and the Fatty Liver Italian Network (FLIN) (Members of FLIN: Gianluca Svegliati-Baroni, Lory S. Crocè, Amalia Gastaldelli, Giulio Marchesini, Fabio Marra, Gianluca Perseghin, and Gianluca Tell)
Fatty liver or hepatic steatosis is a common and often occasional finding for the general practitioner.1 After exclusion of less common causes of fatty liver such as Hepatitis B or C infection, drugs and autoimmune disorders, fatty liver is classified as non-alcoholic (NAFLD) or alcoholic fatty liver disease (AFLD) on the basis of daily ethanol intake. The agreed threshold of ethanol intake used to separate NAFLD from AFLD is 20 g/day, equivalent to 12 drinks per week.2 The term NAFLD covers a wide spectrum of liver injury, ranging from simple steatosis, i.e. fatty infiltration of hepatocytes > 5%, to non-alcoholic steatohepatitis (NASH). At liver biopsy NASH is characterized by necro-inflammation and/or fibrosis, and is histologically indistinguishable from alcoholic steatohepatitis. The histological classification of NAFLD and NASH is being continuously improved and a refined scoring system has been recently proposed by the National Institutes of Health.3
NAFLD is presently regarded as the most common liver disease in Western countries and virtually impacts all fields of clinical medicine, being considered a risk factor for advanced liver disease, type 2 diabetes and cardiovascular disease. Although NAFLD had long been recognized as a frequent clinical entity, only recently its importance as a potential cause of progressive and severe liver disease has been fully acknowledged. NAFLD prevalence in the general population ranges from 16 to 25% in adults4 and from 10 to 19% in children.5 It increases with age, shows different gender specificity according to geographic areas (generally more prevalent in women in US and viceversa in men in Europe and Japan) and varies with ethnicity, with the highest prevalence among the Hispanic population and the lowest among the Afro-American one.6 Prevalence rates of NAFLD are strikingly increased in certain subpopulations, such as patients with obesity, type 2 diabetes mellitus and dyslipidemia, and the whole spectrum of NAFLD is commonly associated with the metabolic syndrome.
The true prevalence of NASH remains largely unknown. According to data derived from autopsy or liver biopsy studies, about 10-15% of NAFLD patients meet the current diagnostic criteria for NASH, pointing to a prevalence of 2-3% in the general population. The incidence and natural course of NAFLD are also difficult to assess because most of the available studies are retrospective and/or performed on clinical series. The few prospective studies are too short to exclude late complications. While simple steatosis has a benign prognosis, NASH may progress to cirrhosis in 30% of cases and is responsible for liver-related death in 3-8%. In clinical series, up to 70% of patients with cryptogenic cirrhosis have clinical features suggestive of NASH.2
NAFLD is considered the hepatic expression of the metabolic syndrome.7 Similarly to the other components of this syndrome, genetic factors can play a role in both the onset and the progression of this disease. However, environmental factors such as high-calorie diet and obesity (especially visceral obesity) are major determinants in the phenotypic expression of NAFLD.
The pathophysiological hallmark of NAFLD is insulin resistance in the liver, muscle and adipose tissue,8 associated with lipid accumulation in ectopic sites, a condition referred to as lipotoxicity.9 The factors responsible for the progression of simple steatosis to NASH are still elusive. The so-called «two-hits» hypothesis suggests that insulin resistance acts as the «first hit» by increasing the efflux of fatty acids from the adipose tissue to the liver. Production of reactive oxygen species, apoptosis dysregulation, and an imbalance between pro-inflammatory (Tumor Necrosis Factor, TNF-α, interleukin-6, leptin) and anti-inflammatory (adiponectin) cytokines would then act as «second hit» leading to necroinflammatory changes and fibrosis.7 However, insulin resistance might also be directly implicated in liver fibrosis suggesting a more complex interplay between the underlying mechanisms.
Early diagnosis of NAFLD, especially in patients at risk of NASH, may help preventing long-term complications. Unfortunately, at present there is no distinctive serological marker for the diagnosis of NAFLD. The most common clinical presentation is hepatomegaly associated with slight elevation of liver enzymes (mostly ALT and gamma-GT) and/or bright liver at ultrasonography.10 However, ALT is a poor marker of liver disease, since normal aminotransferases have been reported in up to 80% of subjects with NAFLD6 and the severity of histological damage is unrelated to ALT levels.7 After exclusion of elevated alcohol intake, viral infections, drugs and autoimmune disorders, NAFLD should be suspected in the presence of any component of the metabolic syndrome. Assessment of body mass index, waist circumference, and blood pressure should be part of the screening procedures. Fasting glucose, triglycerides and HDLcholesterol have to be evaluated in all patients with NAFLD. A test for insulin resistance can be useful. The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), can be easily obtained from fasting insulin and glucose concentrations.11 Longitudinal changes of glucose and insulin during oral glucose tolerance testing are however a better marker of insulin sensitivity. Ferritin may be increased in up to 60% of patients with NAFLD as expression of subclinical inflammation. The «Fatty Liver Index», based on BMI, waist circumference, triglycerides, and gamma-GT activity helps predicting the risk of NAFLD.12 Liver imaging by ultrasound, computed tomography or magnetic resonance spectroscopy may provide supporting evidence of a fatty liver, but the accuracy of these techniques depends upon the degree of steatosis. For ultrasound, the technique most commonly employed, the accuracy is acceptable for values of lobular fat > 30%.2
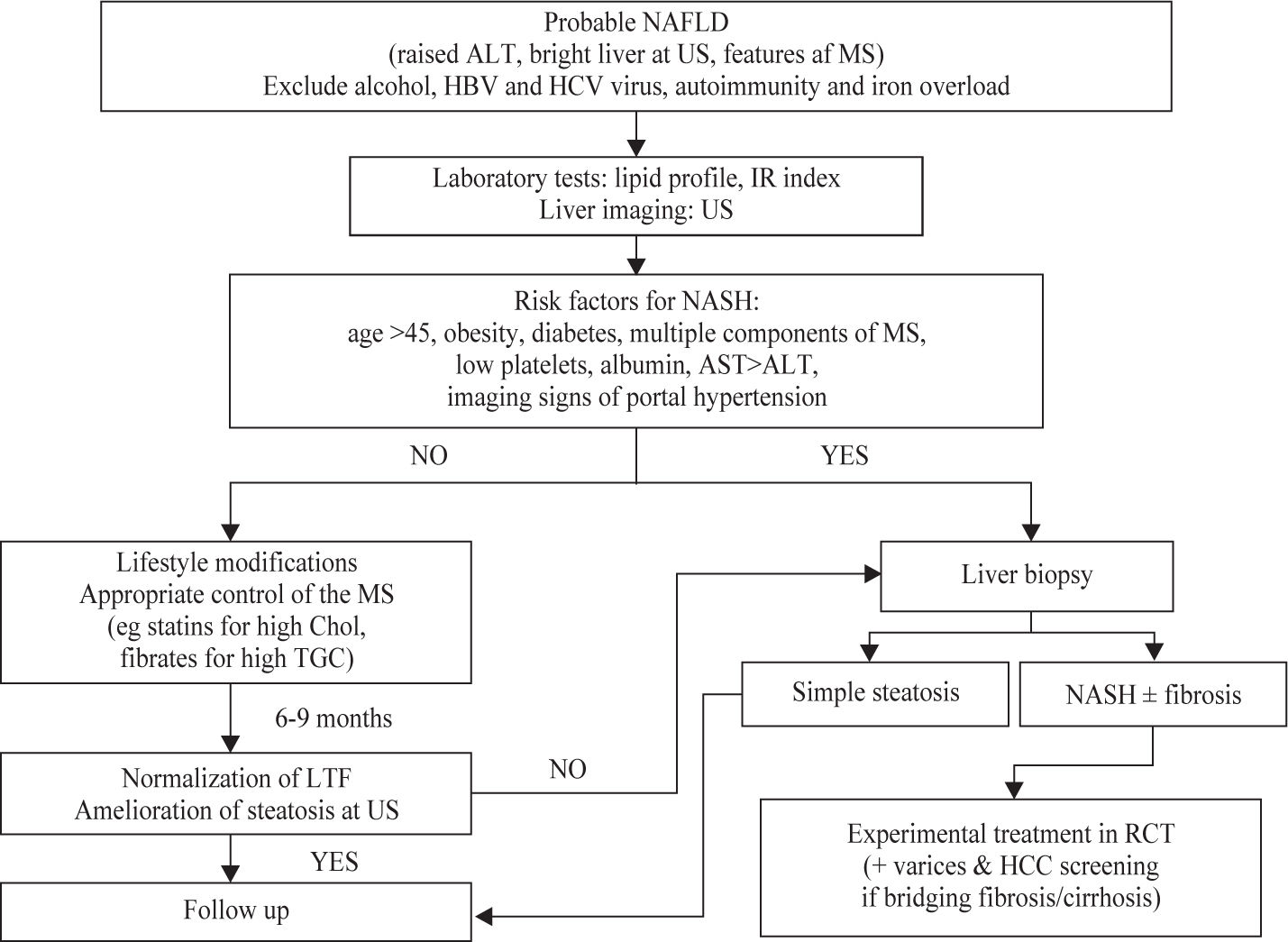
No liver function test or imaging technique can reliably distinguish simple steatosis from NASH. In particular, aminotransferases may be normal despite necro-inflammation and/or fibrosis.13 Liver biopsy is the most sensitive and specific tool to diagnose NASH and to provide important prognostic information. Although there is no consensus on the role of liver biopsy yet, conventional wisdom suggests that it should be utilized in case of uncertainty regarding diagnosis, of initial stigmata of advanced liver disease, or when specific therapy is to be implemented. Less-invasive approaches to identify NASH have been based on biochemical (e.g. platelets and albumin) and ultrasound indicators (e.g. splenomegaly and portal vein dilatation) of advanced liver disease, but their accuracy is still limited. A scoring system composed of routinely measured variables (age, BMI, AST/ALT ratio, albumin, platelet count and glucose) may be useful to predict the absence or the presence of significant fibrosis (stage 3-4) in patients with NAFLD (negative predictive value ≥ 81%, positive predictive value ≥ 77%).14 Transient elastography is a novel method to assess liver fibrosis, but steatosis and obesity hinder its accuracy.15 A suggested diagnostic approach to NAFLD is given in Figure 1
Despite the growing interest for NAFLD, the best treatment is still to be found, since none of the approaches tested so far has shown to be unequivocally effective. Lifestyle is a major risk factor for NAFLD and may also influence the efficacy of drugs. Therefore, nutritional counselling and/or formal diet and exercise should be the initial step in the management of NAFLD. A lowcalorie, low-fat diet is recommended for weight reduction in overweight subjects with an initial target of weight loss ranging between 5-10% of baseline weight. Notably, a weight loss > 1.5 kg/week may promote NASH by massive mobilization of fatty acids to the liver. Lifestyle changes ameliorate liver enzymes in most cases but very few data are available on the potential beneficial effects on histology. The compliance of the patient is essential to reach these goals and cognitive behavioral strategies may be useful in this respect.
The pharmacological treatment of NAFLD is still mostly experimental. Because insulin resistance is the hallmark of NAFLD, insulin-sensitizing agents are expected to be beneficial. Metformin reduces insulin resistance by decreasing glucose production and stimulating mitochondrial beta-oxidation of fatty acids, but the available studies have shown mixed and inconclusive results.16 Thiazolidinediones improve insulin sensitivity in adipose tissue, in the liver and in the muscle by increasing plasma adiponectin levels, stimulating fatty acids oxidation, and inhibiting hepatic gluconeogenesis.17,18 Recently, a randomized controlled trial of pioglitazone in patients with NASH showed a reversal of the metabolic milieu favouring steatosis and an amelioration of all the histological features of steatohepatitis. Although insulin-sensitizing agents can be considered promising therapies for NAFLD, additional randomized trials are needed with adequate sample size and duration. Since pharmacological treatments are likely to be maintained long-term or lifelong in NASH, a careful assessment of the risk-benefit ratio and of the drug-safety profile is of paramount importance.19 Bariatric surgery improves NAFLD in most cases but is reserved to patients with severe obesity or in presence of major co-morbidities.
ConclusionsAlthough NAFLD is becoming a major cause of referral of patients to liver and/or metabolic centres, many questions remain unanswered: 1) what is the burden of disease in high risk subgroups, e.g. diabetics?; 2) what is the contribution of cardiovascular vs. liver disease to morbidity and mortality?; 3) how new accurate and non-invasive diagnostic tools can be developed?; and 4) which treatment works best? The future will keep us certainly busy.
AcknowledgmentsThe finacial and logistic support of the Fondo Studio Malattie del Fegato-Onlus, Trieste, is acknowledged.