Introduction: The clinical and public health implications of the convergence of the human immunodeficiency virus (HIV) epidemic and chronic viral hepatitis in sub-Saharan Africa are poorly understood. This study was designed to determine the seroprevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV), and the impact of co-infection on baseline serum alanine transaminase (ALT), CD4+ T lymphocyte (CD4) count, and plasma HIV-RNA (viral load) in a cohort of HIV-infected Nigerians. Methods: A retrospective study was conducted, on eligible treatment-naive patients who presented between August 2004 and February 2007 to the University College Hospital (UCH), Ibadan, Nigeria. Demographic data and pre-treatment laboratory results (hepatitis B surface antigen (HBsAg), HCV antibodies (anti-HCV), ALT, CD4 count and viral load) were retrieved from the medical records. Fisher’s exact, two sample t-tests, and the Wilcoxon rank sum tests were used to compare groups. A logistic regression model was fitted to explore characteristics associated with co-infection status. Results: A total of 1779 HIV-infected patients (male: female ratio, 1:2) met inclusion criteria. HBsAg was present in 11.9%, anti-HCV in 4.8% and both markers in 1%. HBsAg was more common among males than females (15.4% vs 10.1%, respectively p = 0.001) while anti-HCV was detected in a similar proportion of males and females (5.3% versus 4.6%, respectively p = 0.559). HIV-infected patients with anti-HCV alone had a lower mean baseline CD4 count compared to those without anti-HCV or HBsAg (197 cells/mm3vs247 cells/mm3, respectively p = 0.008). Serum ALT was higher among patients with HBsAg compared to those without HBsAg or anti-HCV (43 International Units (IU) vs. 39 IU, respectively p = 0.015). Male gender was associated with HBV co-infection on logistic regression (OR1.786; 95% CI, 1.306-2.443; p < 0.005). Conclusion: More HIV-infected females than males presented for care in this cohort. We identified a relatively high prevalence of HBV and HCV co-infection in general, and a higher rate of HBV co-infection among males than females. Pre-treatment CD4 count was significantly lower among those with HCV co-infection, while ALT was slightly higher among those with HBV co-infection. Triple infection with HIV, HBV and HCV was present in a small but significant proportion of patients. These findings underscore the importance of testing for HBV and HCV in all HIV-infected persons in our setting.
Acquired immune deficiency syndrome (AIDS) continues to be the single largest cause of mortality in sub-Saharan Africa1. Hepatitis B virus (HBV) and hepatitis C virus (HCV) are major public health concerns in the region as well.2,3 Since there is overlap in risk factors for HIV, HBV and HCV, some patients are infected with multiple viruses. HIV co-infection with HBV and/or HCV is associated with increased risks of liver-related morbidity and mortality.4,5 Combination active antiretroviral therapy may attenuate these risks,6 but chronic hepatitis is a risk factor for drug or immune-mediated liver injury during HIV treatment.7 Such complex interactions underlie the growing importance and upsurge of interest in chronic hepatitis among HIV-infected persons.8
Although HIV, HBV and HCV are endemic in sub-Saharan Africa, little is known about the demographic and clinical features of multiply infected persons in this region. Further, the impact of HBV or HCV on immunologic and virologic parameters of HIV-infected persons, and the impact of HIV on the clinical course of patients with concurrent HBV or HCV infection are poorly characterized. An improved understanding of these issues is necessary to inform prevention and management strategies. Therefore, we undertook this study to determine the prevalence of HBV and HCV infection, and the impact of co-infection or triple-infection on pre-treatment ALT, CD4 count and viral load in a cohort of HIV-infected Nigerians.
Materials and methodsA retrospective study was carried out at the University College Hospital (UCH) Ibadan, one of the clinical sites of the Harvard School of Public Health’s AIDS Prevention Initiative in Nigeria. Baseline laboratory testing conducted on all patients entering the program includes 1) 3rd generation enzyme-linked immunoabsorbent assay (ELISA) for detection of hepatitis B surface antigen (HBsAg), 2) testing for HCV antibodies (anti-HCV) using 3rd generation assays, and 3) measurement of CD4+ T lymphocyte (CD4) count by flow cytometry, serum alanine transaminase (ALT) using enzymatic methods (automated Hitachi 902 machine), and plasma HIV RNA (viral load) by Roche Amplicor RNA PCR assay.
The medical records of 2252 HIV-infected ART-naïve patients • •15 years old who enrolled at UCH between August 2004 and February 2007 were reviewed. A total of 1779 subjects were included in the study after excluding 493 subjects. Demographic information (age and sex) and results of baseline laboratory variables (CD4 count, viral load, ALT, HBsAg, and anti-HCV) were extracted from the medical records of eligible patients. The study was designed according to ethical standards for human studies and approved by the Joint UCH/University of Ibadan Institutional Review Board.
Statistical methodsAll count variables were assessed for normality utilizing visual inspection of box-plots and the Shapiro-Wilk test, and all categorical variables were dichotomized into dummy-variables. The Fisher’s exact test was used to assess differences in frequencies.
Levene’s statistic was used to assess equality of variance between groups before independent two-sample t-tests for equal and unequal variances were utilized for comparisons of means. Given the significant non-normality and non-variance in the HBV and HCV triple-infected patients the non-parametric two-sample Wilcoxon rank-sum (Mann-Whitney) test was performed to determine differences in dependent variable distributions between gender.
Logistic regression models were fitted using stepwise estimation with the log-Likelihood test with a significance level of 0.10 for variable removal and addition.
Forward and backward regression produced the same models. Stata 9.2SE was used in the computational analyses.
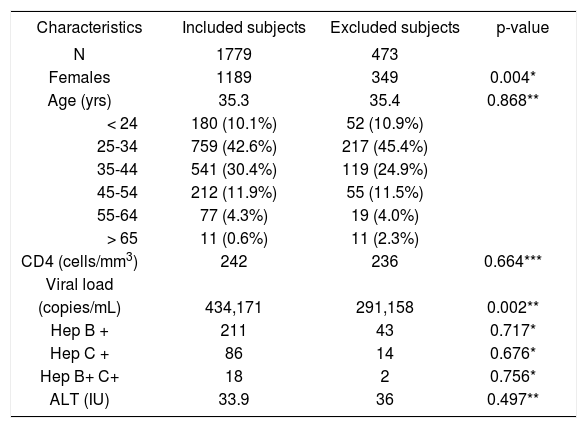
ResultsA total of 2252 HIV/AIDS patients over the age of 15 years presented to UCH during the 30-month study period. Subjects were excluded for incomplete medical records (n = 471) or age < 15 years (n = 2). The 1779 remaining patients were included in the analyses. Baseline demographic and clinical characteristics of included and excluded patients shown in Table I indicates that both groups were comparable, except that excluded population had more females and lower baseline viral loads.
Clinical characteristics of All subjects
| Characteristics | Included subjects | Excluded subjects | p-value |
|---|---|---|---|
| N | 1779 | 473 | |
| Females | 1189 | 349 | 0.004* |
| Age (yrs) | 35.3 | 35.4 | 0.868** |
| < 24 | 180 (10.1%) | 52 (10.9%) | |
| 25-34 | 759 (42.6%) | 217 (45.4%) | |
| 35-44 | 541 (30.4%) | 119 (24.9%) | |
| 45-54 | 212 (11.9%) | 55 (11.5%) | |
| 55-64 | 77 (4.3%) | 19 (4.0%) | |
| > 65 | 11 (0.6%) | 11 (2.3%) | |
| CD4 (cells/mm3) | 242 | 236 | 0.664*** |
| Viral load | |||
| (copies/mL) | 434,171 | 291,158 | 0.002** |
| Hep B + | 211 | 43 | 0.717* |
| Hep C + | 86 | 14 | 0.676* |
| Hep B+ C+ | 18 | 2 | 0.756* |
| ALT (IU) | 33.9 | 36 | 0.497** |
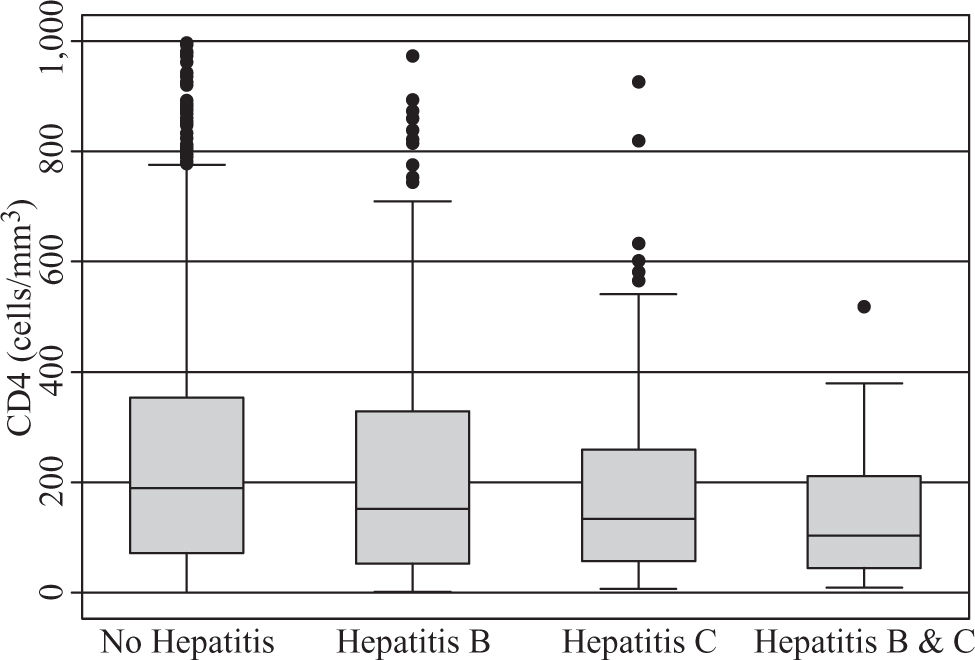
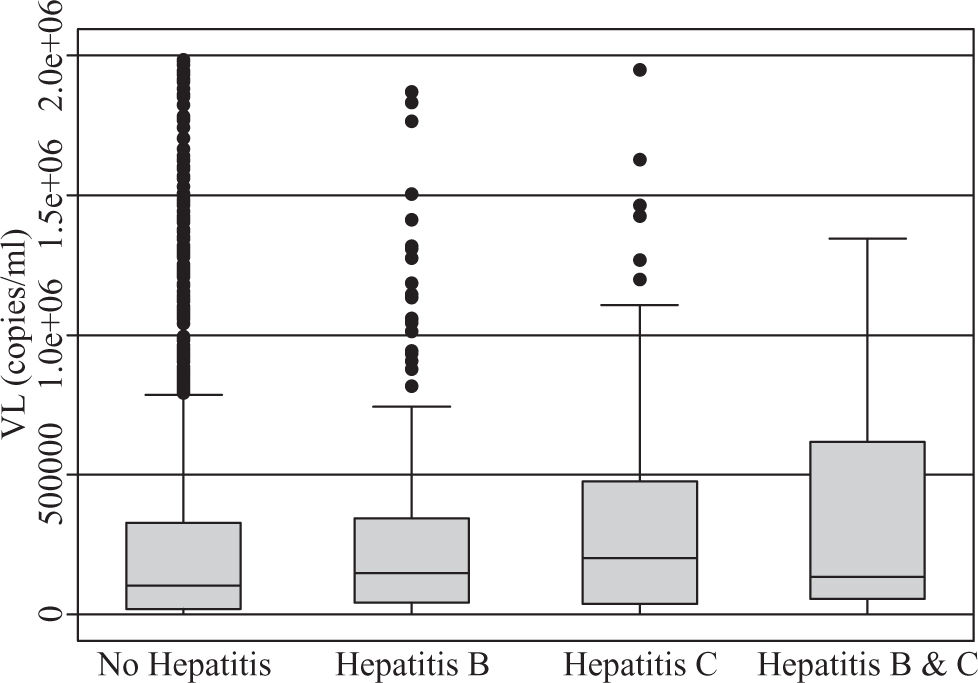
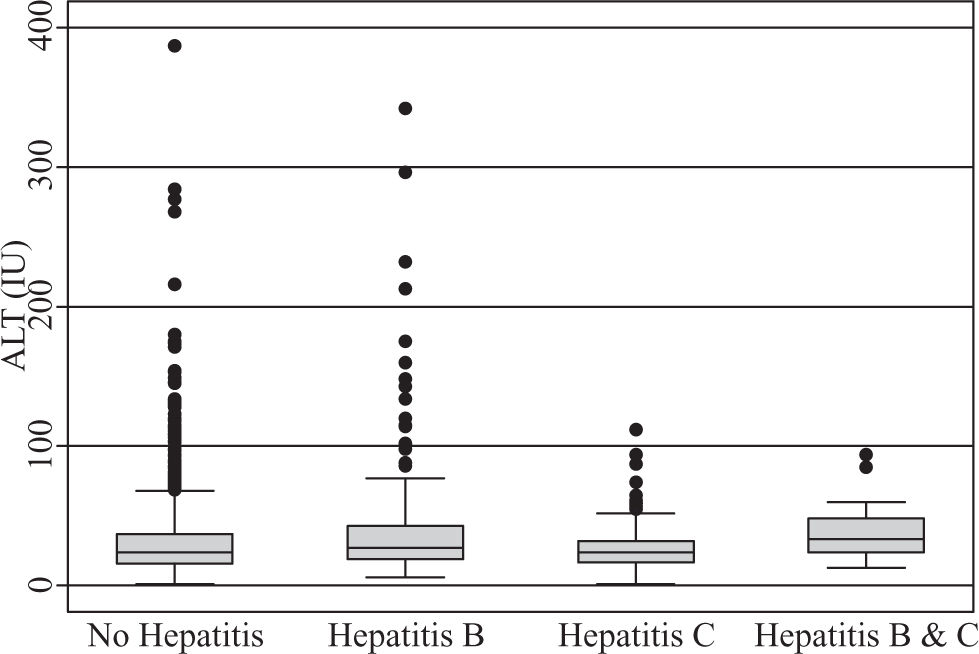
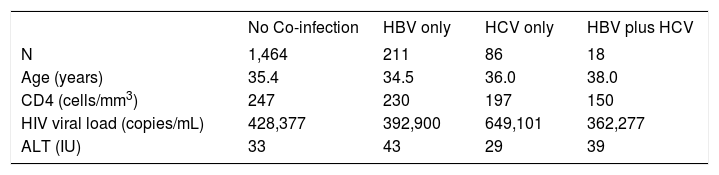
A total of 590 (33.2%) males and 1189 (66.8%) females, a male to female ratio of 1:2 met inclusion criteria. The modal age group of the eligible participants was 25 – 34 years (42.6%) followed by 35-44 years (30.4%). The clinical features of the participants (Table II) demonstrate HBV co-infection based on positive HBsAg in 211/1779 (11.9%) while the prevalence of HCV co-infection based on anti-HCV detection was 86/1779 (4.8%). Triple infection with HIV, HCV and HBV was present in 18/1779 (1%). There was no significant difference in mean CD4 counts between HBV co-infected subjects (230 cells/ mm3) versus those without HBV or HCV (247 cells/mm3), p = 0.288. However, those with anti-HCV had a mean CD4 count of 197 cells/mm3, which was significantly lower than the corresponding value for subjects without HBV or HCV (p = 0.018). The mean CD4 count was also significantly lower among those positive for both HBsAg and anti-HCV (150 cells/mm3) versus those who were negative for both (p = 0.008), (Figure 1). Mean pre-treatment viral loads were comparable between those positive for HBsAg alone (392,900 copies/mL), anti-HCV alone (649,102 copies/mL), both HBsAg and anti-HCV (362,277 copies/mL), and those negative for both markers (428,377 copies/mL), (Figure 2). Serum ALT among patients without HBsAg or anti-HCV was 39 International Units (IU), whereas it was significantly higher (43 IU) for those with HBV co-infection (p = 0.015) and lower (29 IU) for those with anti-HCV alone (p = 0.1217). The mean ALT among those with both HBsAg and anti-HCV was 39 (p = 0.2886), (Figure 3).
Clinical Features of Included Subjects*
| No Co-infection | HBV only | HCV only | HBV plus HCV | |
|---|---|---|---|---|
| N | 1,464 | 211 | 86 | 18 |
| Age (years) | 35.4 | 34.5 | 36.0 | 38.0 |
| CD4 (cells/mm3) | 247 | 230 | 197 | 150 |
| HIV viral load (copies/mL) | 428,377 | 392,900 | 649,101 | 362,277 |
| ALT (IU) | 33 | 43 | 29 | 39 |
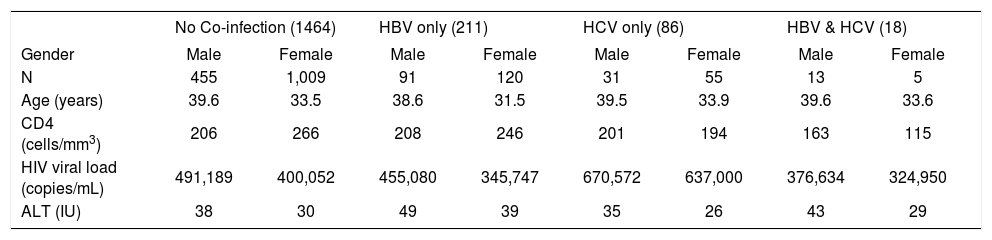
On sex stratification (Table III), 91/540 (15.4%) of males were HBV co-infected versus 120/1189 (10.1%) of females (p = 0.001). Regarding HCV, 31/590 (5.3%) of males were anti-HCV positive compared to 55/1189 (4.6%) of females (p = 0.559). A total of 13/590 (2.2%) males had triple infection with HIV, HBV and HCV compared to 5/1189 (0.4%) of females, p = 0.001). Serum ALT was lower among females compared to males (30.9 IU vs. 39.8 IU, respectively p < 0.005) while mean CD4 count was higher than the value for males (259.9 cells/ mm3vs 204.7 cells/mm3, respectively p < 0.005). There was no gender disparity in baseline viral load (405,217 copies/mL vs 492,521 copies/ml for females vs. males, respectively p = 0.1497). When stratified by groups, women continued to have significantly lower ALT and higher CD4 count than males among patients without any co-infection (n = 1464), (p < 0.005 for both). For patients with anti-HCV alone (n = 86), there remained a difference in ALT between females and males 25.7 cells/mm3vs 35.1 cells/mm3, p = 0.0442. However, among those who had HBV co-infection (n = 211) or triple infection (n = 18), there were no significant differences in CD4 counts and ALT between males and females. On logistic regression, male gender was associated with HBV co-infection (OR1.786; 95% CI, 306-2.443; p < 0.005).
Clinical Features of included subjects separated by gender*
| No Co-infection (1464) | HBV only (211) | HCV only (86) | HBV & HCV (18) | |||||
|---|---|---|---|---|---|---|---|---|
| Gender | Male | Female | Male | Female | Male | Female | Male | Female |
| N | 455 | 1,009 | 91 | 120 | 31 | 55 | 13 | 5 |
| Age (years) | 39.6 | 33.5 | 38.6 | 31.5 | 39.5 | 33.9 | 39.6 | 33.6 |
| CD4 (cells/mm3) | 206 | 266 | 208 | 246 | 201 | 194 | 163 | 115 |
| HIV viral load (copies/mL) | 491,189 | 400,052 | 455,080 | 345,747 | 670,572 | 637,000 | 376,634 | 324,950 |
| ALT (IU) | 38 | 30 | 49 | 39 | 35 | 26 | 43 | 29 |
Chronic viral hepatitis is a leading cause of liver-related deaths among patients with HIV/AIDS worldwide9. According to WHO estimates, the global burden of HIV, HCV and HBV is 33.2 million, 170 million and 400 million, respectively. The number of HIV-infected females who presented to our center for treatment during the study period was approximately twice the number of males. This gender inequality in presentation for therapy is consistent with the sex distribution documented in the majority of treatment centres particularly in the first decade of antiretroviral therapy.10 Women now account for over 50% of people with HIV/AIDS in Africa, a disparity that is even more marked among those aged 15–24 years.11,12 A potential explanation for more females at our center is that women may be more sensitive to changes in their health and may be socially conditioned to seek and receive assistance whereas men may have to prove their masculinity by avoiding the sick role in order to maintain their culturally assigned image as «providers». This, however, does not necessarily imply that in absolute terms more women are infected with HIV in our population. A study in Nigeria actually found that more men were afflicted with HIV/AIDS.13
The co-infection prevalence of 11.9% for HIV and HBV is significant, and confirms that HBV is a major threat to HIV/AIDS patients in Nigeria, as reported in other parts of the world.14 The HBV co-infection rate in this study is higher than the 9.7% reported by Sirisena et al. from an urban population in Northern Nigeria,15 but lower than the 25.9% reported by Uneke et al., who used a similar method of HBV detection in Jos, which is located in Nigeria’s North Central region.16 The factors driving these regional differences are unclear. Gender stratification showed that males had a higher prevalence of HBV (17.9% vs 10.7%). This finding could be linked to an earlier observation that a high proportion of HBV infections in sub-Saharan Africa is acquired vertically or horizontally (from family members and other children) before the age of 5 years.17 Since boys have a predilection for aggressive sports and plays that may result in injury with bleeding, they may be more predisposed to horizontal HBV transmission. Further, societal acceptance of multiple sexual partners for men may contribute to the higher HBV prevalence among HIV-infected men. Importantly, a male preponderance in HBV seropositivity has also been described in HIV seronegative subjects.18 We found higher baseline ALT among HIV-infected patients with HBV co-infection, which is in accord with the finding of other investigators.19
Anti-HCV alone was detected in 4.8% of the patients in this study. In an earlier study, HCV co-infection based on plasma HCV RNA quantification was detected in 8.2% of HIV-infected patients in Northern Nigeria.20 However, cross-study comparisons may be misleading because of the differences in HCV detection techniques. Quantifiable plasma HCV RNA is present only in patients with active HCV replication. In contrast, anti-HCV can be detected in patients with previous HCV exposure, including those with ongoing HCV replication and those whose immune responses curtailed viral replication. There may be very rare cases of falsely negative anti-HCV in patients with advanced immunosuppression.21,22 In the current study, the rates of anti-HCV detection were comparable in males (6.7%) and females (5.2%), and there was an association between detection of anti-HCV and lower CD4 count. This latter association is controversial.23,24,25 HCV by itself has not been shown conclusively to be an independent risk factor for more rapid CD4 decline, although it has been associated with increased occurrence of AIDS-defining events.26,27
Approximately 1% of HIV-infected patients in this study had both HBsAg and anti-HCV. These triply infected patients were disproportionately male (2.2% of males vs 0.4% of females). Although the absolute number (n = 18) was relatively small for analysis, this group appeared to have lower CD4 counts but similar ALT and viral load compared to those without HBsAg or anti-HCV. Studies enrolling a larger number of subjects are needed to elucidate these potential associations further.
This study has several limitations. First, the risk factors for viral hepatitis acquisition were not available. As such we are unable to comment on this important aspect of the epidemiology of HIV, HBV and HCV. Second, plasma HCV-RNA was not quantified in patients who had anti-HCV, making it impossible to distinguish active HCV infection from spontaneously cleared infection. Third, this was a retrospective study, and a substantial proportion of patients were excluded for incomplete medical records. The excluded population, however, largely mirrored the included subjects.
In conclusion, more women than men presented for care in this cohort, but HIV/HBV co-infection and triple-infection with HIV, HBV and HCV were more common in men. The high frequency of HBsAg and anti-HCV confirms the need for routine screening for these markers in HIV-infected patients in our setting. CD4 count was significantly lower, in patients with prior exposure to HCV, while ALT was slightly higher among those positive for HBV infection.
AcknowledgementsWe acknowledge all members of staff and patients who were instrumental in the conduct of this study.