al. identified the combination of cIV and AST as a predictor of both NASH and NASH-related fibrosis [26]. With this score, the authors revealed the AUROCs of 0.857/0.769 for NASH and 0.918/0.842 for NASH-related fibrosis for training/validation data sets, respectively [30]. These AUROCs were higher than those of the NAFIC score [29], BARD score, FIB-4 index and NAFLD fibrosis score.
Although some studies have demonstrated good performance of PC III for fibrosis assessment in patients with NAFLD [31,32], in our study, these biomarkers were not significantly related to the presence of significant or advanced fibrosis. Recently, our group participated in a multicentric study that assessed the performance of PC III as a NASH-fibrosis biomarker diagnostic tool and determined its performance in comparison to established clinical scores and previously reported biomarker panels [27].
HA has long been described as a good biomarker of fibrosis in liver diseases [33,34]. However, some studies have illustrated that serum HA alone has limited accuracy in predicting the severity of liver fibrosis [35,36]. Consequently, some scores using HA with other clinical and laboratory variables such as Enhanced Liver Fibrosis Score (ELF), have been validated [37,38]. Recently, a group from Argentina conducted a small study with biopsy-proven NAFLD patients in which the diagnostic accuracy of HA for significant fibrosis had an AUROC of 0.928 [39]. In the present study, we did not observe good accuracy of HA in detecting significant fibrosis in NAFLD. Despite the fact that LN, another interesting biomarker, did not reach statistical significance at an assumed p value of 0.05, it had a positive relationship (patients with levels above 50 ng/mL had 2.83-times the chance of having advanced fibrosis (F3-F4) with a p value less than 0.10). In 2019, Srivastava et al. demonstrated that noninvasive methods such as FIB-4 followed by ELF in indeterminate cases reduced the cost of primary care for NAFLD patients [40]. Thus, using cIV cutoff above 30 ng/mL, can increase the chance of identifying more advanced fibrosis in NAFLD patients for a low cost. Although, cIV accuracy is similar to FIB-4 accuracy, the sensibility for significant fibrosis and advanced fibrosis are better for cIV when compared with FIB-4 (sensitivity of 73.13% and 64.71%; 92,54% and 87.06% respectively). Since it had no false positives by McNemar's test and both tests are used in screening risk of fibrosis, it is preferable to choose a method with higher sensitivity.
In conclusion, the present study suggests that type IV collagen, a simple serum biomarker, could predict the presence of significant and advanced fibrosis in NAFLD patients and it would be a useful tool in routine clinical practice. Future studies are necessary to confirm or replicate these results before cIV is widely used.
Author’s contributionsConception and design of the study: CPO. Generation, collection, assembly, analysis and/or interpretation of data: LVG, AAAS, DSV, VAFA, AL. Drafting or revision of the manuscript: JTS, FJC, MA, CPO.
Conflicts of interestThe authors have no conflicts of interest to declare. We are grateful for the support of Shenzhen New Industries Biomedical Engineering Co., Ltd., which kindly provided the kits used to carry out the experiments used in this study.