Non-alcoholic fatty liver disease (NAFLD) is a widespread chronic liver disease. It is considered a multifactorial disorder that can progress to liver fibrosis and cause a worldwide public health concern. Coffee consumption may have a protective impact on NAFLD and liver fibrosis. However, the evidence from the previous studies is inconsistent. This meta-analysis summarizes available literature.
Materials and methodsThis study comprises two meta-analyses. The first meta-analysis summarizes the effect of coffee consumption on NAFLD in those who did or did not drink coffee. The second analysis compares the risk of liver fibrosis development between NAFLD patients who did or did not drink coffee. Pooled risk ratios (RR) and confidence intervals (CI) of observational studies were estimated.
ResultsOf the total collected 321 articles, 11 met our eligibility criteria to be included in the analysis. The risk of NAFLD among those who drank coffee compared to those who did not was significantly lower with a pooled RR value of 0.77 (95% CI 0.60–0.98). Moreover, we also found a significantly reduced risk of liver fibrosis in those who drink coffee than those who did not drink in the NAFLD patients with the relative risk (RR) of 0.68 (95% CI 0.68–0.79).
ConclusionsRegular coffee consumption is significantly associated with a reduced risk of NAFLD. It is also significantly associated with decreased risk of liver fibrosis development in already diagnosed NAFLD patients. Although coffee consumption may be considered an essential preventive measure for NAFLD, this subject needs further epidemiological studies.
non-alcoholic fatty liver disease
confidence interval
relative risk
hazard ratio
odds ratio
hepatitis B virus
hepatitis C virus
body mass index
alanine aminotransferase
aspartate aminotransferase
gamma-glutamyl transferase
homeostasis model assessment of insulin resistance
chlorogenic acid
Non-alcoholic fatty liver disease (NAFLD) is currently one of the leading causes of chronic liver disease and elevated liver enzymes in western countries and is characterized by excessive fat deposition in the liver [1,2]. It affects 15–30% of the general population worldwide and approximately 100 million Americans, and it is now considered the second-most common indication for liver transplantation in the United States [3–5]. NAFLD is subdivided into the non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH) based on the presence of inflammation in the liver on histology [6]. NAFL is characterized by the presence of hepatic steatosis without hepatocellular injury. On the other hand, NASH is defined as NAFL, along with the hepatocellular injury with or without hepatic fibrosis. NASH can progress into liver cirrhosis and liver carcinoma [7,8]. The rapidly increasing rates of obesity and metabolic syndrome are the most common risk factors for NAFLD development [9,10]. Several medications, such as pentoxifylline, vitamin E, pioglitazone, and metformin, have been reported to improve the histological features of NAFLD. However, there is minimal evidence to support the efficacy of these drugs [11,12]. None of the therapeutic agents have been approved by the US Food and Drug Administration for NAFLD. Lifestyle modification interventions such as diet control, weight loss, physical activity, and behavioral modification are considered the only potential treatment strategies [7].
Coffee is the most popular beverage worldwide, and it has been estimated that at least half of the adult US population drinks coffee daily [13]. Interestingly, coffee consumption is associated with a reduced risk of metabolic syndrome and type II diabetes [14]. Several epidemiological studies have demonstrated a hepatoprotective effect of coffee consumption on different liver conditions such as NAFLD, liver fibrosis, and hepatocellular carcinoma with inconsistent results. We carried out a detailed systematic review and meta-analysis to validate this probable association in the current study.
2Materials and methods2.1Study selectionThe study methodology was designed and executed to adhere to Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines. We conducted a comprehensive literature search indexed on Google Scholar, Cochrane database, and PubMed of the English language articles published until April 2020. We used the following search terms: “Coffee,” “coffee consumption and non-alcoholic fatty liver disease,” “fatty liver,” “non-alcoholic fatty liver,” and “non-alcoholic liver steatohepatitis.” Additionally, the reference list of published meta-analysis or review articles and included manuscripts were also examined. A detailed flow diagram of the included literature is presented in Fig. 1.
2.2Inclusion criteriaPublished studies with the following criteria were included: (a) case–control, prospective cohort studies, and cross-sectional studies; (b) the epidemiological studies which were published as original articles to evaluate the impact of coffee consumption on NAFLD, or the effect of coffee consumption on the presence of liver fibrosis among the already diagnosed NAFLD patients; (c) odds ratio, hazard ratio, and relative risk or standardized incidence ratio with 95% (CI) were provided. Studies with insufficient statistics were excluded. Two investigators independently determined the study eligibility, and a third reviewer resolved the disagreement on any review. The quality of the study was independently evaluated by each investigator using the Newcastle-Ottawa quality assessment scale (NOS). For cross-sectional studies, we used modified Newcastle-Ottawa for quality assessment [15].
2.3Data extractionWe used a standard extraction method to collect the following data information from the studies: title of the study, year of the study, name of the first author, year of publication, country of original study data, number of total study population along with the number of cases and controls, demographic characteristics of study cohorts, method of identification of coffee consumption among the study population, procedures to verify coffee consumption, presence of NAFLD and degree of liver fibrosis among participants, adjusted effect estimates with 95% confidence interval, and lastly the covariates adjusted in the multivariate analysis. The independent data extraction performed by all investigators further ensured accuracy and reduced bias. Moreover, any data discrepancy was removed by referring to the original article included.
2.4Quality assessment of the studies included and publication biasAmong the study articles included in the first analysis, five articles had NOS of 7–8; however, two articles had six stars according to NOS criteria. As a self-administered questionnaire measuring the coffee intake in all these articles, there was the possibility of reporting bias. Moreover, ultrasonography was the method of diagnosis of NAFLD or NASH in five articles, while liver enzymes (alanine aminotransferase) fluctuation was used as a marker of liver injury in two articles, and one article was based on liver histology and liver alanine aminotransferase. Egger's linear regression test was performed to look for any publication bias. No evidence of publication bias was found (p=0.01).
The study articles included in the second meta-analysis had the Newcastle-Ottawa Scale (NOS) score of 8. Three articles assessed the pre-exposure presence of NAFLD by liver ultrasound, while Bambha et al. used liver biopsy to confirm NAFLD. After exposure, the presence of liver fibrosis was assessed by liver biopsy by Bambha et al., and Anty et al. Additionally, fibroscan was used by Zeiber-Sagi et al. and Soleimani et al. to assess the post-exposure liver fibrosis.
Since the secondary analysis has only four articles, a linear regression test was not performed.
2.5Statistical analysisAs the outcomes of interest (NAFLD, Liver fibrosis) were uncommon, we roughly regarded HR of the cohort studies and OR of the case–control studies as an estimate for relative risk (RR) to improve the precision of the pooled effect estimate. Data analysis was carried out using Review Manager 4.0 software. We used a random-effect model rather than a fixed-effect model to address the high likelihood of between-study variance, as the articles included have different study designs and populations. Cochrane's Q test and I2 statistics were used to measure heterogeneity, which is calculated as the weighted sum-of-squared differences between individual study effects and the pooled effect across studies. A value of I2 0–25% represents an insignificant between-study heterogeneity. More than 25% and less than 50% is a low heterogeneity, and more than 50% to less than 70% is moderate. Any value above 75% represents high between-study heterogeneity.
3ResultsOur search identified a total of 321 articles after the removal of duplicates. After title and abstract screening, 70 articles were included in the full-text read category. According to our inclusion and exclusion criteria, the articles removed incorporated case reports, letters to the editor, review articles, interventional studies. Moreover, studies that did not report the outcomes of interest were also removed, leaving only 11 studies in our meta-analysis. Manual review of the included studies’ references and the selected review articles did not yield any additional research of interest.
There were three case-control studies among the 11 articles selected, four cross-sectional and the remaining four were prospective cohort. Five researchers were from the USA, two from Japan, one from France, one from Iran, and one from Israel. The total study population included 6519 cases and 66,561 non-cases [16–25]. We ran two separate analyses for NAFLD and liver fibrosis regarding coffee consumption.
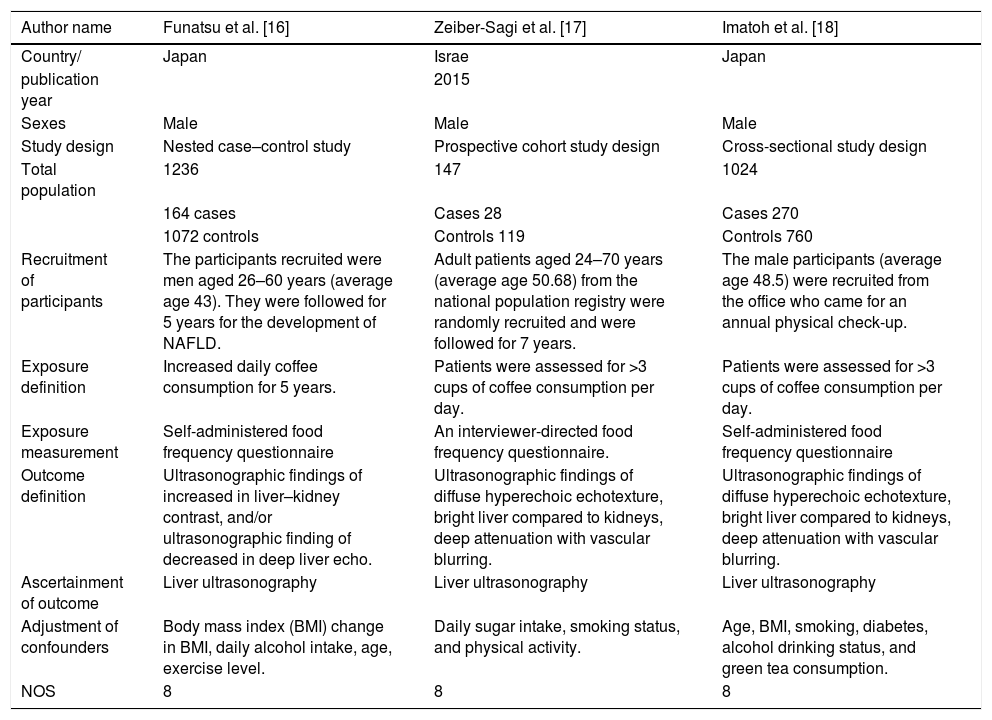
3.1The effect of coffee consumption on non-alcoholic fatty liver disease (NAFLD)Seven epidemiological studies with 71,787 participants with an age range of 20–70 years were included in the first meta-analysis (Table 1). Among the included studies, four were cohort, two were case–control, and one was a cross-sectional study. The participants were recruited from the individual clinics and national population registries. Ruhl et al. and Birendinc et al. recruited the participants from the third US National Health and Nutritional Survey (NHANES). Most of the studies assessed their participants for the presence of NAFLD by liver ultrasonography before the coffee exposure; however, Ruhl et al. and Birendinc et al. used elevated alanine aminotransferase (ALT) as a measure of liver injury (NAFLD) in their study populations. The study participants were asked through a questionnaire about coffee exposure as the number of cups (0 to >3) consumed per day. NAFLD was the outcome of interest ascertained by the liver ultrasound in five studies except for Ruhl et al. and Birendinc et al., who determined the outcome by measuring the change in liver enzymes (Table 1). Studies were adjusted for the confounders, including body mass index (BMI) change in BMI, daily alcohol intake, daily sugar intake, sex, physical activity, age, smoking, diabetes, alcohol drinking status, education status, green tea consumption, and risk factors for liver injury. Newcastle-Ottawa Scale (NOS) was used to assess the quality of all included studies.
Main characteristics of the studies included in the meta-analysis of the impact of the coffee consumption and the presence of non-alcoholic fatty liver disease (NAFLD).
| Author name | Funatsu et al. [16] | Zeiber-Sagi et al. [17] | Imatoh et al. [18] |
|---|---|---|---|
| Country/ | Japan | Israe | Japan |
| publication year | 2015 | ||
| Sexes | Male | Male | Male |
| Study design | Nested case–control study | Prospective cohort study design | Cross-sectional study design |
| Total population | 1236 | 147 | 1024 |
| 164 cases | Cases 28 | Cases 270 | |
| 1072 controls | Controls 119 | Controls 760 | |
| Recruitment of participants | The participants recruited were men aged 26–60 years (average age 43). They were followed for 5 years for the development of NAFLD. | Adult patients aged 24–70 years (average age 50.68) from the national population registry were randomly recruited and were followed for 7 years. | The male participants (average age 48.5) were recruited from the office who came for an annual physical check-up. |
| Exposure definition | Increased daily coffee consumption for 5 years. | Patients were assessed for >3 cups of coffee consumption per day. | Patients were assessed for >3 cups of coffee consumption per day. |
| Exposure measurement | Self-administered food frequency questionnaire | An interviewer-directed food frequency questionnaire. | Self-administered food frequency questionnaire |
| Outcome definition | Ultrasonographic findings of increased in liver–kidney contrast, and/or ultrasonographic finding of decreased in deep liver echo. | Ultrasonographic findings of diffuse hyperechoic echotexture, bright liver compared to kidneys, deep attenuation with vascular blurring. | Ultrasonographic findings of diffuse hyperechoic echotexture, bright liver compared to kidneys, deep attenuation with vascular blurring. |
| Ascertainment of outcome | Liver ultrasonography | Liver ultrasonography | Liver ultrasonography |
| Adjustment of confounders | Body mass index (BMI) change in BMI, daily alcohol intake, age, exercise level. | Daily sugar intake, smoking status, and physical activity. | Age, BMI, smoking, diabetes, alcohol drinking status, and green tea consumption. |
| NOS | 8 | 8 | 8 |
| Author name | Catalano et al. [19] | Wendy et al. [20] | Ruhl et al. [21] |
|---|---|---|---|
| Country | US | US | US |
| publication year | 2010 | 2017 | 2005 |
| Sexes | Male 147 | Male | Male |
| Female 163 | Female | ||
| Study design | Case–control study | Prospective cohort study design | Prospective cohort study design |
| Total population | 310 | 44,576 | 5944 |
| 157 cases | 2786 cases | 508 cases | |
| 153 controls | 41,790 controls | 5436 controls | |
| Recruitment of participants | Adult patients (average age 48.7) with a history of gastroenterology disease were recruited from a gastroenterology and nutrition unit clinic. | The participants recruited were men aged 45–75 years (average age 61), who were enrolled in multiethnic Medicare free for service. | Adult patients >20 years. were recruited from the third US National Health and Nutritional Survey (NHANES) from 1984 to 1994. |
| Exposure definition | Patients were assessed for 0 to >3 cups of coffee consumption per day. | Patients were assessed for 0 to >3 cups of coffee consumption per day. | Patients were assessed for 0 to >3 cups of coffee consumption per day. |
| Exposure measurement | Calculated average cups of coffee consumed/day | Standardized quantitative food frequency questionnaire | A self-reported questionnaire was used. |
| Outcome definition | Ultrasonographic appearance of fatty liver assessed as a bright liver score (BLS) >1. | A decrease in liver enzyme function and reduced histological activity. | A decrease in the liver enzyme (ALT). |
| Ascertainment of outcome | Liver ultrasonography | Liver enzyme, Liver histology. | Measurement of a liver enzyme (ALT) |
| Adjustment of confounders | Alcohol intake, diabetes, Liver enzymes such as ALT. | BMI, diabetes, daily alcohol intake, education status, smoking status. | Age, sex, race, smoking status, physical activity, and risk factors for liver injury. |
| NOS | 7 | 6 | 6 |
| Author name | Birerdinc et al. [22] |
|---|---|
| Country/ | US |
| publication year | 2011 |
| Sexes | Male |
| Study design | Cross-sectional study design |
| Total population | 18,550 |
| 1782 cases | |
| 16,768 controls | |
| Recruitment of participants | Adult patients (average age 55) were recruited from the third US National Health and Nutritional Survey (NHANES) from 2001–2008. In this study, NAFLD was defined as an elevation of liver aminotransferases. |
| Exposure definition | Patients were assessed for >3 cups of coffee consumption per day. |
| Exposure measurement | Self-administered dietary intake questionnaire |
| Outcome definition | A decrease in liver enzymes. |
| Ascertainment of outcome | Measurement of liver aminotransferases. |
| Adjustment of confounders | Age, gender, ethnicity, diabetes, and metabolic syndrome, alcohol drinking status. |
| NOS | 7 |
NAFLD: non-alcoholic fatty liver disease. BLS: bright liver score. BMI: body mass index. ALT: alanine aminotransferase.
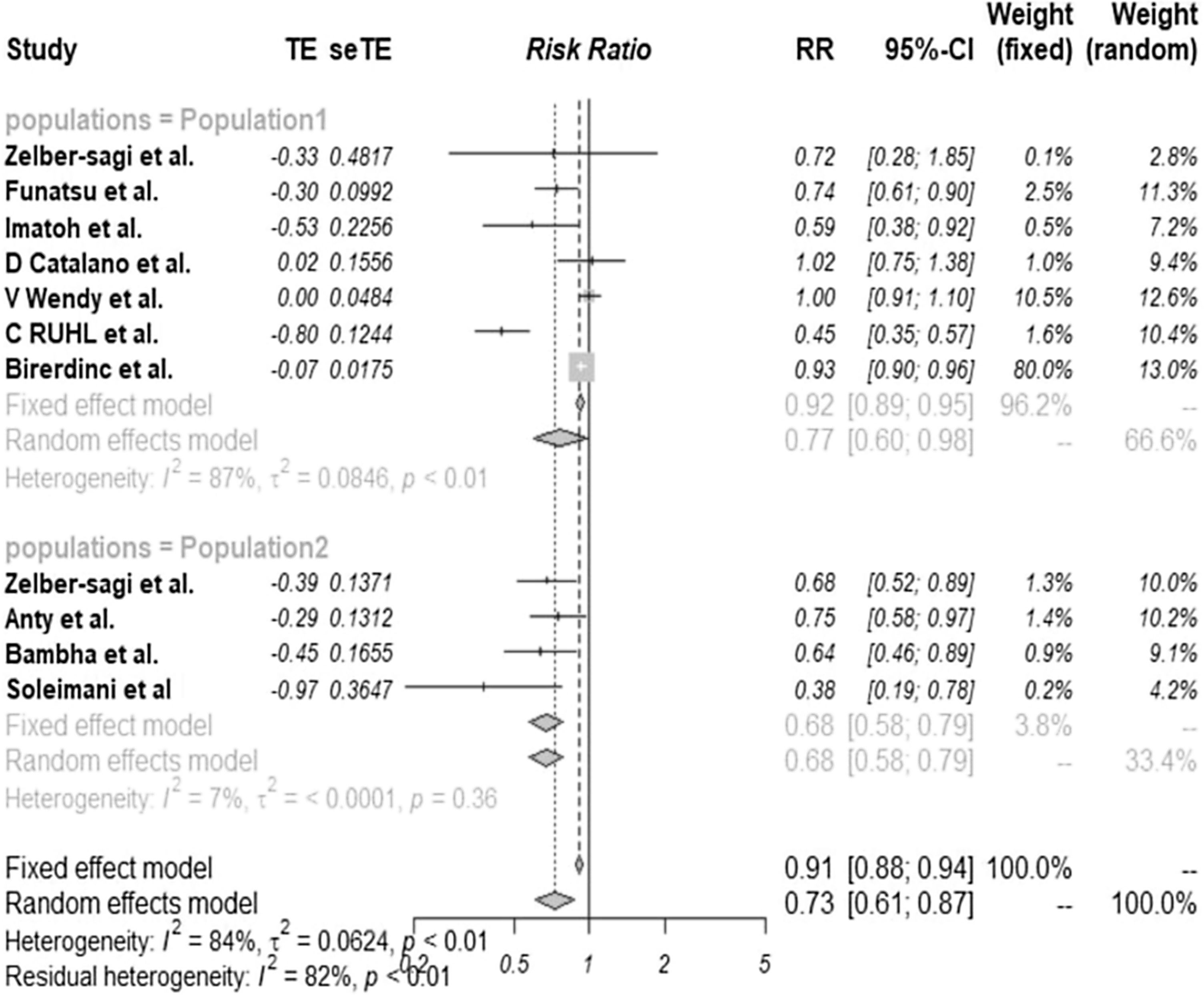
We observed a significantly decreased risk of NAFLD among the patients who drank coffee regularly than those who did not, with a pooled risk ratio of 0.77 (95% CI 0.60–0.98). The statistical heterogeneity was significant, with the I2 of 87% (Fig. 2, population 1). The clinical characteristics of these articles and the quality assessment have also been described in Table 1.
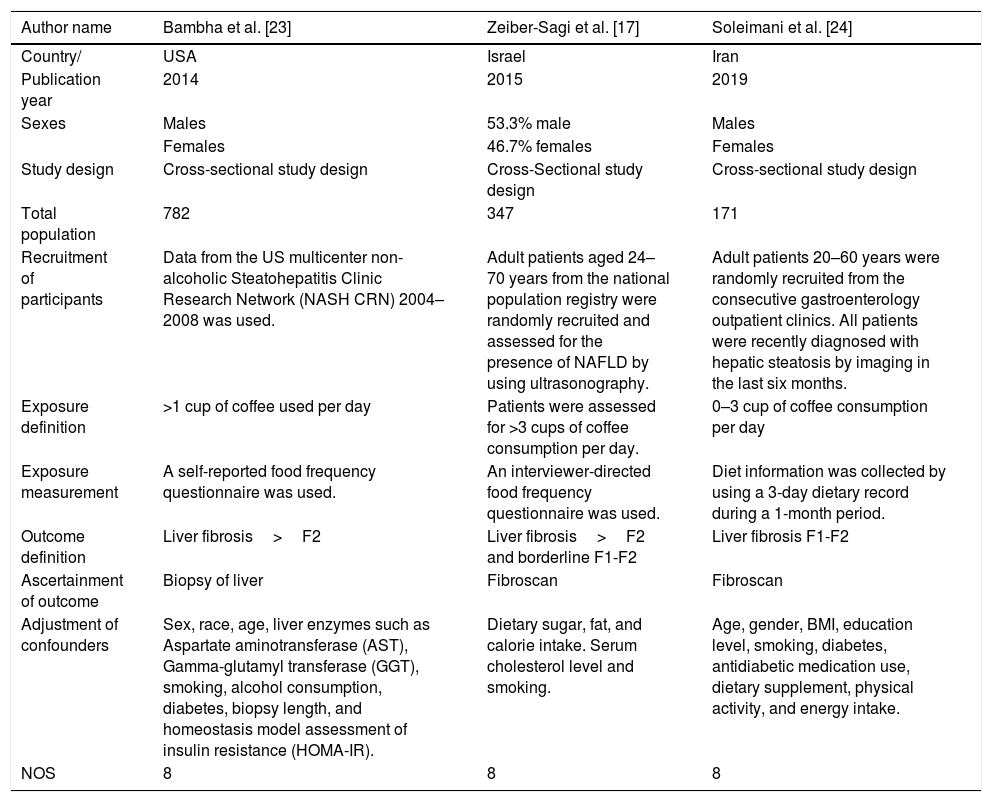
3.2The effect of coffee consumption on liver fibrosisFour cross-sectional studies, including 1338 participants with an age range of 20–70 years, were included in this meta-analysis (Table 2). The participants were recruited from the individual clinics except for Bambha et al., which used data from the US multicenter non-alcoholic Steatohepatitis Clinic Research Network (NASH CRN) 2004–2008. The participants were assessed for the presence or absence of NAFLD by liver ultrasonography before the coffee exposure; however, Bambha et al. included participants with biopsy-confirmed NAFLD. The study participants were asked through a questionnaire about coffee exposure as the number of cups (0 to >3) consumed per day. Liver fibrosis was the outcome of interest ascertained by the liver biopsy by Bambha et al. and Anty et al. Moreover, Zeiber-Sagi et al. and Soleimani et al. assessed the presence of liver fibrosis by liver fibroscan after coffee exposure (Table 2). According to the NASH Clinical Research Network, Scoring System, outcome liver fibrosis was defined as F>2. Moreover, all the studies were adjusted for the confounders including body mass index (BMI) change in BMI, daily alcohol intake, daily sugar intake, sex, gender, dietary fat, calorie intake, serum cholesterol level, smoking status, physical activity, age, diabetes, alcohol drinking status, education status, liver enzymes such as Aspartate aminotransferase (AST), Gamma-glutamyl transferase (GGT), biopsy length, and homeostasis model assessment of insulin resistance (HOMA-IR). Newcastle-Ottawa Scale (NOS) was used to assess the quality of all included studies.
Main characteristics of the studies included in the meta-analysis of the impact of coffee consumption on liver fibrosis in patients with existing non-alcoholic fatty liver disease (NAFLD).
| Author name | Bambha et al. [23] | Zeiber-Sagi et al. [17] | Soleimani et al. [24] |
|---|---|---|---|
| Country/ | USA | Israel | Iran |
| Publication year | 2014 | 2015 | 2019 |
| Sexes | Males | 53.3% male | Males |
| Females | 46.7% females | Females | |
| Study design | Cross-sectional study design | Cross-Sectional study design | Cross-sectional study design |
| Total population | 782 | 347 | 171 |
| Recruitment of participants | Data from the US multicenter non-alcoholic Steatohepatitis Clinic Research Network (NASH CRN) 2004–2008 was used. | Adult patients aged 24–70 years from the national population registry were randomly recruited and assessed for the presence of NAFLD by using ultrasonography. | Adult patients 20–60 years were randomly recruited from the consecutive gastroenterology outpatient clinics. All patients were recently diagnosed with hepatic steatosis by imaging in the last six months. |
| Exposure definition | >1 cup of coffee used per day | Patients were assessed for >3 cups of coffee consumption per day. | 0–3 cup of coffee consumption per day |
| Exposure measurement | A self-reported food frequency questionnaire was used. | An interviewer-directed food frequency questionnaire was used. | Diet information was collected by using a 3-day dietary record during a 1-month period. |
| Outcome definition | Liver fibrosis>F2 | Liver fibrosis>F2 and borderline F1-F2 | Liver fibrosis F1-F2 |
| Ascertainment of outcome | Biopsy of liver | Fibroscan | Fibroscan |
| Adjustment of confounders | Sex, race, age, liver enzymes such as Aspartate aminotransferase (AST), Gamma-glutamyl transferase (GGT), smoking, alcohol consumption, diabetes, biopsy length, and homeostasis model assessment of insulin resistance (HOMA-IR). | Dietary sugar, fat, and calorie intake. Serum cholesterol level and smoking. | Age, gender, BMI, education level, smoking, diabetes, antidiabetic medication use, dietary supplement, physical activity, and energy intake. |
| NOS | 8 | 8 | 8 |
| Author name | Anty et al. [25] |
|---|---|
| Country/ | France |
| publication year | 2012 |
| Sexes | Males 34 |
| Females 161 | |
| Study design | Cross-sectional study design |
| Total Population | 38 |
| Recruitment of participants | Adult patients with a history of NAFLD were recruited from the bariatric clinic. They were assessed for NAFLD by using ultrasonography. |
| Exposure definition | Patients were assessed for 0–3 cups of regular coffee and espresso consumption per day |
| Exposure measurement | An interviewer-directed food frequency questionnaire was used. |
| Outcome definition | Liver fibrosis>F2 |
| Ascertainment of outcome | Liver biopsy during bariatric surgery |
| Adjustment of confounders | Metabolic syndrome, liver enzymes such as AST, HOMA-IR. |
| NOS | 8 |
NAFLD: non-alcoholic fatty liver disease. BMI: body mass index. ALT: alanine aminotransferase. AST: aspartate aminotransferase. GGT: gamma-glutamyl transferase. HOMA-IR: homeostasis model assessment of insulin resistance.
NASH Clinical Research Network Scoring System Definition and Scores in Study set: where; F0: no fibrosis. F1a: mild sinusoidal fibrosis in zone 3. F1b: moderate zone 3 sinusoidal fibrosis. F1c: periportal sinusoidal fibrosis. F2: periportal sinusoidal fibrosis and zone 3 sinusoidal fibrosis. F3: bridging fibrosis in the liver. F4: cirrhosis.
We found a significantly reduced risk of liver fibrosis in patients with already diagnosed NAFLD who consumed coffee regularly compared to those who did not. The pooled risk ratio was 0.68 (95% CI 0.68–0.79). The statistical heterogeneity was insignificant here, with the I2 of 7% (Fig. 2, population 2). The clinical characteristics of these articles with the quality assessment score are described in Table 2.
4DiscussionThis study assessed the potential protective effect of coffee consumption on the development and progression of NAFLD. Our meta-analysis results revealed a 23% decreased risk of development of NAFLD among those who drank coffee regularly; this relationship was found to be statistically significant. Furthermore, in patients who had a previous diagnosis of NAFLD, there was a 32% reduced risk in developing fibrosis in patients who drank coffee daily compared to those who did not.
This protective effect of coffee was statistically significant, and numerous research trials support the potential beneficial impact of coffee for reducing liver fibrosis [26,27,40]. Studies have shown that high coffee consumption plays a protective role in non-alcoholic liver disease and significantly reduces fibrosis risk among those already diagnosed with NASH [28]. Moreover, more than two cups of coffee consumption per day were significantly associated with the lower risk of liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [29,30]. It is also associated with decreased mortality due to chronic liver diseases [30]. A retrospective cross-sectional study has shown the effect of coffee consumption on relieving the liver stiffness, suggesting less fibrosis and inflammation in patients with liver diseases such as NAFLD, HBV, and HCV [31]. Of note, Anty et al. performed multivariate analysis to find the types of coffee as the independent parameters associated with liver fibrosis. They reported that caffeine intake from regular coffee consumption is an independent and significant factor and is protective against liver fibrosis compared to other caffeine sources such as espresso coffee, soft drinks, chocolate, and tea [25].
Nevertheless, two previous studies were unable to establish the correlation of the effect of coffee consumption and the risk of NAFLD and liver fibrosis. The first meta-analysis revealed that coffee drinkers have a lower risk of developing NAFLD. They are at reduced risk of developing liver fibrosis in the presence of chronic liver diseases. However, the meta-analysis was comprised of only three studies to support such association [32]. The limitation of the number of studies included in this meta-analysis makes the results more conservative. The second meta-analysis demonstrated a dose-response relationship between coffee intake and reduced risk of NAFLD [33]. According to this study, more than three cups of coffee per day were associated with the reduced risk of non-alcoholic fatty liver disease (NAFLD). They concluded that the risk of development of NAFLD was inversely associated with coffee consumption; however, they did not focus on the association of dose of coffee consumption and liver fibrosis in already diagnosed NAFLD patients [33].
In contrast, our meta-analysis included seven studies to elucidate the hepatoprotective effects of coffee consumption on NAFLD. We also included four other studies demonstrating the beneficial effect of coffee on liver fibrosis in patients who have already been diagnosed with NAFLD. Therefore, our present study is unique because we have included more updated articles with a significantly higher patient population, thus increasing the study's strength. The numerical significance, along with the quality of the included articles, explicated the protective effect of coffee on NAFLD and fibrosis, consequently distinguishing our review from the previous studies.
There are a few possible explanations for the protective effect of coffee consumption on liver fibrosis. First, the leading coffee compound may have a hepatoprotective effect, and this evidence has been biologically supported in the literature. Coffee is a mixture of several chemical compounds, including caffeine, potassium, diterpenes, niacin, and contains some antioxidants such as chlorogenic acid (CGA) [34]. These compounds’ anti-fibrotic and antioxidant properties may have an attenuative effect on chronic liver diseases such as cirrhosis, fibrosis, fatty liver disease, and carcinoma [30,35]. A high concentration of antioxidants (CGA) in coffee has a significant role in modulating glucose intolerance, reducing lipid accumulation in hepatocytes, and improving fatty liver disease in animal models such as rats [36].
Moreover, the caffeine component also acts as a potent antioxidant and could help attenuate oxidative stress and inflammation in the liver [37,38]. Some in vitro studies also have reported the anti-fibrotic effect of caffeine through inhibition of adhesion kinase enzyme. Caffeine also causes induction of filamentous actin and C-AMP (cyclic adenosine monophosphate) expression and promotes apoptosis of stellate cells in the liver [37]. In animal studies, caffeine has shown anti-inflammatory and anti-fibrotic effects. It inhibits liver fibrosis through several mechanisms such as downregulating the expression of transforming growth factor-β, promoting the breakdown of SMAD2 (Small Mothers Against Decapentaplegic) protein, inhibiting the SMAD3 protein phosphorylation, and upregulating the peroxisome-activated receptor-γ [39]. It also downregulates the expression of connective tissue growth factor in the liver [40,41]. Additionally, other compounds in coffee such as kahweol and cafestol, etc. also have antioxidant effects and can protect the liver by preventing inflammatory reactions by downregulating inflammatory markers [40]. According to newer studies, coffee consumption is associated with a decreased leptin level, which is an essential mediator of fibrosis and inflammation in the liver [18]. These findings solidify the potential anti-inflammatory role of coffee compounds other than caffeine. Lastly, many non-caffeine compounds such as uridine diphosphate glucanosyltransferase and chlorogenic acid are potent antioxidants. They also have been associated with inhibition of the accumulation of lipid fats in hepatocytes. They are also shown to promote insulin sensitivity and reduce the inflammatory response [42,43].
Our study included more articles and a higher number of participants to highlight coffee consumption associated with reduced risk of NAFLD and liver fibrosis among those already diagnosed with NAFLD, compared to the previous meta-analyses. We found quintessential findings that coffee use is protective for NAFLD and liver fibrosis.
4.1LimitationsWe used high-quality study articles for this meta-analysis as predicted by the high-quality assessment score. However, we also acknowledge some limitations in this review. Firstly, the definition of coffee consumption was varied between the included articles. Additionally, the relevant information about coffee consumption, such as the type of coffee used, brewing method, coffee components, whether it is caffeinated or decaffeinated coffee and time of drinking coffee, were not clearly stated in the articles. Therefore, the threshold to define the amount of coffee to achieve the hepatoprotective effect cannot be established with certainty. Secondly, we have evaluated publication bias only for those articles showing the impact of coffee consumption on NAFLD. We could not perform this evaluation for studies showing the association of beneficial coffee consumption effect on liver fibrosis because of the small number of studies included. Therefore, there could be a possible publication bias present in this study. Thirdly, all the articles included in the meta-analysis are observational studies showing only association, but not a causal relationship of coffee as a hepatoprotective agent. Lastly, most of the studies included in the meta-analysis to determine the coffee effect on NAFLD were conducted on males. It reduces the strength of findings and is a possible source of selection bias, as they do not represent the general population. Moreover, all the studies included in this meta-analysis have adjusted their effect estimates for possible confounders; however, we cannot rule out several other confounders that may have affected this association.
In conclusion, our study has demonstrated a significantly reduced risk of non-alcoholic fatty liver disease among those who drank coffee regularly and a substantially decreased risk of liver fibrosis among coffee drinkers who already were diagnosed with NAFLD. Whether to consider coffee consumption as a preventive measure against NAFLD and liver fibrosis needs to be investigated further by epidemiological studies.
Authors’ disclosuresThe authors have nothing to disclose.
Financial supportThere was no financial support
Conflict of interestThe authors declare that they have no conflict of interest regarding the publication of this article.





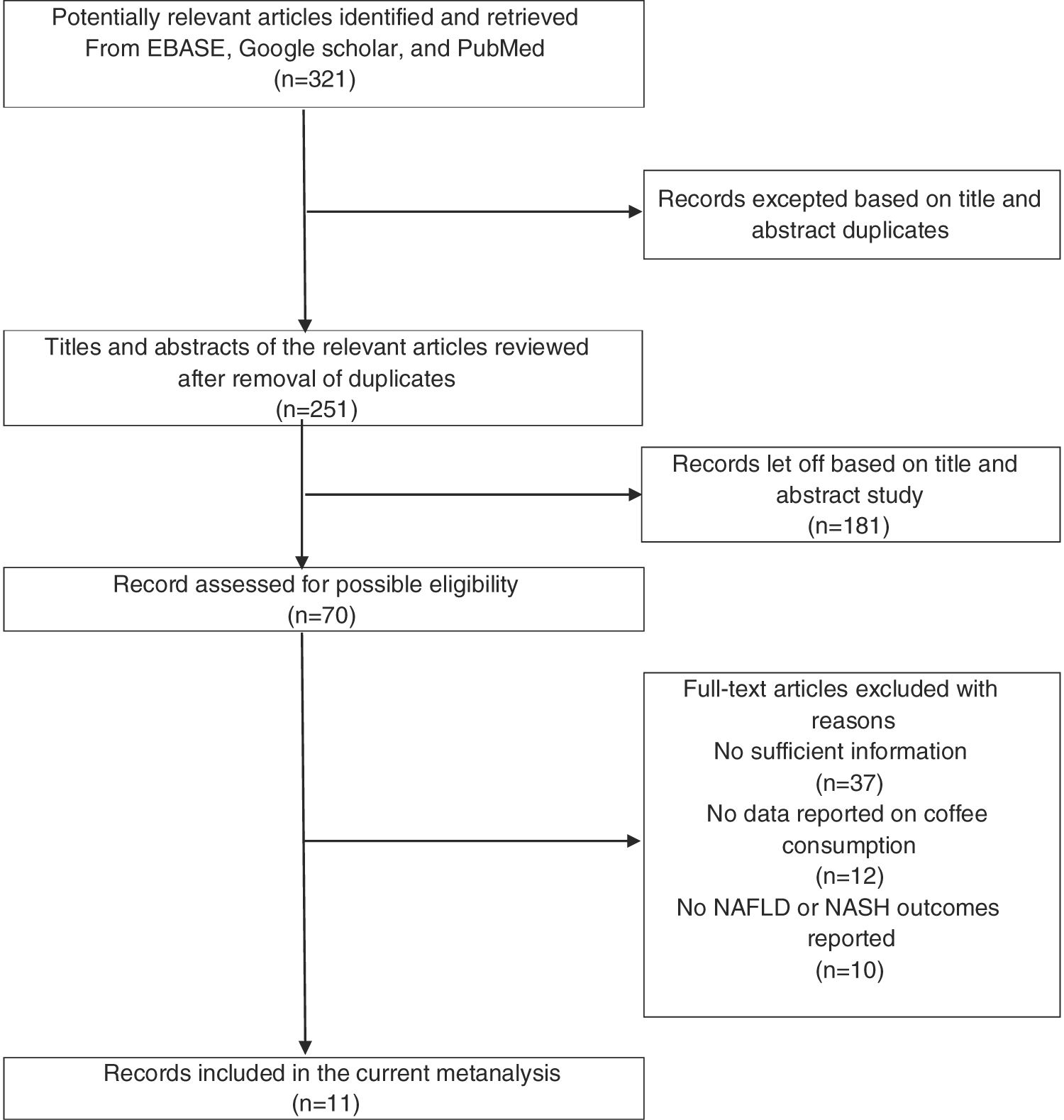
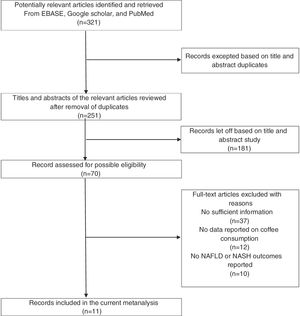 NAFLD; non-alcoholic fatty liver disease, NASH; non-alcoholic steatohepatitis.' title='Literature review process.
NAFLD; non-alcoholic fatty liver disease, NASH; non-alcoholic steatohepatitis.' title='Literature review process. 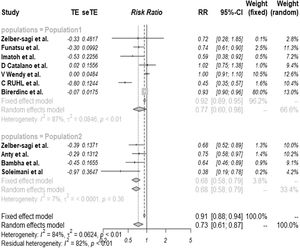 NAFLD (population 1) and liver fibrosis (population 2).
NAFLD (population 1) and liver fibrosis (population 2). 



