Introduction. This work focuses on ammonia metabolism of Liver Microorgans (LMOs) after cold preservation in a normothermic reoxygenation system (NRS). We have previously reported the development of a novel preservation solution, Bes-Gluconate-PEG 35 kDa (BG35) that showed the same efficacy as ViaSpan® to protect LMOs against cold preservation injury. The objective of this work was to study mRNA levels and activities of two key Urea Cycle enzymes, Carbamyl Phosphate Synthetase I (CPSI) and Ornithine Transcar-bamylase (OTC), after preservation of LMOs in BG35 and ViaSpan® and the ability of these tissue slices to detoxify an ammonia overload in a NRS model.
Material and methods. After 48 h of cold storage (0°C in BG35 or ViaSpan®) LMOs were rewarmed in KHR containing an ammonium chloride overload (1 mM). We determined ammonium detoxification capacity (ADC), urea synthesis and enzyme activities and relative mRNA levels for CPSI and OTC.
Results. At the end of reoxygenation LMOs cold preserved in BG35 have ADC and urea synthesis similar to controls. ViaSpan® group demonstrated a lower capacity to detoxify ammonia and to synthesize urea than fresh LMOs during the whole reoxygenation period which correlated with the lower mRNA levels and activities for CPSI and OTC observed for this group.
Conclusion. We demonstrate that our preservation conditions (48 hours, BG35 solution, anoxia, 0°C) did not affect ammonia metabolism of cold preserved LMOs maintaining the physiological and biochemical liver functions tested, which allows their future use as biological component of a BAL system.
Liver transplantation is the treatment of choice for patients with irreversible liver disease. However, this procedure has become a victim of its own success: there are too many patients on waiting lists to receive an organ.1 Hence, different strategies have been used to either bridge patients until transplantation or support hepatic self-regeneration, one of them being the bioartificial liver (BAL).2
Liver Microorgans (LMOs) are intact pieces of tissue that maintain a normal microarchitecture, including hepatic cellular types and cell to cell contacts and communication, and can be used as an alternative to whole-organ models.3 We have recently become interested in evaluating the performance of rat LMOs as the metabolically active component of a bioartificial liver (BAL) model. The “ideal” biological component should perform the functions of the damaged liver and then LMOs are suitable candidates because they bear all the characteristics of a liver lobule.4
The urea cycle (UC) is the metabolic pathway responsible for ammonium removal. Carbamyl Phosphate Synthetase I (CPSI) and Ornithine Transcarbamylase (OTC) catalyze the first and second committed steps of waste nitrogen metabolism in the urea cycle, respectively. Recently, our group has focused on the investigation of the maintenance of ammonia detoxification capacity and, mRNA level and activity of CPSI and OTC during cold preservation of hepatocytes.5 In this work we focus on ammonia metabolism of LMOs after cold preservation in a normothermic reoxygenation system (NRS). It is a simple model as well as an easy way to evaluate the ammonia capacity of preserved tissues before switching to a more complex model as it is the BAL. Accordingly, we have already reported the development of a novel preservation solution, Bes-Gluconate-PEG 35 kDa (BG35) that showed the same efficacy as ViaSpan® to protect LMOs against cold preservation injury.6
Since the urea cycle is the major pathway of ammonia removal, the objective of this work was to study relative mRNA levels and activities of two key enzymes CPSI and OTC after LMOs preservation in BG35 and ViaSpan® and the ability of these tissue slices to detoxify an ammonia overload in a NRS model.
Material and MethodsAnimalsMale Wistar rats weighing 250-300 g were used in all the experiments. The rats were allowed access to standard laboratory food and water ad libitum prior to the experiments, and received care in compliance with international regulations. The National Scientific Council from Argentine (CONICET) approved the protocols used involving animals.
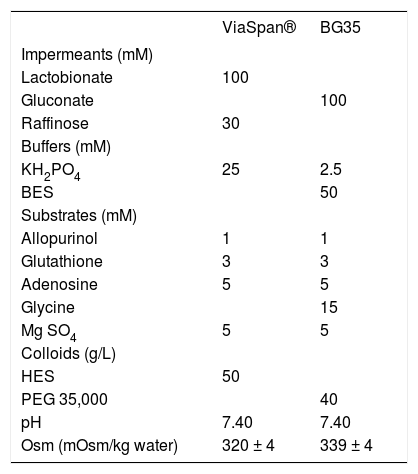
Liver extractionAnimals were anesthetized using 8% chloral hydrate in water solution (i.p. injection of 0.5 mL/ 100 g of corporal weight) and administered 200 U of sodium heparin through the femoral vein, immediately before surgery. An abdominal incision was made to access the portal vein which was cannulated using a 16G catheter (Abbocath-T, Abbott Ireland Ltd.). The liver was washed with 20 mL of Krebs-Henseleit Reoxygenation Media (KHR) (control group), or with 20 mL of each preservation solution (for preserved groups; the solutions compositions are shown in table 1. The composition of KHR was as follows: 114 mM NaCl, 25 mM NaHCO3, 4.8 mM KCl, 1.5 mM CaCl2, 10 mM HEPES, 5 mM glucose, 1 mM allopurinol, 3 mM glycine; pH = 7.40, 310 mOsm/kg water.
Composition of the preservation solutions ViaSpan® and BG35.
| ViaSpan® | BG35 | |
|---|---|---|
| Impermeants (mM) | ||
| Lactobionate | 100 | |
| Gluconate | 100 | |
| Raffinose | 30 | |
| Buffers (mM) | ||
| KH2PO4 | 25 | 2.5 |
| BES | 50 | |
| Substrates (mM) | ||
| Allopurinol | 1 | 1 |
| Glutathione | 3 | 3 |
| Adenosine | 5 | 5 |
| Glycine | 15 | |
| Mg SO4 | 5 | 5 |
| Colloids (g/L) | ||
| HES | 50 | |
| PEG 35,000 | 40 | |
| pH | 7.40 | 7.40 |
| Osm (mOsm/kg water) | 320 ± 4 | 339 ± 4 |
BES: N, N-bis (2-hydroxyethyl)-2-aminoethanesulfonic acid. HES: hydroxyethyl starch. PEG: polyethyleneglycol. Dexamethasone 16 mg/L, insulin 40 UI/L and penicillin G 200,000 UI/L were added to Viaspan® before use. Streptomycin 0.25 mg/mL and penicillin G 10 UI/mL were added to BG35 before use. All the solutions were bubbled with 100% N2 for 45 min at 0 oC before use.
Simultaneously, the inferior cava vein was cut at the abdominal level, right underneath the renal veins, to eliminate the blood and the washing fluid and avoiding in this way, an intra-hepatic pressure rise. Finally, the animal was sacrificed by cutting its diaphragm, and the liver was carefully extracted. The organ was externally washed with cold KHR solution to eliminate the remaining blood, and it was put into a flask containing 50 mL of that same solution at 0 °C.
Preparation of LMOsLMOs were prepared using “free-hand” techniques, by cutting the liver first into blocks and then into slices of 338 ± 27 μm thickness (n = 50), using a blade. We worked on ice to reduce tissue deterioration, and over a paper filter to prevent the slippage of the hepatic blocks that could complicate the precise cutting of the tissue slices.
After hand slicing, LMOs were placed in different solutions. Fresh LMOs (controls) were placed in KHR and immediately submitted to reoxygenation step. LMOs that underwent the preservation process were placed in the different preservation solutions used.
LMOs’ preservationOnce cut, LMOs were placed in ViaSpan® (UW Bristol-Myers-Squibb, Bruxelles, Belgium) or BG35. Approximately 50 LMOs were stored for up to 48 h at 0 oC in a 100 mL Boeco glass bottle containing 50 mL of the preservation solution.6 The compositions of the different solutions are listed in table 1.
LMOs’ reoxygenationAfter 48 h of cold storage, LMOs were washed thoroughly with a rinse solution previously described by our group7 to remove residual preservation solution that could interfere during the subsequent reoxygenation step. The composition of this rinse solution was as follows: 114 mM NaCl, 25 mM NaHCO3, 4.8 mM KCl, 1.5 mM CaCl2, 10 mM HEPES, 5 mM glucose, 1 mM allopurinol, 3 mM glycine; 3 mM glutathione, 1 mM methionine pH = 7.40, 300 mOsm/kg water.
This step was carried out at 37 °C during 120 min in a Dubnoff metabolic shaker in KHR under carbogen atmosphere (95%O2:5% CO2) in 6-well culture plates. Figure 1 shows the arrangement used for LMOs, that were placed in pairs plus 5 mL of KHR per well. Control LMOs were reoxygenated immediately after sliced.
Arrangement used in the normothermic reoxygenation system. Distribution adopted by LMOs during the normothermic reoxygenation step. This step was carried out at 37 °C during 120 min in a Dubnoff metabolic shaker in KHR under carbogen atmosphere (95% O2:5% CO2) in 6-well culture plates. We placed two LMOs plus 5 mL of KHR on each well.
To evaluate all the parameters related to ammonia metabolism, LMOs were rewarmed in KHR containing an ammonium chloride overload (1 mM final concentration).8
Aliquots of the KHR and tissue samples were removed at 0, 60 and 120 min of the rewarming period to assess the different viability and functional parameters described below.
Viability and functional assays- •
Tissue water content (TWC). TWC content was determined by a desiccation method (12 h in a hot oven at 105 °C). TWC was expressed as mL water/g dry tissue and performed in quadruplicate for each experiment to produce a mean value.
- •
Lactate dehydrogenase release (LDH). The LDH activity was measured in the incubation medium and in the tissue, as described previously.9 Two LMOs per time point were homogenized with 3 mL of a lysis solution containing 0.1% (v/v) Triton X-100, 0.9% NaCl and 0.1% BSA, and were used to measure enzymatic activity in the tissue. Five hundred microlitres of the corresponding supernatant were used to determine the enzymatic activity of LDH released into the medium. Results were expressed as the percentage of the released total enzyme activity with respect to the total activity calculated as the sum of homogenate plus supernatant activities.
- •
RNA isolation, reverse transcription, and semi-quantitative real time PCR. Total RNA was extracted using Tri Reagent™ (Sigma Chem. Co. S. Louis, USA) according to the manufacturer’s instructions. Reverse transcription and semi-quantitative real time PCR were made as previously described.10Table 2 shows the primers designed for each gene analyzed. Relative expression analysis was made using the 2-ΔΔCT method, normalizing the values with respect to β-Actin, 18S rRNA and Glyceraldehide Phosphate Dehydrogenase RNA levels as housekeeping genes. The initial quantity of template cDNA for each sample was expressed relative to a reference sample, considered as 1X. The chosen reference sample was one belonging to the control group, at the beginning of the reoxygenation period. All the Real Time RT-PCR experiments were performed in triplicates.
Table 2..Primers designed for gene expression analysis.
CPSI Sense Antisense 5’-ATC TGA GGA AGG AGC TGT CT-3’ 5’- AAA ACC ACT TGT CAA TGG AT-3’ OTC Sense Antisense 5’-ATG ACA GAT GCA GTG TTA GC-3’ 5’-CAG GAT CTG GAT AGG ATG AT-3’ β-actin Sense Antisense 5’-CAA CCT TCT TGC CAG CTC-3’ 5’-GAC GAG CGC AGC GAT ATC-3’ GAPDH Sense Antisense 5’-CCA TCA CCA TCT TCC AGG AG-3’ 5’-CCT GCT TCA CCA CCT TCT TG-3’ 18S rRNA Sense Antisense 5’-TAA CCC GTT GAA CCC CAT T-3’ 5’-CCA TCC AAT CGG TAG TAG CG-3’ - •
Ammonia detoxification. Ammonia was determined enzymatically according to the method described by van Anken, et al.11
Ammonia detoxification capacity (ADC) represents the quantity of ammonia detoxified per gram of LMOs. This parameter was calculated from the measured values of ammonia amount as follows:
ADC = (Q0-Qt)/LMO mass
Where Q0 and Qt are the ammonia content of the medium (in μmoles) at time 0 and t (60 or 120 min) of incubation and LMO mass corresponds to the grams of wet tissue, respectively.
- •
Urea synthesis. Urea was spectrophotometrically determined in the incubation medium with diacetyl monoxime and thiosemicarbazide in the presence of sulphuric acid, phosphoric acid and ferric chloride as previously described.12 Results are expressed as μmol of urea synthesized per gram of LMOs (wet tissue).
- •
CPSI and OTC activities. CPSI activity was determined using the rapid colorimetric assay described by Pierson.13 The method utilizes the chemical conversion of carbamyl phosphate into hydroxyurea by the action of hydroxylamine instead of employing a coupling enzyme. The hydroxyurea was quantified by an improved colorimetric assay for ureido compounds by measuring the absorption of the resulting chromophore at 458 nm. CPSI activity was expressed as U/g of wet tissue, where U represents the μmol of carbamyl phosphate synthesized per minute at 37 °C.
OTC activity was evaluated through the determination of the citrulline produced, by the diacetylmonoxime-antipyrine reaction. This method was described by Ceriotti,14 and is based on the following reaction catalyzed by OTC:
Carbamyl phosphate + ornithine → citruline + Pi
OTC activity was expressed as U/g of wet tissue, where U equals the µmol of substrate reacting per minute at 37 °C.
- •
Histology. Samples of all the experimental groups were fixed in 10% formaldehyde in PBS (pH = 7.40) and processed for paraffin embedding. Slices of 5 μm thick were cut and stained with hematoxilin & eosin. Afterward, they were analyzed with a light field microscopy (Olympus Co, LTD. Model U-MDOB, 20X objectives, equipped with a digital camera Olympus model D-360 Zoom-3.2 megapixels of resolution) taking into account hepatocyte cords integrity, presence of vacuoles, blebs or necrotic focus, sinusoidal endothelial cells shape, and the general morphological aspect of the hepatic lobules.
Statistical differences between values were assessed by analysis of variance (ANOVA) followed by Scheffe’s multiple range test. A p value < 0.05 was considered statistically significant.
ResultsEvolution of LMOs viability during the cold preservation periodIn a previous work,6 we have demonstrated that LMOs cold preserved in BG35 and ViaSpan® solutions were able to keep their total water and glycogen content and oxygen consumption stable for up to 48 h of cold preservation. As this work focused on LMOs ammonia detoxification during the reoxygenation period, in order to assess their future use as the biological component of a BAL system, in this work we have tested LMOs viability during the cold preservation period using only LDH Release. This test constitutes a simple, fast and precise method that we have shown to correlate with other viability parameters employed to assess state of LMOs.
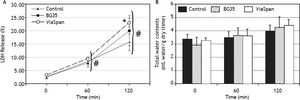
A significant increase in LDH Release during cold storage was observed for LMOs preserved in the two evaluated solutions (BG35: 4.6 ± 0.4%; 7.6 ± 0.6% and 18.9 ± 1.2%, and ViaSpan®: 6.3 ± 0.7%; 16.9 ± 0.7% and 21.1 ± 1.8%, after 0, 24 and 48 h, respectively). Both preserved groups started the preservation period with similar values of LDH Release. However, after 24 and 48 h of cold storage, the amount of this enzyme released by LMOs preserved in ViaSpan® was statistically higher than that observed for BG35 group (p < 0.05).
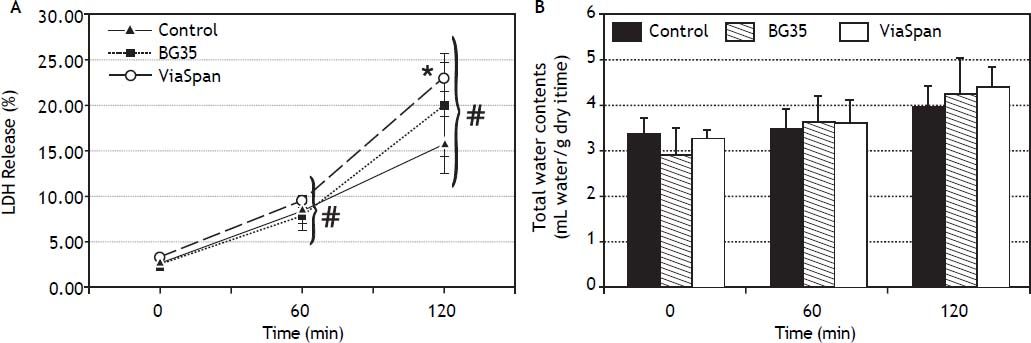
Evolution of viability of LMOs during the reoxygenation periodAfter 48 h of preservation, LMOs were submitted to the rewarming incubation. LDH Release suffered a statistically significant increase (p < 0.05) during the reoxygenation period for all the studied groups (Figure 2A), reaching the following values after 120 min: 15.8 ± 3.1% for control, 20.1 ± 5.6% for BG35 and 23.1 ± 1.7% for ViaSpan®. Again, after 2 h of rewarming, LMOs cold preserved in ViaSpan® showed a LDH Release significantly higher than controls (p < 0.05), whereas no difference was observed between controls and BG35. Figure 2B shows the TWC of controls and cold preserved LMOs. No significant differences were observed between all the studied groups, indicating that cold preserved LMOs can regulate their TWC similarly to controls. The values of TWC (mL water/g dry tissue) after 120 min of rewarming for each group were 4.0 ± 0.5 for control; 4.2 ± 0.8 for BG35, and 4.4 ± 0.5 for ViaSpan®.
A.Time course of LDH Release during 120 min of reoxygenation determined for fresh and cold preserved LMOs in BG35 and ViaSpan® solutions. Data are expressed as mean ± SD for 5 LMOs preparations. * Different from control, p < 0.05. f Different from all the other reoxygenation times, p < 0.05. B. Time course of total water content during 120 min of reoxygenation determined in fresh and 48 h cold preserved LMOs in BG35 and ViaSpan® solutions. Each bar represents the mean ± SD for 5 LMOs preparations.
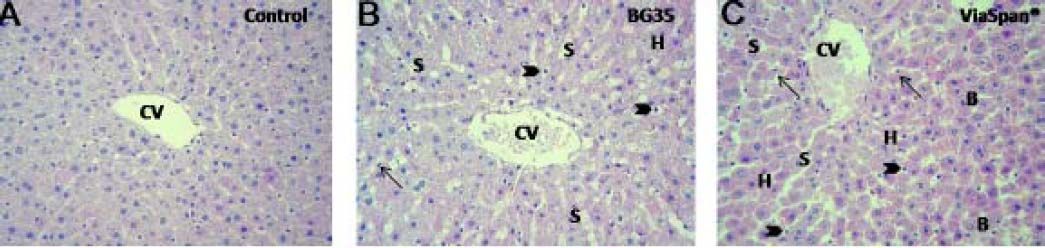
LMOs from control and preserved groups (BG 35 and ViaSpan®, 48 h at 0 °C), followed by normothermic reoxygenation were analyzed. Control group showed conserved hepatocyte cords architecture with endothelial cells attached to the perisinusoidal matrix. The shape of these cells was normal and the hepatic lobules preserved their architecture (Figure 3A). After 120 min of reoxygenation the preserved groups showed conserved hepatocyte cord integrity with sinusoides markedly opened (Figure 3B and 3C). Most of the endothelial cells were swollen and inside the sinusoidal lumen.
LMOs morphology. Samples were taken from control and preserved groups at the end (t = 120 min) of the normothermic reoxygenation step. Control: normal morphology through the hepatic parenchyma surrounding a central vein (CV). LMOs preserved in BG35 and in ViaSpan®: presented opened sinusoids (S); swollen hepatocytes (H); rounded (arrow heads) and fusiform (arrows) endothelial cells. ViaSpan® presented blebs (B) and gratest amount of swollen hepatocytes (H). Magnifications 20X.
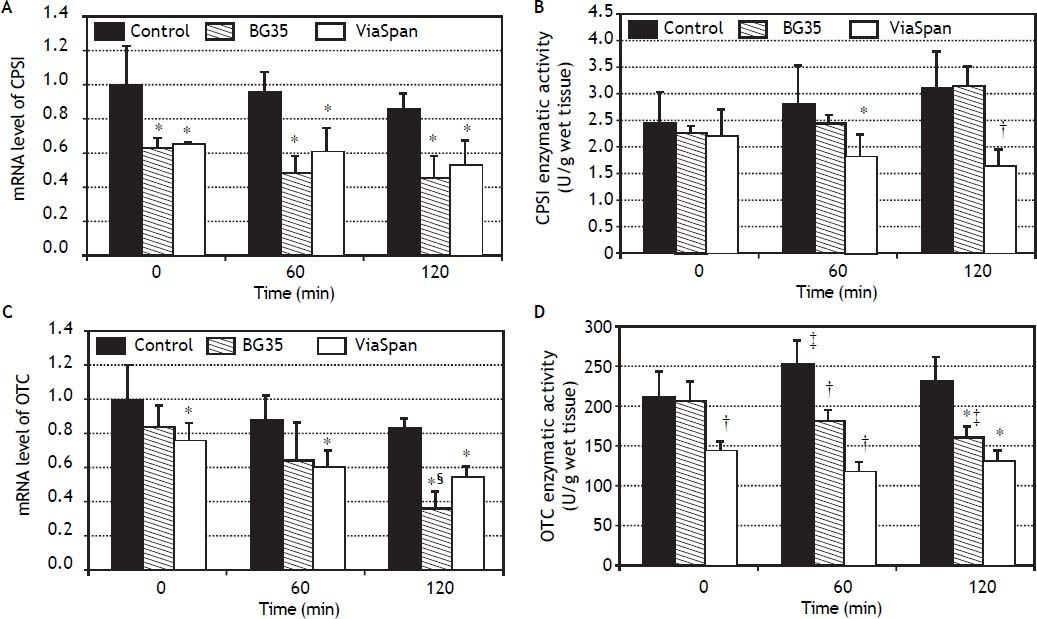
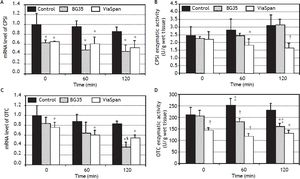
mRNA level and enzymatic activity analyses of the major urea cycle enzymes, CPSI and OTC, are shown in figure 4.
Time course evolution of: A. mRNA level of CPSI. B. CPSI enzymatic activity. C. mRNA level of OTC. D. OTC enzymatic activity. All parameters were determined in fresh and cold preserved LMOs in BG35 and ViaSpan® solutions at times 0, 60 and 120 min of rewarming. Each bar represents the mean ± SD for 3 LMOs preparations. * Different from control group, p < 0.05. † Different from the other groups, p < 0.05. ‡ Different from time 0, p < 0.05. § Different from the other reoxygenation times, p < 0.05.
Both preserved groups initially faced the reoxygenation period with transcript levels of CPSI gene lower than control group, and this statistical difference was maintained during the whole reoxygenation period (controls: 1.00 ± 0.24, 0.96 ± 0.12 and 0.86 ± 0.10; BG35: 0.63 ± 0.06, 0.48 ± 0.11 and 0.45 ± 0.14, and ViaSpan®: 0.65 ± 0.01, 0.61 ± 0.14 and 0.53 ± 0.15, p < 0.05). Though, it is worth noting that, for all the studied groups, there was no decrease in CPSI mRNA levels with increasing rewarming time (Figure 4A). Despite the transcript level differences found, all groups had similar quantities of CPSI enzymatic activity at the beginning of rewarming but, after 60 min, LMOs preserved in ViaSpan® showed a CPSI activity lower than controls (1.82 ± 0.44 vs. 2.82 ± 0.81 U/g of wet tissue, respectively, p < 0.05) and, after 120 min of reoxygenation, the activity level was not only different from fresh LMOs but also from BG35 group (controls: 3.12 ± 0.72; BG35: 3.16 ± 0.38, and ViaSpan®: 1.64 ± 0.35 U/g of wet tissue, p < 0.05, see figure 4B). As could be seen in figure 4C, only LMOs cold preserved in ViaSpan® solution showed a transcript level of OTC gene lower than fresh LMOs at all the times analyzed (control group: 1.00 ± 0.20, 0.87 ± 0.16 and 0.82 ± 0.07, and ViaSpan®: 0.76 ± 0.10, 0.60 ± 0.10 and 0.54 ± 0.07, p < 0.05). Regarding BG35 group, the OTC transcript level after 120 min showed a significant decrease compared to time 0 and 60 (0.36 ± 0.10 vs. 0.84 ± 0.12 and 0.64 ± 0.23, respectively, p < 0.05), reaching a value statistically different from that of the control group (p < 0.05) (Figure 4C).
ViaSpan® group started the reoxygenation period with lower OTC activity than fresh and BG35 preserved LMOs (141.5 ± 13.9; 209.7 ± 33.2, and 204.5 ± 25.13 U/g of wet tissue, respectively; p < 0.05). After one hour of rewarming, preserved groups maintained their OTC activities and controls had a higher activity than that of time 0 (control: 251.7 ± 29.4; BG35: 180.3 ± 14.4, and ViaSpan®: 115.4 ± 14.0 U/g of wet tissue; p < 0.05). At the end of the reoxygenation period, only BG35 group had decreased OTC activity with respect to time 0 and both preserved groups had inferior activity levels compared to fresh LMOs (control: 228.7 ± 32.7; BG35: 159.7 ± 14.9, and ViaSpan®: 128.4 ± 15.7 U/g of wet tissue; p < 0.05 (Figure 4D). Still, BG35 preserved LMOs retained better OTC activity after 120 min of reoxygenation.
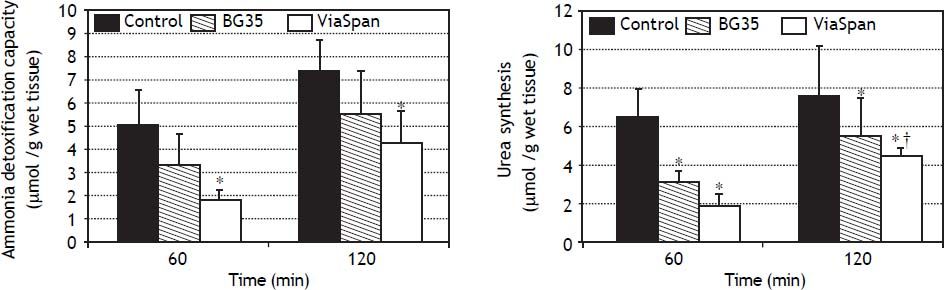
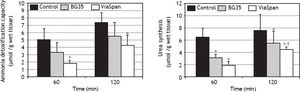
Evaluation of ammonia metabolism during the reoxygenation stepADC is shown in figure 5A. LMOs cold preserved in BG35 behaved similarly to controls, whereas ViaSpan® group demonstrated a significant lower capacity to detoxify ammonia than fresh LMOs during the whole reoxygenation period (controls: 5.0 ± 1.6 and 7.4 ± 1, and ViaSpan®: 1.8 ± 0.5 and 4.3 ± 1.4 μmol/g of wet tissue, at 60 and 120 min, respectively, p < 0.05).
Time course evolution of: A. Ammonia detoxification capacity, and B. Urea synthesis. All parameters were determined in fresh and cold preserved LMOs in BG35 and ViaSpan® solutions after 60 and 120 min of rewarming. Each bar represents the mean ± SD for 3 LMOs preparations. * Different from control group, p < 0.05. †Different from the other reoxygenation times, p < 0.05.
Regarding urea synthesis (Figure 5B), after 60 min of reoxygenation, both preserved groups had a statistically lower capacity to synthesize this metabolite than controls. At the end of the rewarming period, BG35 group was able to revert this situation and showed a level of urea synthesis similar to controls, while ViaSpan® group could not, though its synthesis ability significantly increased in comparison to time 60 (controls: 6.4 ± 0.8 and 7.5 ± 2.6; BG35: 3.1 ± 0.5 and 5.4 ± 2.0, and ViaSpan®: 1.8 ± 0.7 and 4.4 ± 0.4 μmol/g of wet tissue, at 60 and 120 min, respectively, p < 0.05).
DiscussionThe UC is an enzymatic pathway that catalyzes the elimination of ammonia produced mainly by amino acid metabolism, thereby converting ammonia into urea. The UC consists of five enzymes, carbamyl phosphate synthetase I (CPSI), ornithine transcarbamylase (OTC), argininosuccinate lyase (ASL), argininosuccinate synthetase (ASS), and arginase (ARG). Although several tissues express some UC enzymes, only hepatocytes have the metabolic capacity of detoxifying ammonia into urea. CPSI is a highly conserved mitochondrial enzyme that catalyzed the first committed step of waste nitrogen metabolism in the UC. OTC is the other UC enzyme at the mitochondrial matrix whose deficiency in humans is related with the most common and severe inborn error of the UC.15
In this work we focused on the investigation of relative mRNA level and activities of the urea cycle enzymes CPSI and OTC, ADC and urea synthesis after cold preservation because correct detoxification of ammonia is of most importance when LMOs are envisioned to be destined to BAL devices. After 48 h of cold storage of LMOs in BG35 and ViaSpan®, we evaluated if the mRNA levels and activities of CPSI and OTC suffer any changes when compared to controls LMOs, and the correlation of these parameters with ammonia metabolism (ADC and urea synthesis). We found a better conservation of the activities of CPSI and OTC during the reoxygenation step when LMOs had been preserved in BG35 solution. Even though the underlying mechanism was not determined, it is possible that the reduction of messenger RNA and activities were due to degradation during storage that was somehow prevented better by BG35 than ViaSpan®.16 Specifically, CPSI activity, but not mRNA levels, was maintained better (Figure 4A and 4B) and, both, mRNA levels and activity of OTC was well retained (Figure 4C and 4D) during reoxygenation. Noteworthy is the fact that immediately after preservation (t = 0 min of reoxygenation) both enzyme activities present similar amounts of those observed for freshly isolated control LMOs. In our model of cold preservation followed by a restoration of physiological temperatures and oxygenation it is more probable that uncontrolled break up of macromolecules occurs instead of proper turnover of these species. Furthermore, we found that transcript levels and enzymatic activities are either maintained or decreased along reoxygenation indicating that a mechanism of conservation/degradation is taking place instead of de novo transcription or protein synthesis.
When we studied the ammonia detoxification capacity and urea synthesis (Figure 3A and 3B) no differences were found between preserved groups, however, only LMOs cold stored in BG35 showed values similar to controls. Previously,6 we have demonstrated that LMOs cold stored in BG35 were the only preserved group that showed an oxygen consumption rate similar to fresh LMOs. Also formerly, we have shown that immediately after preservation ATP levels are severely decreased but are actively replenished during rewarming.17 This fact can explain our observation that urea synthesis was increased at the end of reoxygenation in both groups of LMOs. Together, these constitute an index of mitochondrial function preservation.
In a previous work we studied the ammonia detoxification capacity of cold preserved isolated hepatocytes and we found that after 2 h of reoxygenation they showed an ammonia detoxification capacity of 10.3 ± 1.4 μmol/g of wet tissue.8 This value was similar to that obtained by preserved LMOs (Figure 5A). This point is crucial because we have already probed isolated hepatocytes in an experimental BAL device with good results.18 Even though mass comparisons between isolated hepatocytes and LMOs are artful, our results point to a successful use of these tissue slices as well. LMOs have the important advantage over isolated hepatic cells that invasive cell-isolation techniques are not required. They also retain the original architecture and thus, cell-to-cell and cell-to-matrix interactions are preserved. LMOs allow the direct and rapid analysis of tissue under standardized conditions in a reproducible manner while maintaining the characteristics of the original organ.19
The objective of this work was not to compare the efficacy of the two preservation solutions employed: ViaSpan® is the gold standard in liver preservation20 and BG35 was developed in our laboratory to be used with LMOs and in the future to study the cold preservation of the whole liver. The use of BG35 solution for the storage of LMOs may facilitate liver research in situations in which the more complex and expensive ViaSpan® is not available since the cost of one litter of BG35 is about one third of that of an equal volume of ViaSpan®.17
Together, these data strongly demonstrate that our preservation conditions (48 h, BG35 preservation solution, 0 °C, and anoxia) did not affect ammonia metabolism and that cold preserved LMOs maintain the physiological and biochemical liver functions tested, which allows their future use as biological component of a BAL system. These results will allow us to advance in the development of a BAL model suitable to use fresh and cold preserved rat LMOs as it biological component.
FundingThis work was funded by the National Council of Research (Conicet), PIP 112-200801-01208 and Progetti Alta Rilevanza, Ministero degli Esteri, Italy, Prot. 269/P/0093044 and prot. 269/P0114337. M.G. Mediavilla, J.V. Rodriguez and M.E. Mamprin are members of CONICET.
Abreviations- •
ADC: ammonia detoxification capacity.
- •
BAL: bioartificial liver.
- •
BG35: bes-gluconate-PEG 35 kDa preservation solution.
- •
CPSI: carbamyl phosphate synthetase I.
- •
KHR: Krebs Henseleit reoxygenation media.
- •
LDH: lactate dehydrogenase.
- •
LMOs: liver microorgans.
- •
NRS: normothermic reoxygenation system.
- •
OTC: ornithine transcarbamylase.
- •
TWC: tissue water content
- •
UC: urea cycle.