Radical resection remains the only curative treatment for liver tumors. Although associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) can increase the resection rate, huge controversy exists for high reported mortality and morbidity. This study was to evaluate the efficacy and safety of modified ALPPS procedure.
Patients and MethodsPatients who were performed ALPPS in single-center in recent 5 years were retrospectively reviewed. The modified strategy included strict patient selection, precise future liver remnant (FLR) assessment and operation planning, and usage of minimally invasive methods. Data including clinical records, functional FLR increase, complications, and overall survival (OS) were analyzed.
ResultsSixty patients underwent modified ALPPS procedure and recovered well. No severe complications happened after the 1-stage operation, and the increasing FLR was 179.3 cm3(±72.4 cm3), with similar functional FLR increase. The OS was 20.0 months (±4.5month).
ConclusionsALPPS could be a feasible treatment for complex liver tumors by risk-reduced modification. It could be expected to provide long-term survival for patients without enough FLR.
For patients with primary or metastatic liver tumors, radical resection still remains the only definitely curative treatment. However, because of obvious liver cirrhosis in hepatocellular carcinoma (HCC) or multiple lobes involved in metastatic liver tumors, the consideration of insufficient future liver remnant (FLR) and subsequently occurred post-hepatectomy liver failure (PHLF) severely restricted the performance of radical resection, and only 30% patients had benefited from the radical hepatectomy. [1,2] Portal vein embolization (PVE) and planned 2-stage hepatectomy within 2–8 weeks provided an alternative for regeneration of the remnant liver, and has been studied as an effective way to completely remove bilobar liver tumors [3,4]. In order to promote FLR hyperplasia and shorten the interval, a new 2-stage hepatectomy named as associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been developed since 2007. ALPPS can predictably induce an accelerated FLR growth of 40%-160% in 6–9 days, a short period in which major tumor progression is unlikely and R0 resection can be performed without troublesome adhesions, thus broadening the indications for curative resection without risk of PHLF [5].
However, ALPPS has also been accompanied by huge controversy because of high reported mortality (up to 25%) and morbidity (up to 40%) [6]. Severe sepsis originating in the necrotic liver focus after the first operation and correlated liver failure from insufficient functional FLR seemed so inevitable that some surgeons had to limit ALPPS only in strictly selected patients with good liver function [7]. Therefore, we formulated and applied a risk-reduced strategy, including reasonable adjustments in patients selection, precise FLR assessment and operation planning, and usage of minimally invasive techniques to perform ALPPS since 2015. Here we retrospectively described our 5-year experience in modified ALPPS procedures, with the purpose to clarify the effect of ALPPS in treating complex liver tumors.
2Patients and methodsAll patients who underwent ALPPS in Zibo Central Hospital, a regional specialized center of hepatobiliary surgery, between March 1, 2015 and February 29, 2020 were reviewed. They were diagnosed on basis of clinical features, laboratory tests, and imaging findings, and proved by pathological results. All patients had been referred to the current treatment of liver tumor, including liver transplantation, hepatectomy, radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), targeted drug therapy. After informed of detailed benefits and risks of ALPPS, each patient included in this study made the final choice and signed informed consents. This study conformed to the current Declaration of Helsinki, and the Ethics Committee of Zibo Central Hospital approved access to the database and methods used for data retrieval and analysis (No.2018- ZCH-E013). Zibo Central Hospital Reviewer Board recognized this retrospective study (No.2018-ZCH-R0457).
3Principle in patient selectionExcept for routine preoperative evaluation (including blood routine test, coagulation test, biomedical test and assessment of cardiopulmonary function), the Child-Pugh classification system combined with Model of End-Stage Liver Disease (MELD) scoring system was used for preoperative evaluation of the liver function. Only patients without obvious anemia, coagulopathy, disturbance of internal environment and vital organ dysfunction could be potential candidates, while those with Grade C liver function or MELD scoring above 24 were all excluded. The first criteria of FLR was: ①FLR/Standard liver volume (SLV)<30% in normal liver; ② FLR/SLV<40% in abnormal liver; ③FLR/SLV<20% in colorectal liver metastasis without chemotherapy or<30% with chemotherapy (details of FLR and SLV calculations in next section). The second criteria of FLR was ①FLR/Body weight (BW)>0.5% in normal liver; ②FLR/BW>0.8% in abnormal liver. Liver biopsy and pathological examination were performed for every patient and the liver condition was evaluated according to the NASH CRN scoring system [5–7]: F0 = no fibrosis; F1 = perisinusoidal or portal/periportal fibrosis, F2 = perisinusoidal and portal/periportal fibrosis, F3 = bridging fibrosis, and F4 = cirrhosis. Abnormal liver was defined as F ≥ 2. The third criteria of FLR was jointly used with indocyanine green plasma disappearance rate (ICGPDR), and the cutoff value was defined as [(FLR/SLV) × ICGPDR]<5% [8]. Functional FLR was also performed before ALPPS and compared with FLR (details in next section).
Contraindications of ALPPS were identified as: ①unresectable tumor on FLR; ②unresectable extrahepatic tumor accompanied; ③liver cirrhosis with severe portal hypertension (>70% decrease in portal vein volume, routinely evaluated by Doppler’s ultrasound as preoperative hemodynamics assessment of portal vein for patients with liver cirrhosis); ④unavailable R0 margin on liver; ⑤unendurable to anesthesia or surgical trauma. All these patients were reviewed by a multidisciplinary tumor board consisting of hepatobiliary and colorectal surgeons, medical oncologists, radiation oncologists, pathologists and interventional radiologists and were deemed unresectable by a single-staged operation owing to inadequate FLR. Complications were defined according to the Clavien–Dindo classification, with severe complications defined as grade IIIb and above. [2,5–7]
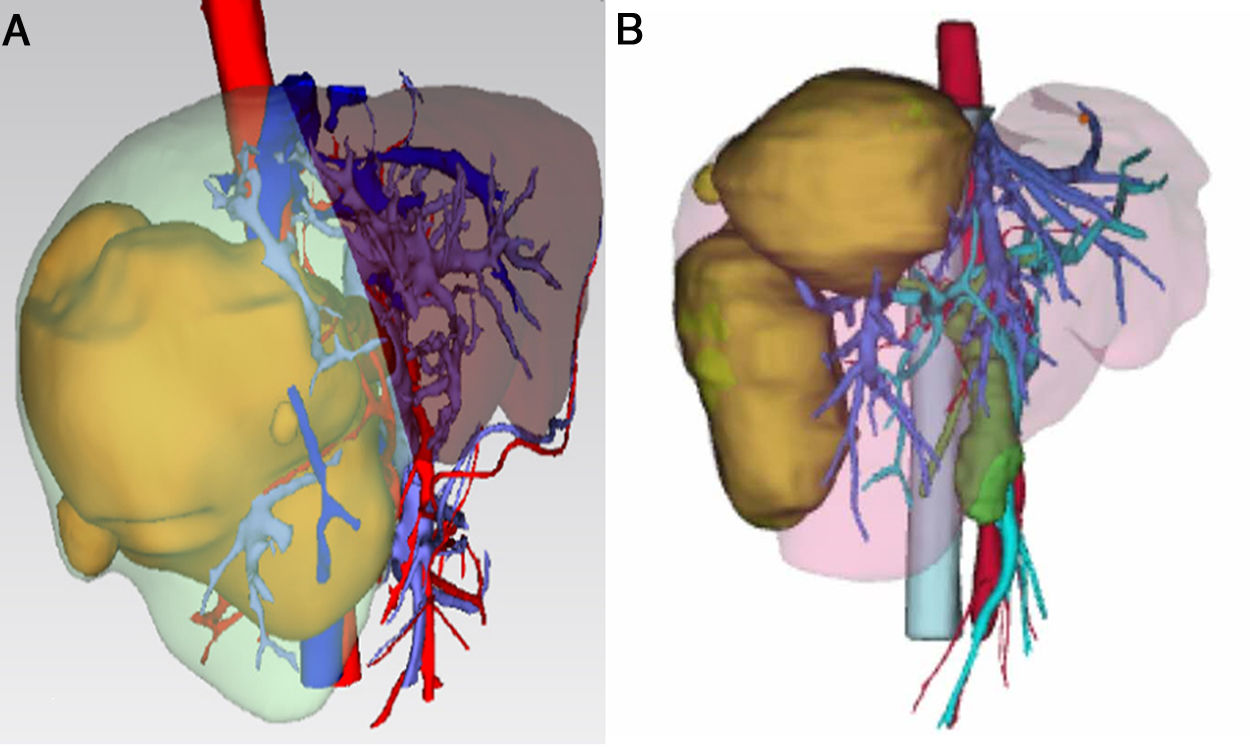
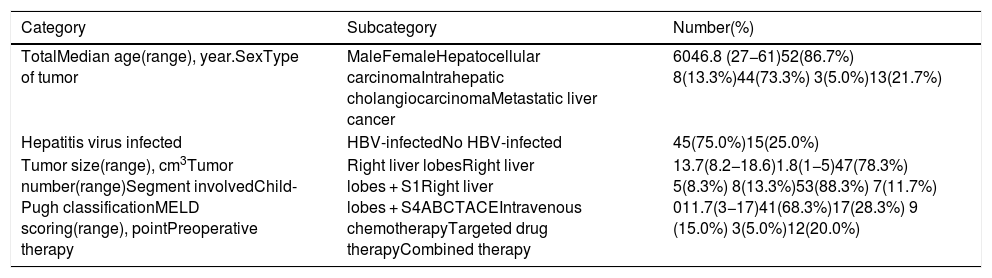
3.1Precise FLR assessment and operation planningBody surface area (BSA) was calculated according to Stevenson’s formula: BSA (m2) = 0.0061×body height (cm) + 0.0128 × BW (kg). SLV was calculated according to Urata’s formula: SLV (mL) = 706.2×BSA+2.4.. FLR/BW ratio reflected the remnant liver weight calculated as percentage of BW, and ratio below the cutoff value (0.5% in normal liver or 0.8% in abnormal liver) proved to be correlated with adverse outcome. [8,9]. Data of FLR was obtained from multi-spiral computed tomography (MSCT) and three-dimensional (3D) imaging reconstruction. A 64-slice MSCT (Siemens, Germany) was used to provide fundamental information for 3D reconstruction. The MSCT scanning parameters were set as a slice thickness of 1.25 mm, a pitch of 0.984 mm, 0.6-sec scanning time per rotation, table speed of 13.5 mm/rotation, and 2 mm interval for reconstruction. Compound meglumine diatrizoate injection (Hansen Pharmaceutical Co.Ltd, China) was infused at 3.5 ml/s for enhanced scanning, and images were obtained at 5 s for arterial phase, at 20 s for portal phase, at 70 s for venous phase after arriving at the peak aortic enhancement. After MSCT examination, all images including pre-contrast, arterial, portal, and venous phases were collected and analyzed using 3D processing software (3D Hepa Vision System, Germany). The region-growing method was used to perform 3D reconstruction of the liver, tumor, pancreas and spleen. The segmentation based on threshold method was used to perform 3D reconstruction of the portal vein, hepatic artery and hepatic vein. Simulated surgery was then performed using the information obtained from the 3D reconstruction, including tumor size, tumor location, proximity and relation of the tumor to its surrounding major blood vessels, and abiding by the principle of R0 resection. Precise preoperative evaluation was based on the anatomy and variations of the portal vein, hepatic artery and hepatic vein according to Cheng's Standard, Michel's Standard, and Nakamura's Standard, and the goal was to achieve complete tumor resection [6–10]. After segmentation of the 3D liver model and simulated surgery were performed using the built-in software, the volumes of the entire liver, tumor, resected liver and FLR were calculated (Fig. 1).
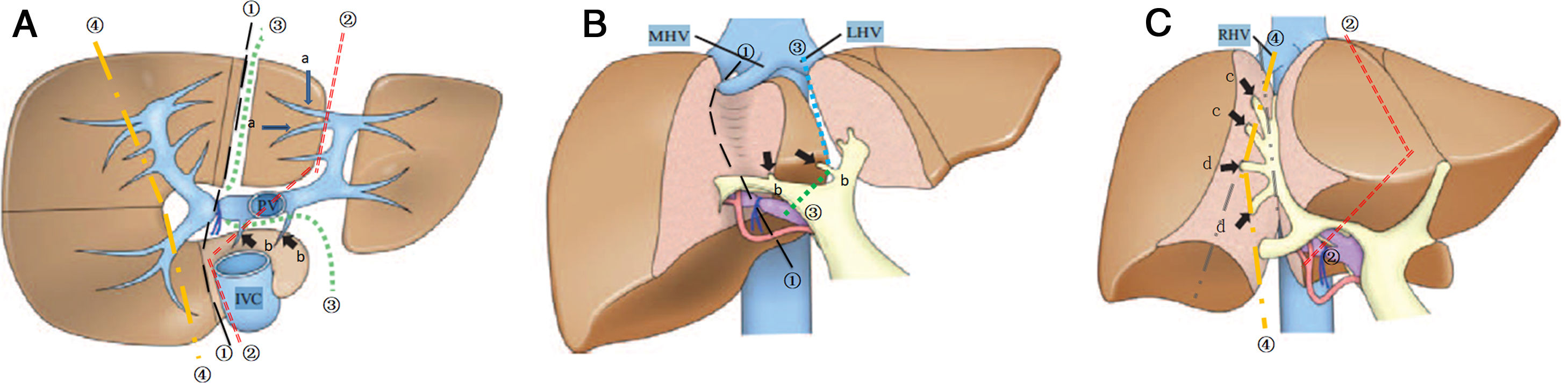
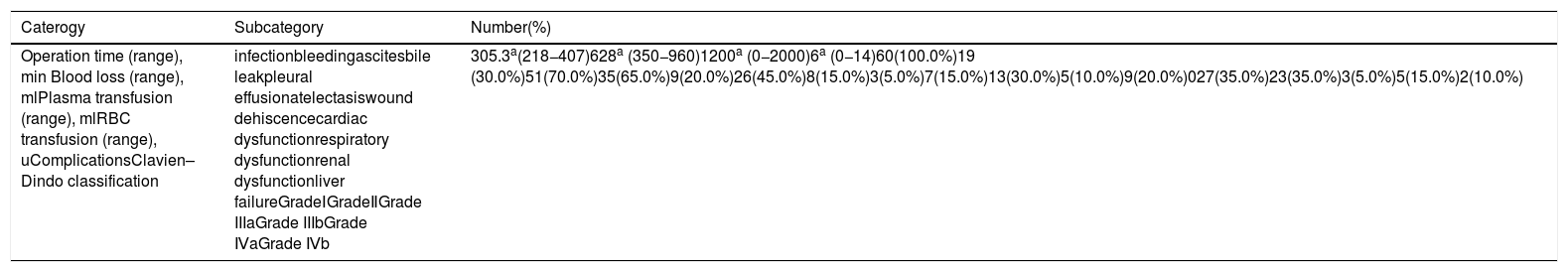
3.2Surgical procedure of ALPPSLaparoscopic in-situ partial parenchyma splitting assisted by RFA was performed as minimally invasive methods in the 1-stage operation. The anterior approach was used to dissect the right hepatic artery, portal vein and bile duct separately in Glisson’s sheath near the porta hepatis. After retrohepatic tunnel was established, through which liver-hanging maneuver could be performed by an elastic strap, liver parenchyma partition was performed using RFA (Habib 4X, RITA 4401 L, Angio Dynamics Inc, USA), guided by laparoscopic ultrasound (LUS) (EUP-OL334, ALOKA, Japan). For patients planned for right hemiliver hepatectomy, only the right portal vein was ligated, and liver parenchyma was divided along the right side of the median fissure just to the right of the middle hepatic vein. For patients plus S4 resection, the liver was divided at the right side of the umbilical portion of the left portal vein, with S1 portal pedicles preserved. For patients plus S1 resection, the liver was divided just to the left of the middle hepatic vein, which continuing towards the ductus venosum with the caudate portal pedicles preserved. For patients planned for extended right lateral sectoriectomy, transection of the liver parenchyma was slightly lateral to the main portal pedicle of the right paramedian sector, preserving the portal pedicles by ligating the portal vein branch of the right lateral sector. [10,11] Details of operation planning were shown in Fig. 2. Working power of Habib 4X was set as 70–80 Watt, and the ablation time in each point was 3−6 min, with a point-to-point distance of 2.5−3 cm and the depth just arriving at the front wall of the strap. Finally 0# absorbable silks (PDS) were left as labels for the right hepatic artery, bile duct, and the retrohepatic tunnel.
The transection line of ablation in the 1-stage operation. A shows transverse section of the liver parenchyma in the 1-stage operation. B and C show coronogram of the 1-stage operation.①indicates the transection line (black short lines) for hepatectomy of right hemiliver; ②indicates the transection line (red dotted line) for right hepatectomy plus S4 resection; ③indicates the transection line (green dotted line) for right hepatectomy plus S1 resection; ④indicates the transection line (yellow short lines) for right lateral sectoriectomy. Arrows(a ) indicate the preserved portal pedicles in S4 for patients undergoing right hepatectomy plus S1 resection. Arrows (b) indicate the preserved portal pedicles in S1 for patients undergoing right hepatectomy plus S4 resection. IVC indicates inferior vena cava; PV, portal vein; LHV, left hepatic vein; MHV, middle hepatic vein; RHV, right hepatic vein.
The 2-stage operation was performed by open surgery, which started from the reverse l-shaped incision under right costal margin. After the right hepatic artery and bile duct were transected, the PDS labeled for the retrohepatic tunnel was lifted, allowing for hepatectomy along the demarcated line. The atrophied lobes were transected until to the anterior wall of the vena cava. After complete hemostasis, silicone drains were placed and the operation was finished.
3.3Liver volume and functional liver volume evaluationMSCT was performed from day 7 after the 1-stage operation to calculate the FLR, and was repeated at an interval of 6–8 days until the FLR and FLR/BW reached the cutoff values, safe enough to perform the 2-stage operation. Fig.3 showed the FLR increase after the 1-stage operation. Kinetic growth rate (KGR), which was defined as the degree of hypertrophy at initial volume assessment divided by number of weeks elapsed after the 1-stage operation, was calculated [12].
Before ALPPS and 1 week after the 1-stage operation, Symbia T16 scanner (Siemens, Germany) was used to perform SPECT for evaluation of functional FLR. Twenty minutes after intravenous injection of sodium phytate, dynamic scintigraphy and SPECT images were obtained with a low-energy, high-resolution collimator. The scanning parameters were set as 128 × 128, and a frame was collected every 25 s. A total of 64 frames of images were collected around the human body. After image reconstruction, the functional liver volume was calculated by classical edge-tracing method and representational pixel method respectively. By means of the edge-tracing method, the region of interest was delineated on each slice image along the liver boundary defined by the threshold, then the number of pixels in each slice was added together, so the liver volume could be calculated as total pixels × edge length per pixel. Representational Pixel Method was used by determining total hepatic radioactivity count and representational pixel radioactivity count firstly, and then liver volume could be calculated as total hepatic radioactivity count ÷ representational pixel radioactivity count × side length per pixel. Average value calculated through both methods was taken as the final result, and the % functional FLR was estimated as the average FLR value/the whole live volume. [13,14] Correlation analysis was performed with FLR.
3.4Follow-up and statistical analysisAll patients were followed up routinely every 4–6 weeks after discharged from our department until death or end of the study. Patients’ continuing medical history and the results of physical examination and laboratory tests were all recorded. Descriptive data was expressed as mean ± SD and median (range values). Patient survival rate was calculated by the Kaplan–Meier method. The significance of difference was determined by the log-rank test. P < 0.05 was considered significant.
4Results4.1Patient characteristicsA total of 60 patients with average age of 44.7 ± 11.2, including 52 males and 8 females, had underwent this modified ALPPS procedures and were included in this retrospective study. Average tumor size was 10.4 ± 5.1 cm. Forty-five patients were HBV-infected with liver cirrhosis, including HCC in 42 patients and intrahepatic cholangiocarcinoma in 3. Two patients were HCC without viral hepatitis, and 13 were hepatic metastases secondary to colorectal cancer. Single tumor was found in 36 patients, while multiple tumors were found in 24. Except for the right liver lobes involved, S1 was invaded in 5 patients, and S4 was invaded in 8. The MELD score averaged 12.4 ± 4.6. According to Child-Pugh classification, 53 patients were grade A and 7 were grade B. Seventeen patients with HBV-infected HCC had underwent preoperational TACE, 3 such patients had underwent preoperational sorafenib therapy, and 9 with colorectal liver metastases had underwent preoperational intravenous chemotherapy. Combined preoperational therapy was performed in 12 patients, including TACE combined with apatinb in 8 with HBV-infected HCC, and intravenous chemotherapy combined with cetuximab in 4 with liver metastases. The baseline characteristics were shown in Table 1.
Characteristics of the patients included in this study.
| Category | Subcategory | Number(%) |
|---|---|---|
| TotalMedian age(range), year.SexType of tumor | MaleFemaleHepatocellular carcinomaIntrahepatic cholangiocarcinomaMetastatic liver cancer | 6046.8 (27−61)52(86.7%) 8(13.3%)44(73.3%) 3(5.0%)13(21.7%) |
| Hepatitis virus infected | HBV-infectedNo HBV-infected | 45(75.0%)15(25.0%) |
| Tumor size(range), cm3Tumor number(range)Segment involvedChild-Pugh classificationMELD scoring(range), pointPreoperative therapy | Right liver lobesRight liver lobes + S1Right liver lobes + S4ABCTACEIntravenous chemotherapyTargeted drug therapyCombined therapy | 13.7(8.2−18.6)1.8(1−5)47(78.3%) 5(8.3%) 8(13.3%)53(88.3%) 7(11.7%) 011.7(3−17)41(68.3%)17(28.3%) 9 (15.0%) 3(5.0%)12(20.0%) |
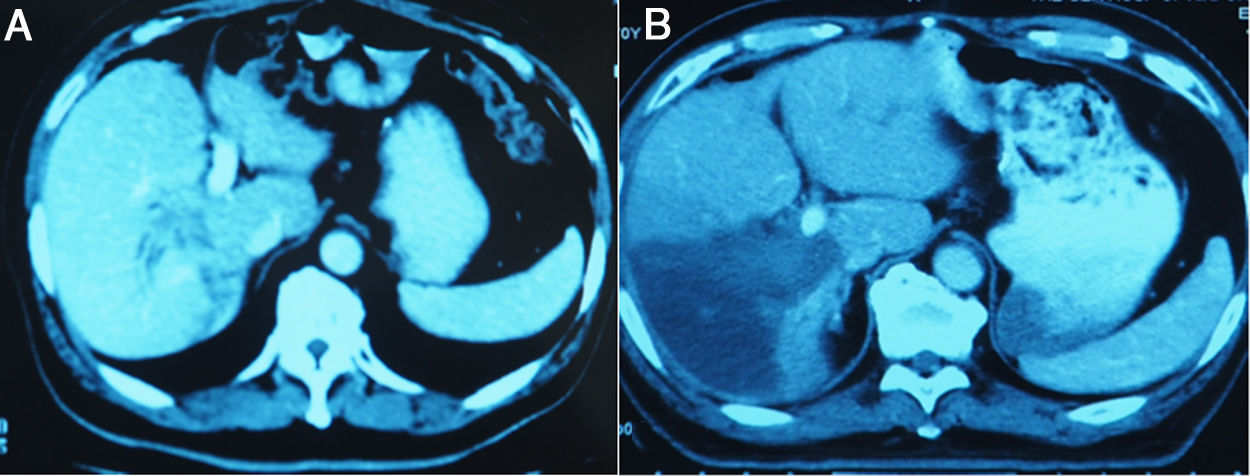
The liver tumors were reconstructed in the 3D way, which included the tumor site and the metastasis situation (Fig. 1).The CT showed the patients’ HCC situation, Fig. 3 was showing one patent’s CT image.
4.2Surgical information and complications of the 1-stage operationThe operation time of the 1-stage operation was 146.3 ± 22.7 min, and the blood loss was 148.5 ± 27.8 mL. The transection line of ablation in the 1-stage operation was showed in the Fig. 2. Drug therapy for liver protection was routinely applied, and elevated ALT/AST could recover in 5–7 days. Thirty-four patients developed various complications, nevertheless no severe or life threatening complications happened. The details were shown in Table 2. Plasma transfusion was performed in 7 patients because of mild intrahepatic bleeding on basis of coagulopathy or ascite related to hypoalbuminemia, but no red blood cell (RBC) transfusion was performed. Infection happened in 9 patients with postoperative fever and white blood cell (WBC) count elevated, in which 6 were diagnosed by CT as intrahepatic bile leak and bile lake formation. They were treated by ultrasound-guided percutaneous drainage, and categorized into Grade IIIa by Clavien–Dindo classification. The other 3 infected patients were given parenteral nutrition for 3–5 days because of accompanied mild hypoalbuminemia and bad appetite, and together with the 7 who underwent plasma transfusions, these 10 patients were categorized into Grade Ⅱ. Hypoumbilical hernia of the Trocar wound happened in 2 patient, partly because of hypoalbuminemia and ascites formation, who were treated by hernia repair under Local anesthesia and albumin transfusion, and were categorized into Grade IIIa.
Outcome of the 1-stage operation of ALPPS.
| Caterogy | Subcategory | Number(%) |
|---|---|---|
| Operation time (range), min Blood loss (range), mlComplicationsClavien–Dindo classification | Infectionbleedingascitesbile leakpleural effusionwound herniaGradeⅠGradeⅡGrade IIIa | 156.8a (102−227)165a (80−280)34(56.7%) 9(15.0%) 5(8.3%)17(28.3%) 6(10.0%) 9(15.0%) 2(3.3%)16(26.7%)10(16.7%) 8(13.3%) |
The preoperative FLR was388.6 cm3 ±122.5 cm3, the preoperative FLR/SLV (% FLR) was 33.2% ±8.6%, and the preoperative FLR/BW was 0.78%±0.14%. The interval day between two stages was 16.4 ± 5.7 d (median of 17.6 d, range of 12–26 d). While FLR before the 2-stage operation was 567.9 cm3±132.3 cm3 and the FLR/SLV before the 2-stage operation was 51.6%±12.7%, and the FLR/ BW before the 2-stage operation was 0.94%±0.22%.So, the increasing volume was 179.3 cm3 ±72.4 cm3 with the increasing ratio of 45.7% ±22.9%. The KGR was 13.2 ± 7.6 cm3/day. These are showed in Table 3. Functional liver volume was calculated using SPECT/CT. Mean % functional FLR volume before ALPPS was 34.7%, similar to the preoperative % FLR/SLV. One week after the first operation, mean % functional FLR was 45.8%, also similar to the % FLR/SLV (47.2%, P = 0.376).
FLR assessment at the interval of two stages of ALPPS.
| Variables | Mean ± SD |
|---|---|
| Preoperative FLRPreoperative FLR/SLVPreoperative FLR/BW. Interval time. FLR before stage 2FLR/SLV before stage 2FLR/ BW before stage 2Increasing volume Increasing ratio KGR | 388.6 ± 122.5 (cm3)33.2 ± 8.6 (%)0.78 ± 0.14 (%)16.4 ± 5.7 (day)567.9 ± 132.3 (cm3)51.6 ± 12.7 (%)0.94 ± 0.22 (%)179.3 ± 72.4 (cm3)45.7 ± 22.9 (%)13.2 ± 7.6 (cm3/day) |
Extended right lateral sectoriectomy was performed in 15 patients, hepatectomy of right hemiliver in 32, right hepatectomy plus S4 resection in 8, and right hepatectomy plus S1 resection in 5. The 2-stage operation time was 287.5 ± 48.2 min, and the blood loss was 615.7 ± 62.4 mL, with intraoperative RBC transfusion in 13 patients. The hospital stay after the 2-stage operation was 23.4 ± 7.4 d, and 10 patients (16.7%) developed severe complications, including 3 of Grade Ⅲb, 5 of Grade Ⅳa, and 2 of Grade Ⅳb. The details were shown in Table 4. Fifty-six patients (93.3%) underwent plasma transfusions during the recovery period (1250 ± 350 mL), of whom 51 underwent RBC transfusion (7.2 ± 3.5 u). Wound dehiscence because of severe hypoproteinemia and ascites happened in 3 patients, and tension-reducing suture under general anesthesia was performed in emergency (Grade Ⅲb). Seven patients were sent to intensive care unit (ICU) because of acute respiratory distress syndrome (ARDS) or acute left heart failure, or acute liver failure (5 of Grade Ⅳa), and multiple organ dysfunction syndrome (MODS, 2 of Grade Ⅳb), and they all recovered well.
Outcome of the 2-stage operation of ALPPS.
| Caterogy | Subcategory | Number(%) |
|---|---|---|
| Operation time (range), min Blood loss (range), mlPlasma transfusion (range), mlRBC transfusion (range), uComplicationsClavien–Dindo classification | infectionbleedingascitesbile leakpleural effusionatelectasiswound dehiscencecardiac dysfunctionrespiratory dysfunctionrenal dysfunctionliver failureGradeⅠGradeⅡGrade IIIaGrade IIIbGrade ⅣaGrade Ⅳb | 305.3a(218−407)628a (350−960)1200a (0−2000)6a (0−14)60(100.0%)19 (30.0%)51(70.0%)35(65.0%)9(20.0%)26(45.0%)8(15.0%)3(5.0%)7(15.0%)13(30.0%)5(10.0%)9(20.0%)027(35.0%)23(35.0%)3(5.0%)5(15.0%)2(10.0%) |
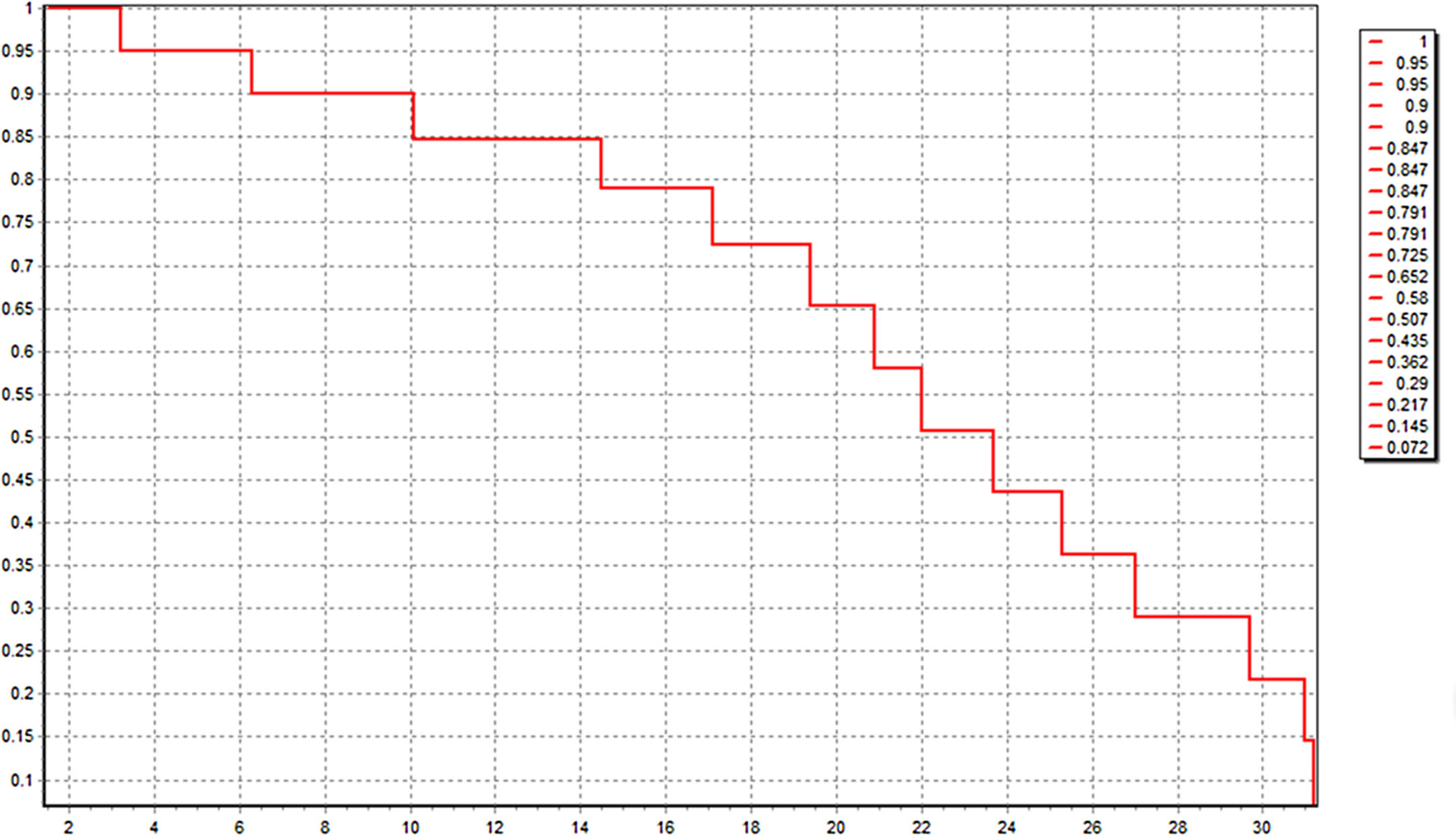
All these 60 patients have been well followed up. The follow-up time was 20.0 ± 2.1 month, and 21 patients (35.0%) were still alive just at the time of data analysis. The median OS was 22.4 (3.2–31.4) month. Kaplan-Meier survival curve was shown in Fig. 4. Six patients of HCC died in 6 months after operation because of lung metastasis or brain metastasis, 2 of colorectal liver metastasis died of cerebro-vascular accident in 12 months after operation, and the other 31 all died of tumor recurrence.
5DiscussionAlthough complete resection remains the best treatment for liver tumor, the concern of no enough FLR to maintain normal liver function or no strategy to induce rapid liver hypertrophy has been the main limitation for radical resection [15,16]. How to make the residual liver reach the standard of safe tumor resection has been a hot spot in the field of liver surgery. The commonly used methods such as TACE, portal vein embolism (PVE), and portal vein ligation (PVL) will take at least 4–6 weeks (8–12 weeks in TACE) to increase the FLR by 20%-40%. Therefore, no more than 10% patients can really take advantages of such methods to undergo radical resection, and many patients may lose the chance of radical resection because of wide metastasis during the long wait for FLR increase [17]. Since the first performance in 2007 by Dr. Schlitt in German, ALPPS has been proved effective in rapid FLR increase, and the reported interval between two stages was only 12.3 d, obviously shorter than post-PVE or post-PVL resection, therefore, the tumor progressionduring the interval was negligible and the resection rate can be definitely improved [18]. However, during the initial ALPPS practice, the 1-stage operation was always performed by right portal vein ligation and complete liver parenchyma disconnection through open surgery, often leading to severe complications and even death, which were the most outstanding defects of ALPPS [6]. The 1-stage operation in initial ALPPS practice produced two large liver stumps and doubled the incidence of bile leakage and severe infection, while disconnection of perihepatic ligaments and liver parenchyma resulted in extensive adhesion, making the 2-stage operation very difficult to perform. Another important factor was that massive bleeding or repeated porta hepatis occlusion during the 1-stage operation had significant impact on functional liver recovery, especially for patients with liver cirrhosis. [19,20] Therefore, ALPPS had once been tentatively practiced in few institutions and only for patients of metastatic liver tumors with normal liver condition. Most surgeons believed that the key to reduce the morbidity and mortality lied in the balance of rapid FLR increase and trauma reduction of the 1-stage operation [21]. In this study, we provided a feasible risk-reduced strategy for ALPPS modification and obtained ideal results for patients with different degrees of liver damage.
In patient selection, we didn’t exclude those with liver cirrhosis deliberately, but put emphasis on the preoperative evaluation of liver function and performed ALPPS for patients with huge HCC in Grade B liver condition. On one hand we combined Child-Pugh classification and MELD scoring methods to exclude those unendurable to liver surgery and used parameters of FLR/SLV and FLR/BW as indications for ALPPS, on the other hand, we tested functional FLR and ICGPDR of FLR to accurately evaluate residual liver function and find potential ALPPS candidates. This modified patient selection expanded the range of indications for ALPPS, especially in advanced fibrotic liver or liver cirrhosis, and decreased the incidence of PHLF. Therefore our study contained not only the largest cases (n = 60), but also the highest proportion of HBV-infected liver fibrosis or cirrhosis (75.0%) through literature review up to February 2020. Precise FLR assessment and operative planning had provided basis for the modified patient selection. By means of 3D reconstruction, meticulous liver anatomy and intrahepatic vascular systems were reconstructed, and could be presented individually or as a whole. Therefore, the crucial information for ALPPS, including the blood supply system of tumor, the reflux system of the segments occupied by tumor, and the outline of FLR could be taken into careful account before operation [22]. Not only the arterial blood supply, portal vein supply and hepatic vein circumfluence of the tumor could be observed, but also the distance between tumor and the secondary or tertiary branches of hepatic artery and portal vein could be measured accurately, which determined the surgical methods and resection range, and ensured the venous return of the residual liver [23]. The purpose of ALPPS is to remove the liver tumor as clean as possible, so FLR assessment and operation planning should be more accurately. In this study, we used 3D reconstruction to extract the central line from the branches of portal vein and calculate the blood supply by Nearest Neighbor Approximate Segmentation (NNAS) method, thus assessed the FLR and designed operations of each stage more accurately according to Couniaud segmenting principle. [23]
It has been reported that partial disconnection of the liver parenchyma (50% -80%) could achieve the same effect of rapid liver hyperplasia as complete disconnection, which was also proved by animal models [24–26]. Based on this theory, we used RFA to take place of the complete liver partition in the 1-stage operation. Guided by LUS, a blood-free zone formed between healthy and diseased lobes without liver cross-section formation, thus decreased the incidence of bile leak and blood loss, and provided ideal condition for the 2-stage operation. The mean operation time was only146.3 min, and median blood loss was only 148.5 mL, without severe complications occurring or RBC transfusion performed. Liver damage was mild and recovery was fast. In our study, the mean FLR before the 2-stage operation was 567.9 cm3 and the mean FLR/SLV before the 2-stage operation ratio was 51.6%. The mean increasing volume was179.3 cm3 and the mean increasing ratio was 45.7%. Although the increasing ratio was slightly less than some studies, functional FLR increase was evident and it was enough for patients to endure this procedure. The mean interval day in our study was 16.4 days, which was also little longer than the interval of initial ALPPS but much less than the interval of 4–6 weeks for PVE. Just as literature reported, the overall incidence of postoperative complications and mortality of ALPPS were 16%-64% and 12%-23%, and nearly two thirds of the patients who had underwent ALPPS had no liver cirrhosis [20,22,25]. While in this study, although two thirds patients were HBV-related liver cirrhosis, even the liver function was Grade B in 7 (11.7%) of them, all patients underwent the 2-stage operation successfully. No patient died in the perioperative period. The primary deadly reason for initial ALPPS was bile leakage combined with severe infection, while in comparison, except 3 patients of wound dehiscence, severe complications after the 2-stage operation were mainly connected with the surgical trauma or liver cirrhosis.
The median OS ofthis modified ALPPS group was 22.0 months, similar with literature report, but in consideration of the clinical staging of liver tumor and the factor of liver cirrhosis, we can conclude that this modified ALPPS procedure has a satisfactory long-term survival. It was reported by international ALPPS registry that the duration of the 1-stage operation and intraoperative RBC transfusion were independent risk factors for OS [27], which could also be reflected in our risk-reduced strategy The minimally invasive methods of laparoscopic in-situ partial splitting of liver parenchyma using RFA guided by LUS in the 1-stage operation, the combined use of which were rarely reported in literature, decreased bleeding, reduced squeezing and twisting of the tumor, and achieved coagulative necrosis of the liver tissue by delivering high amount of thermal energy, thus avoided intraoperative tumor metastasis.
Because of the retrospective and cross-sectional nature of this single-center study, control group was lack and many sources of bias couldn’t be avoided. The long-term survival would be more convincing if randomized controlled study could be conducted. Although ALPPS with modified procedure has been increasing in thses years, most literature only report ALPPS in strictly defined and selected patients containing no more than 20 cases. ALPPS is still not mature, and surgeons are still trying their best to standardize this procedure. We will cooperate with multiple centers and collect more appropriate cases to conduct randomized controlled trial.
6ConclusionsAccording to this study, ALPPS could be a feasible treatment for patients with huge live tumors by risk-reduced modification, and it could be expected to provide a long-term survival for patients without enough FLR. We present more of our experience with the use of this strategy for selected patients, and demonstrate a satisfactory effect.
Declaration of interest statementNo benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
We thank Dr. Ya-Lin Kong and Dr. Hong-Yi Zhang of Chinese PLA Air Force Medical Center (Chinese PLA Air Force General Hospital) for help with the study design and data collection.