Background. Radiofrequency ablation (RFA) has been performed as a first line curative treatment modality for patients with hepatocellular carcinoma (HCC) within the Milan criteria currently. However, prognosis of hepatitis B- and hepatitis C-related HCC after RFA remains debatable. This study aimed to assess the impact of viral etiology on the prognosis of HCC patients undergoing RFA.
Material and methods. One hundred and ninety-two patients with positive serum HBV surface antigen (HBsAg) and negative serum antibody against HCV (anti-HCV) were enrolled as the B-HCC group and 165 patients with negative serum HBsAg and positive anti-HCV as the C-HCC group. Post-RFA prognoses were compared between the two groups using multivariate and propensity score matching analyses.
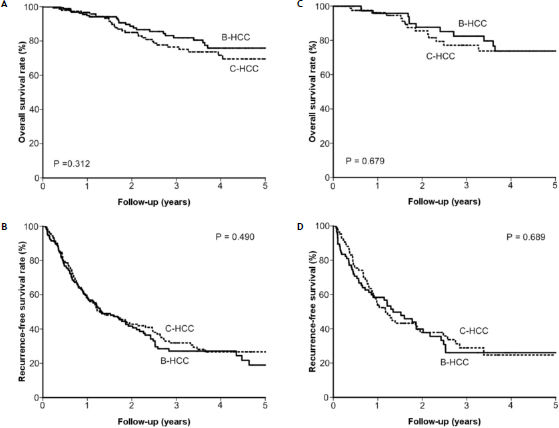
Results. The B-HCC group had higher male-to-female ratio and better liver functional reserve than the C-HCC group. After a median follow-up of 23.0 ± 22.7 months, 55 patients died and 189 patients had tumor recurrence after RFA. The cumulative five-year survival rate was 75.9% and 69.5% in the B-HCC and C-HCC groups, respectively (p = 0.312), while the five-year recurrence-free survival rate was 19.0% and 26.6%, respectively (p = 0.490). After propensity-score matching, the B-HCC group still had comparable overall survival rate (p = 0.679) and recurrence-free survival rate (p = 0.689) to the C-HCC group. For 132 patients with Barcelona-Clinic Liver Cancer stage 0, the five-year overall survival and recurrence-free survival rates were also comparable between the two groups (p = 0.559 and p = 0.872, respectively).
Conclusion. Viral etiology is not essential for determining outcome in HCC patients undergoing RFA.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy in the world and its incidence rate is still rising in Western countries.1–3 Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the major risk factors4 while current curative treatment modalities include liver transplantation, surgical resection, and local ablation therapies.5,6 Although liver transplantation is closest to cure among these modalities, it is often hampered by the shortage of donors. Surgical resection is the choice for patients with early-stage HCC and well preserved liver function.1,4 However’ tumor recurrence occurs in more than 70% of patients within 5 years after resection and early recurrence (within two years post-operatively) is noted in most cases.7 Surgical resection followed by liver transplantation in cases of tumor recurrence (salvage transplantation) may be considered,8 but a substantial number of patients fail to adopt this strategy due to tumor recurrence beyond the Milan criteria or due to co-morbidities that bar further liver transplantation. Consequently, local ablation therapies have become widely applied as treatment of small HCC for both treatment-naïve patients and those with tumor recurrence.
Among local ablation therapies, percutaneous radiofrequency ablation (RFA) is considered the best because of fewer therapy sessions, better local tumor control, and higher overall survival rate.9,10 Recent studies comparing RFA and resection surgery in HCC patients within the Milan criteria show that resection is superior to RFA in recurrence-free survival but may not provide better survival benefit than RFA in Barcelona-Clinic Liver Cancer (BCLC) stage 0 patients (single tumor with size ≤ 2 cm).1,11,12 Thus, RFA is suitable as first-line therapy for small HCC.
Several studies have compared the post-operative prognosis of HBV-related HCC and HCV-related HCC. However, whether or not the viral etiology is an independent prognostic factor of HCC remains controversial,13–19 mainly because of differences in demographic data, liver functional reserve, tumor factors, and treatment modalities. For patients with small HCC who undergo RFA, tumor size, tumor number, prothrombin activity, platelet counts, serum albumin, and serum α-fetoprotein (AFP) levels have been associated with tumor recurrence and survival.20–23 Unfortunately, there is paucity of data regarding the impact of viral etiology on longterm outcome. This study aimed to compare the prognosis between HBV- and HCV-related HCC patients who underwent RFA.
Material and MethodsPatients and follow-upThis cohort study retrospectively reviewed HCC patients who were unable or refused to undergo resection surgery or liver transplantation, and underwent RFA at Taipei Veterans General Hospital between January 1, 2002 and September 30, 2011. The choice of RFA was made by the patients after thorough discussion with their physicians regarding the advantages, side effects, and prognosis of various therapeutic modalities, including resection surgery, liver transplantation, local ablation therapy, trans-arterial chemo-embolization (TACE), and chemotherapy.
After review of medical records, 451 consecutive treatment-naïve HCC patients who fulfilled the diagnostic criteria of HCC by the American Association for the Study of Liver Disease (AASLD consensus, 2005)24 and who underwent RFA as the first treatment modality were enrolled. Consequently, the diagnosis of HCC in our cohort was established either by liver biopsy or by imaging [dynamic computed tomography (CT) or magnetic resonance imaging (MRI)]. For imaging diagnosis, HCC was diagnosed in nodules above 2 cm showing the radiological hallmark of HCC (hypervascular with washout in portal/venous phase) either by CT or MRI. In the setting of nodules of 1-2 cm in diameters, two coincidental image modalities (CT and MRI) with typical HCC vascular pattern were needed. These diagnostic criteria have been validated in two prospective studies.25,26
The inclusion criteria were tumor size < 5 cm without extra-hepatic metastasis;3 or less number of tumors; and grade A or B Child Pugh classification of liver function. The exclusion criteria were:
- •
Age < 18 years.
- •
Terminal illness of a major organ (e.g., severe heart failure, chronic obstructive pulmonary disease.
- •
HCC beyond the Milan criteria.
- •
Grade C Child Pugh classification.
- •
Other types of hepatitis (e.g., autoimmune hepatitis, Wilson’s disease, hemochromatosis).
- •
Platelet count < 70,000/mm3.
The device and procedure of RFA were as previously described.11 The number and size of tumors was measured by CT or MRI. If two or more tumors were found, the maximum size of tumors was recorded. This study complied with the standards of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB).
Among the 451 patients, 192 were assigned to the B-HCC group based on positive serum HBV surface antigen (HBsAg) and negative serum antibody against HCV (anti-HCV). Another 165 patients were enrolled in the C-HCC group based on negative serum HBsAg and positive serum anti-HCV. The remaining 94, including 15 who were positive for serum HBsAg and anti-HCV and 79 who were negative for serum HBsAg and anti-HCV, were excluded.
Dynamic CT scan was done one month after complete tumor ablation by RFA. MRI was performed in patients who were allergic to the contrast medium of CT, those with renal insufficiency, or those with inconclusive CT scan diagnosis. For confirmed residual tumors, a second RFA session was conducted. Viable tumor persisting after two RFA sessions was considered treatment failure.
If no viable tumor was detected, the patient was followed-up with testing for serum liver function, AFP levels, and ultrasonography every three months, and CT scan or MRI every 6 months until December 31, 2011. The starting date of follow-up for tumor recurrence was the day when all of the tumors ablated by RFA were confirmed by CT or MRI.
Tumor recurrence was suspected if there was elevated serum AFP level > 20 ng/mL) or a new lesion was detected by surveillance ultrasonography. These were confirmed and diagnosed by dynamic CT or MRI.
Biochemical and serologic markersSerum hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) were tested using radio-immunoassay (Abbott Laboratories, North Chicago, IL) while antibody to hepatitis C virus (HCV) was measured using second-generation enzyme immunoassay (Abbott Laboratories, North Chicago, IL). Serum biochemistry tests were measured by the Roche/Hitachi Modular Analytics Systems (Roche Diagnostics GmbH, Mannheim, Germany). Serum AFP level was measured by radio-immunoassay (Serono Diagnostic SA, Coinsin/VD, Switzerland).
Anti-viral therapy after RFANational reimbursed anti-viral therapy for chronic viral hepatitis was implemented in Taiwan since 2003. Antiviral therapy could be described for patients with chronic hepatitis B if fulfill one of the following indications:
- •
Positive HBsAg with hepatic decompensation defined as prothrombin time prolongs ≥ 3 seconds or bilirubin ≥2 mg/dL.
- •
Cirrhosis with serum HBV DNA levels ≥ 2,000 IU/mL.
- •
Positive HBsAg for more than 6 months with positive HBeAg and ALT ≥ 5 times upper limit of normal (ULN).
- •
Positive HBsAg for more than 6 months with positive HBeAg and ALT levels between 2 and 5 times ULN for 2 times apart from 3 months and HBV DNA ≥ 20,000 IU/mL.
- •
Positive HBsAg for more than 6 months with negative HBeAg and ALT ≥ 2 times ULN for 2 times apart from 3 months and HBV DNA ≥ 2,000 IU/mL.
Regarding patients with chronic hepatitis C, antiviral therapy could be prescribed if the patients fulfill all of the following indications:
- •
Positive anti-HCV antibody in serum for more than 6 months.
- •
Positive sera HCV RNA by PCR method.
- •
Serum ALT levels larger than ULN for 2 times apart from 3 months.
- •
Well compensated liver functional reserve.
The baseline characteristics to be evaluated with outcomes were selected by the 2001 European Association for the Study of the Liver (EASL) guidelines.27 Pearson chi-square analysis or Fisher’s exact test were used to compare categorical variables, while the Mann-Whitney U-test was used to compare continuous variables. The overall survival rate and recurrence-free survival rate were estimated by the Kaplan-Meier method and compared using the Cox’s proportional hazards model.
Propensity scores were used to control for selection bias.11,28,29 Variables entered in the propensity model were age, sex, anti-viral therapy, serum albumin, bilirubin, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (Alk-P) levels, prothrombin time international normalized ratio (PT-INR), platelet count, tumor size and number, and AFP. Subsequently, a one-to-one match between the B-HCC and C-HCC groups was obtained using the nearest-neighbor matching method.28 Survival analysis was repeated to analyze the overall survival and recurrence-free survival amended from these confounding factors.
Variables with statistical significance (p < 0.05) or proximate to it (p < 0.1) by univariate analysis underwent multivariate analysis via forward stepwise Cox regression model. A two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS 17.0 for Windows (SPSS. Inc., Chicago, IL, USA).
ResultsComparison of the clinical demographic data for B-HCC and C-HCC groupsThe mean age of the C-HCC group (68.4 ± 9.6 years) had a trend of being older than that of the B-HCC group (66.1 ± 12.7 years, p = 0.097) (Table 1). The proportion of male patients was higher in the B-HCC group (82.8 vs. 53.3%, p < 0.001), which also had higher serum albumin level and platelet count, and lower serum ALT and AST levels. Nevertheless, considering tumor factors, the B-HCC group had larger tumor size and lower serum AFP level than the C-HCC group.
Comparison of the demographic data of B-HCC and C-HCC patients undergoing RFA.
| Parameter | Total (n = 357) | B-HCC (n = 192) | C-HCC (n = 165) | pa |
|---|---|---|---|---|
| • Patient demographics. | ||||
| Age (y/o) (mean ± SD). | 67.2 ± 11.5 | 66.1 ± 12.7 | 68.4 ± 9.6 | 0.097 |
| Sex (M/F) (%). | 247/110 (69.2/30.8) | 159/33 (82.8/17.2) | 88/77 (53.3/46.7) | < 0.001 |
| Antiviral agents (yes/no) (%). | 106/251 (29.7/70.3) | 73/119 (38.0/62.0) | 33/132 (20.0/80.0) | < 0.001 |
| • Serum biochemistry and liver function tests.b | ||||
| Albumin (g/dL).c | 3.8; 3.3–4.1 | 3.9; 3.4–4.2 | 3.7; 3.3-4.1 | 0.017 |
| Total bilirubin (mg/dL).c | 0.8; 0.6–1.2 | 0.8; 0.6–1.2 | 0.8; 0.5–1.3 | 0.772 |
| ALT (U/L).c | 51.0; 34.0–87.0 | 40.0; 30.0–63.8 | 69.0; 45.0-116.0 | < 0.001 |
| AST (U/L).c | 55.0; 35.0–85.0 | 41.0; 30.5–63.0 | 71.0; 48.0-109.0 | < 0.001 |
| Creatinine (mg/dL).c | 0.9; 0.8–1.1 | 1.0; 0.8–1.1 | 0.9; 0.8-1.1 | 0.152 |
| Alk-P (U/L).c | 95.0; 74.8–126.0 | 93.0; 73.8–124.3 | 96.0; 75.0-126.0 | 0.624 |
| PT-INR.c | 1.1; 1.0–1.2 | 1.1; 1.0-1.2 | 1.1; 1.0–1.1 | 0.082 |
| Platelet (mm3).c | 118,000; 82,000 – 195,500 | 141,000; 87,250 – 220,750 | 101,000; 70,500 – 149,000 | < 0.001 |
| • Tumor factors.b | ||||
| Tumor size (cm).c | 2.2; 1.6–2.9 | 2.3; 1.8–2.9 | 2.1; 1.6-2.8 | 0.048 |
| Single tumor/ multi-nodularity (%) | 285/72 (79.8/20.2) | 159/33 (82.8/17.2) | 126/39 (76.4/23.6) | 0.167 |
| AFP (ng/mL).c | 24.2; 8.5–82.0 | 16.5; 6.3–69.3 | 28.7; 13.0-85.7 | 0.001 |
SD: standard deviation. ALT: alanine aminotransferase. AST: aspartate aminotransferase. PT-INR: prothrombin time-international normalized ratio. AFP: α-fetoprotein.
After a median follow-up of 23.0 ± 22.7 months, 55 patients died and 302 were alive by the last visit. The overall cumulative survival rates at 1, 2, 3, and 5 years were 94.8%, 88.6%, 81.9%, and 75.9%, respectively, in the B-HCC group and 96.6%, 85.0%, 76.5%, and 69.5%, respectively, in the C-HCC group. The overall survival rates were comparable between the two groups (p = 0.312) (Figure 1A).
The overall survival rate (p = 0.312) (A) and recurrence-free survival rate (p = 0.490) (B) between the B-HCC and C-HCC groups were comparable. After propensity score matching, the overall survival rate (p = 0.679) (C) and recurrence-free survival rate (p = 0.689) (D) between the two groups were still comparable.
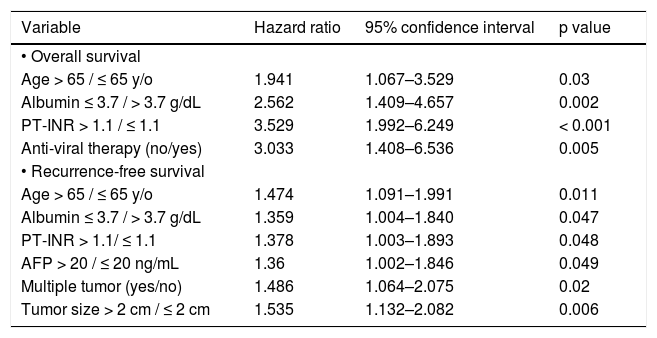
By multivariate analysis, age > 65 years (Hazard ratio [HR] 1.941, 95% confidence interval [95% CI] 1.067–3.529; p = 0.030), serum albumin level < 3.7 g/ dL (HR 2.562, 95% CI: 1.409–4.657; p = 0.002), PT-INR > 1.1 (HR 3.529, 95% CI: 1.992–6.249; p < 0.001), and no anti-viral therapy (HR 3.033, 95% CI: 1.408–6.536; p = 0.005) were independent risk factors associated with poor overall survival (Table 2).
Multivariate analysis of prognostic factors for overall survival and recurrence-free survival in B-HCC or C-HCC group (n = 357).
| Variable | Hazard ratio | 95% confidence interval | p value |
|---|---|---|---|
| • Overall survival | |||
| Age > 65 / ≤ 65 y/o | 1.941 | 1.067–3.529 | 0.03 |
| Albumin ≤ 3.7 / > 3.7 g/dL | 2.562 | 1.409–4.657 | 0.002 |
| PT-INR > 1.1 / ≤ 1.1 | 3.529 | 1.992–6.249 | < 0.001 |
| Anti-viral therapy (no/yes) | 3.033 | 1.408–6.536 | 0.005 |
| • Recurrence-free survival | |||
| Age > 65 / ≤ 65 y/o | 1.474 | 1.091–1.991 | 0.011 |
| Albumin ≤ 3.7 / > 3.7 g/dL | 1.359 | 1.004–1.840 | 0.047 |
| PT-INR > 1.1/ ≤ 1.1 | 1.378 | 1.003–1.893 | 0.048 |
| AFP > 20 / ≤ 20 ng/mL | 1.36 | 1.002–1.846 | 0.049 |
| Multiple tumor (yes/no) | 1.486 | 1.064–2.075 | 0.02 |
| Tumor size > 2 cm / ≤ 2 cm | 1.535 | 1.132–2.082 | 0.006 |
PT: prothrombin time. INR: international normalized ratio. AFP: α-fetoprotein.
For patients in the B-HCC group, serum albumin ≤ 3.7 g/dL (p = 0.003) was an independent risk factor associated with mortality in multivariate analysis. In the C-HCC group, age > 65 years (p = 0.019), serum albumin < 3.7 g/dL (p = 0.050), PT-INR > 1.1 (p < 0.001), AST > 45 U/L (p = 0.034), serum creatinine > 1.2 mg/dL (p = 0.009), AFP > 20 ng/mL (p = 0.012), and no anti-viral therapy (p = 0.022) were associated with poor overall survival in multivariate analysis (Table 3).
Multivariate analysis of prognostic factors for overall survival and recurrence-free survival in B-HCC (n = 192) and C-HCC (n = 165) group.
| Variable | Hazard ratio | 95% confidence interval | p value |
|---|---|---|---|
| Overall survival. | |||
| • B-HCC. | |||
| Albumin ≤ 3.7 / > 3.7 g/dL. | 3.482 | 1.513–8.015 | 0.003 |
| • C-HCC. | |||
| Age > 65 / ≤ 65 y/o. | 3.432 | 1.229–9.581 | 0.019 |
| Albumin ≤ 3.7 / > 3.7 g/dL. | 2.519 | 0.999–6.350 | 0.05 |
| PT-INR > 1.1/ ≤ 1.1. | 5.875 | 2.543–13.572 | < 0.001 |
| AST > 45 / ≤ 45 U/L. | 2.793 | 1.078–7.246 | 0.034 |
| Creatinine > 1.2/ ≤ 1.2 mg/dL. | 4.162 | 1.431–12.106 | 0.009 |
| AFP > 20 / ≤ 20 ng/mL. | 3.481 | 1.310–9.251 | 0.012 |
| Anti-viral therapy (no/yes). | 6.503 | 1.316–32.142 | 0.022 |
| Recurrence-free survival. | |||
| • B-HCC. | |||
| Age, > 65 / ≤ 65 y/o. | 1.888 | 1.249–2.853 | 0.003 |
| Albumin ≤ 3.7 / > 3.7 g/dL. | 1.644 | 1.060–2.549 | 0.026 |
| PT-INR > 1.1 / ≤ 1.1. | 1.908 | 1.227–2.966 | 0.004 |
| Multiple tumors (yes/no). | 1.916 | 1.166–3.147 | 0.01 |
| • C-HCC. | |||
| AFP > 20 / ≤ 20 ng/mL. | 2.101 | 1.311–3.366 | 0.002 |
| Multiple tumors (yes/no). | 1.625 | 1.021–2.585 | 0.041 |
| Tumor size > 2 cm / ≤ 2 cm. | 1.906 | 1.245–2.918 | 0.003 |
AST: aspartate aminotransferase. PT: prothrombin time. INR: international normalized ratio. AFP: α-fetoprotein.
After RFA, 189 patients had tumor recurrence, with 10.5 ± 18.0 months median time of development. The recurrence-free survival rates at 1, 2, 3, and 5 years were 58.3%, 41.7%, 27.1%, and 19.0%, respectively, in the B-HCC group and 57.5%, 42.8%, 31.9%, and 26.6%, respectively, in the C-HCC group. The recurrence-free survival rates in the B-HCC and C-HCC groups were not significantly different (p = 0.490) (Figure 1B). Taken together, the influence of viral etiology did not impact on the outcomes of RFA in the whole groups of HCC patients in terms of overall survival and recurrence-free survival.
By multivariate analysis, age > 65 years (HR 1.474, 95% CI: 1.091-1.991; p = 0.011), serum albumin level ≤ 3.7 g/dL (HR 1.359, 95% CI: 1.004–1.840; p = 0.047), PT-INR > 1.1 (HR 1.378, 95% CI: 1.003– 1.893; p = 0.048), AFP > 20 ng/mL (HR 1.360, 95% CI: 1.002–1.846; p = 0.049), multiple tumors (HR 1.486, 95% CI: 1.064–2.075; p = 0.020), and tumor size > 2 cm (HR 1.535, 95% CI: 1.132–2.082; p = 0.006) were independent risk factors associated with recurrence-free survival after RFA (Table 2).
In the B-HCC group, multivariate analysis revealed that age > 65 years (p = 0.003), albumin < 3.7 g/dL (p = 0.026), PT-INR > 1.1 (p = 0.004), and multiple tumors (p = 0.010) were independent risk factors associated with tumor recurrence. For the C-HCC group, AFP > 20 ng/mL (p = 0.002), multiple tumors (p = 0.041), and tumor size > 2 cm (p = 0.003) were related to recurrence-free survival after RFA (Table 3).
Comparison of prognosis after propensity score correction with one-to-one nearest-neighbor matching methodPropensity analysis with one-to-one nearest-neighbor matching method was applied to minimize confounding factors like age, sex, anti-viral therapy, serum albumin, bilirubin creatinine, ALT, AST, and Alk-P levels, PT-INR, platelet counts, tumor size, tumor number, and AFP. Eighty-six patients were matched in each group, with the previously mentioned factors appearing to be well-matched between these two groups (Table 4). After matching, the overall survival rate and recurrence-free rate remained comparable [p = 0.679 (Figure 1C) and p = 0.689 (Figure 1D), respectively].
Comparison of the demographic data of B-HCC and C-HCC patients undergoing RFA, by Propensity analysis with one-to-one nearest-neighbor matching method.
| Parameter | Total (n = 172) | B-HCC (n = 86) | C-HCC (n = 86) | Pa |
|---|---|---|---|---|
| • Patient demographics. | ||||
| Age (y/o) (mean ± SD). | 67.6 ±11.2 | 66.5 ± 12.0 | 68.8 ± 10.2 | 0.168 |
| Sex (M/F) (%). | 122/50 (70.9/29.1) | 61/25 (70.9/29.1) | 61/25 (70.9/29.1) | 1 |
| Anti-viral agents (yes/no) (%). | 47/125 (27.3/72.7) | 29/57 (33.7/66.3) | 18/68 (20.9/79.1) | 0.087 |
| • Serum biochemistry tests and liver function tests.b | ||||
| Albumin (g/dL).c | 3.8; 3.2–4.1 | 3.9; 3.4–4.2 | 3.6; 3.1–4.0 | 0.149 |
| Total bilirubin (mg/dL).c | 0.8; 0.5–1.3 | 0.8; 0.6–1.2 | 0.8; 0.5–1.3 | 0.286 |
| ALT (U/L).c | 55.0; 37.0–84.0 | 53.0; 36.0–85.5 | 57.0; 37.0–84.5 | 0.863 |
| AST (U/L).c | 59.0; 36.0–85.6 | 52.5; 35.0–85.0 | 65.5; 41.8-89.8 | 0.631 |
| Creatinine (mg/dL).c | 0.9; 0.8–1.2 | 0.9; 0.7–1.1 | 1.0; 0.8-1.2 | 0.287 |
| Alk-P (U/L).c | 93.0; 71.0–119.5 | 93.0; 76.0–125.0 | 93.0; 65.0-117.0 | 0.318 |
| PT-INR.c | 1.1; 1.0–1.1 | 1.1; 1.0–1.2 | 1.1; 1.0-1.1 | 0.46 |
| Platelet (mm3).c | 120,000; 82,000–196,000 | 143,500; 98,750– 24,9250 | 104,500; 69,250-166,750 | 0.14 |
| • Tumor factors.b | ||||
| Tumor size (cm).c | 2.2; 1.6–2.8 | 2.2; 1.7–2.9 | 2.1; 1.6-2.8 | 0.166 |
| Single tumor/multi-nodularity (%). | 138/34 (80.2/19.8) | 69/17 (80.2/19.8) | 69/17 (80.2/19.8) | 1 |
| AFP (ng/mL).c | 21.0; 0.3–80.4 | 18.1; 8.7–86.4 | 26.0; 10.4-80.1 | 0.137 |
SD: standard deviation. ALT: alanine aminotransferase. AST: aspartate aminotransferase. PT-INR: prothrombin time-international normalized ratio. AFP: α-fetoprotein.
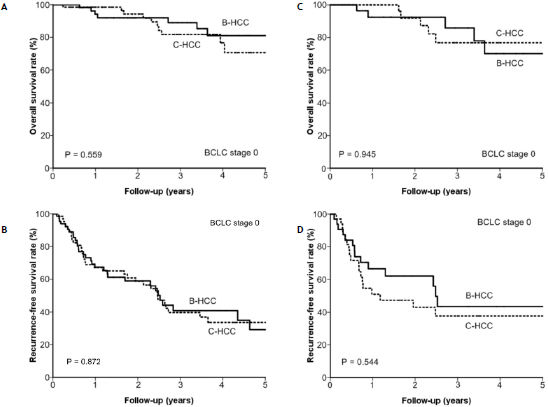
In this cohort, 132 patients with solitary HCC ≤ 2 cm in size were defined as very early small HCC and BCLC stage 0.1 The B-HCC group and C-HCC groups each had 66 such patients. After a median follow-up of 29.5 ± 23.6 months, 17 patients with BCLC stage 0 HCC died and 115 were alive. The overall cumulative survival rates at 1, 2, 3, and 5 years were 96.4%, 93.2%, 85.0%, and 75.8%, respectively. Stratified by viral etiology, the overall cumulative survival rates were 94.1%, 92.0%, 89.0%, and 81.0%, respectively, in the B-HCC group and 98.4%, 94.2%, 81.7%, and 70.7%, respectively, in the C-HCC group (p = 0.559) (Figure 2A).
for patients with BCLC stage 0, the (A) overall survival rate (p = 0.559) and (B) recurrence-free survival rate (p = 0.872) between the B-HCC and C-HCC groups were comparable (p = 0.752). After propensity score matching, the overall survival rate (p = 0.945) (C) and recurrence-free survival rate (p = 0.544) (D) between the two group remained comparable.
Multivariate analysis showed that age > 65 years (HR 5.206, 95% CI: 1.409–19.236; p = 0.013), serum albumin level ≤ 3.7 g/dL (HR 4.271, 95% CI: 1.342– 13.592; p = 0.014), PT-INR > 1.1 (HR 6.273, 95% CI: 1.771–22.212; p = 0.004), and no anti-viral therapy (HR 6.074, 95% CI: 1.507–24.484; p = 0.011) were independent risk factors associated with poor overall survival (Table 5).
Multivariate analysis of prognostic factors for overall survival and recurrence-free survival in B-HCC or C-HCC group in BCLC stage 0 (n = 132).
| Variable | Hazard ratio | 95% confidence interval | p value |
|---|---|---|---|
| • Overall survival. | |||
| Age > 65 / ≤ 65 y/o. | 5.206 | 1.409 – 19.236 | 0.013 |
| Albumin ≤ 3.7 / > 3.7 g/dL. | 4.271 | 1.342 – 13.592 | 0.014 |
| PT INR > 1.1/ ≤ 1.1. | 6.273 | 1.771 – 22.212 | 0.004 |
| Antiviral therapy (no/yes). | 6.074 | 1.507 – 24.484 | 0.011 |
| • Recurrence-free survival. | |||
| Albumin ≤ 3.7 / > 3.7 g/dL. | 2.906 | 1.728 – 4.889 | < 0.001 |
PT: prothrombin time. INR: international normalized ratio. AFP: α-fetoprotein.
Regarding recurrence, 62 patients with BCLC stage 0 HCC developed recurrence after RFA in a median time of 14.7 ± 21.6 months. The recurrence-free survival rates at 1, 2, 3, and 5 years were 67.3%, 58.9%, 40.8%, and 29.1%, respectively. The B-HCC and C-HCC groups had similar rates of developing recurrence after RFA (p = 0.872) (Figure 2B). By multivariate analysis, only serum albumin level ≤ 3.7 g/dL (HR 2.906, 95% CI: 1.728– 4.889; p < 0.001) was associated with recurrence-free survival for patients with BCLC stage 0 HCC after RFA (Table 5).
After propensity score matching, patients in the B-HCC and C-HCC groups still had similar prognosis in both overall survival (p = 0.945) (Figure 2C) and recurrence-free survival (p = 0.544) (Figure 2D).
DiscussionFor HCC, HBV and HCV infections represent major risk factors.30 There is increasing evidence that these infections may have different mechanisms of hepatocarcinogenesis.31,32 In patients with chronic hepatitis B, HBV DNA can integrate into the host genome and directly induce HCC without cirrhosis.33 In contrast, the persistent chronic inflammatory status induced by viral infection seems to be the major route of hepatocarcinogenesis in HCV-induced HCC.34 Given the distinct oncogenic pathway and natural history, differences in the prognosis of HCC should also be expected according to its etiology. However, evidence has remained equivocal because of heterogeneous selection criteria, treatment methods, and unbalanced age, liver function, and cancer stage between these two groups in most studies.13–19
RFA is an alternative first-line therapy for small HCC. Unfortunately, there have been very few studies that compared post-RFA prognosis between B-HCC and C-HCC. Shiina, et al. reported that C-HCC was a poor indicator of overall survival and distant recurrence after RFA.35 This study was from an area where chronic HCV infection was the predominant etiology for HCC and 28% of patients in their cohort were treated with a combination of TACE and RFA as the initial treatment modality. The impact of viral factors on post-RFA outcomes still needed to be elucidated.
The current study is the first to directly compare post-RFA prognosis in patients with HCC caused by HBV and HCV. As the demographic characteristics are quite different between these two groups, it is difficult to evaluate the true impact of viral etiology on the outcomes of patients with HCC after RFA therapy. In this study, both multivariate analysis and propensity score matching analysis have been conducted to reduce the influence of confounding factors on prognosis. Consistent with the result of multivariate analysis, patients in the B-HCC group have comparable outcomes to those in the C-HCC group in overall survival rate and in recurrence-free survival rate after adjustments by propensity score matching analysis.
Moreover, in patients with HCC in BCLC stage 0 (very early stage), there is still no difference in post-RFA prognosis between the two groups. This is similar to results of our previous study in which the long-term outcomes were shown to be comparable between patients with HBV- and HCV-related HCC undergoing resection surgery.19 This also suggests that viral etiology is not crucial in determining the prognosis of patients with early stage HCC receiving curative therapies, regardless of resection surgery or RFA.
Regarding host factors, patients in the C-HCC group in the present cohort have a trend of older age, lower male-to-female ratio, lower platelet count and serum albumin, and higher ALT and AST levels than those in the B-HCC group. These data are similar to those of previous reports in Taiwan.13,14,17,19 In Taiwan, HCV exposure is usually acquired in adulthood whereas HBV infection is through vertical transmission during delivery or acquired in early childhood.36 As the process of hepatocarcinogenesis is complex and requires time to accumulate sufficient mutations, it is reasonable that patients in the C-HCC group are older than those in the B-HCC group. Furthermore, HCC is more prevalent in males than in females in the B-HCC group. This male predominance in HBV-related HCC has been proposed to be related to the higher blood testosterone levels and more active androgen receptor.37 A recent study has further demonstrated that the HBx protein can interact with the androgen receptor signaling pathway to enhance hepatocarcinogenesis.38
The higher serum ALT and AST levels and lower platelet count and serum albumin level may reflect more hepatic inflammation and advanced liver fibrosis in C-HCC.39 Such findings imply that patients in the C-HCC group have relatively poorer liver functional reserve than those in the B-HCC group. However, total bilirubin levels and prothrombin time are similar between these two groups. This may be due to the selection criteria for RFA, which exclude patients with jaundice and coagulopathy.
Liver functional reserve, as well as prothrombin time and serum albumin, are strong predictors of overall survival and recurrence-free survival rates, similar to results of previous studies.20,21,23,35 While tumor factors like serum AFP level, tumor number, and tumor size are predictors of recurrence-free survival, they are not associated with overall survival. This was probably because recurrent tumors are small, can be detected by close surveillance, and can be completely ablated by repeat local ablation therapy.40 Consequently, the outcome of patients with tumor recurrence after RFA remains acceptable if they are followed-up regularly.
The effect of anti-viral therapy on reducing HCC recurrence after curative treatment remains controversial.41–43 In the present cohort, patients receiving anti-viral therapy after RFA have better overall survival than those without therapy, although this has no effect on recurrence-free survival. The use of anti-viral therapy after RFA may improve clinical outcomes due to the restored liver functional reserve, which substantially influences overall survival in patients with small HCC undergoing RFA. Nevertheless, the administration of anti-viral therapy in Taiwan should fulfill the rules of National Health Insurance which anti-viral therapy is given while persistent viral activity and hepatitis is evidenced by liver biochemistry data and molecular assays. It implied that patients without anti-viral therapy may not have evidence of persistent viral activity. However, patients in the C-HCC group with high HCV viral loads and active hepatic necro-inflammation may not receive pegylated interferon and ribavirin combination therapy due to the concern of side effects and compliance. Consequently, these may have bias in evaluating the true effect of anti-viral therapy due to retrospective analysis. Further large-scale prospective studies are warranted to investigate the impact of anti-viral therapy on tumor recurrence after RFA.
This study has several limitations. First, this is a retrospective study and some confounding factors like alcoholism were not evaluated. Second, the prognosis of HCC with HBV and HCV dual infections, and non-HBV, non-HCV related HCC were not compared due to the small case numbers. Third, the effects of anti-viral therapy after RFA warrant further large-scale trials with longer observation times.
In conclusion, viral etiology does not impact on the outcome of small HCC patients who undergo RFA. Liver functional reserve is, however, crucial in determining post-RFA prognosis in HCC patients. Anti-viral therapy may improve overall survival.
DisclosuresThere is no potential conflict of interest involved in this paper.
Grant SupportThis study was supported by grants from the National Science Council, Taiwan (98-2314-B-075-030-MY2, 100-2314-B-075-073), Taipei Veterans General Hospital (V98C1-120, V100A-034, V100C-034, V101C-083), and the Center of Excellence for Cancer Research at TVGH (DOH100-TD-C-111-007), Taipei, Taiwan.
ContributionsDrs. PH Chen and WY Kao contributed equally to this work.
Author Contribution- •
Ping-Hsien Chen: study concept and design; analysis and interpretation of data; drafting of the manuscript.
- •
Wei-Yu Kao: study concept and design; analysis and interpretation of data; drafting of the manuscript.
- •
Yi-You Chiou: performance of the radiofrequency ablation therapy.
- •
Hung-Hsu Hung: study concept and design; analysis and interpretation of data.
- •
Chien-Wei Su: overall study concept and design; analysis and interpretation of data; drafting of the manuscript.
- •
Yi-Hong Chou: performance of the radiofrequency ablation therapy.
- •
Teh-Ia Huo: study concept and design, valuable discussion and support.
- •
Yi-Hsiang Huang: valuable discussion.
- •
Wen-Chieh Wu: study concept and design, valuable discussion and support.
- •
Yee Chao: study concept and design, valuable discussion and support.
- •
Han-Chieh Lin: study concept and design, valuable discussion and support.
- •
Jaw-Ching Wu: overall study concept and design, analysis and interpretation of data; critical revision of the manuscript for important intellectual content and final drafting of the manuscript.