Background and rationale. The post-Liver Transplant Quality of Life (pLTQ) questionnaire, developed in the United States, is a disease-targeted instrument designed to evaluate health-related quality of life (HRQoL) in liver transplant recipients. Our study sought to validate a version of the pLTQ for use in the Brazilian population. Translation and cross-cultural adaptation were carried out in accordance with international standard practices for questionnaire validation. Validity was measured by means of convergent validity (correlations between pLTQ domains and WHOQOL-Bref domains). Reliability was assessed by measurement of internal consistency (Cronbach’s alpha coefficient), reproducibility (intraclass correlation coefficient), sensitivity to change (effect size), and floor and ceiling effects.
Results. The study sample comprised 160 liver transplant recipients, with a mean age of 56.9 ± 10.4 years, treated at a tertiary hospital in Southern Brazil. The sample was largely male (62.5%), and the predominant indication for liver transplant was hepatocellular carcinoma (49.4%). Only two questionnaire items were modified during the translation and cross-cultural validation stage. The mean total pLTQ score was 5.58 ± 0.9, with < 20% floor/ceiling effect. Correlations between pLTQ and WHOQOL-Bref domains were acceptable (r = 0.37 - 0.40). For similar dimensions, the correlations between WHOQOL-Bref and pLTQ were statistically significant (p ≤ 0.001). Cronbach’s alpha for the total score was 0.91 (95% CI 0.89 - 0.93), with a range of 0.51 to 0.77 across domains. Reproducibility was 0.90, and sensitivity to change was 0.84.
Conclusion. In conclusion, the Brazilian Portuguese version of the pLTQ exhibited good psychometric performance, suggesting that it can be a useful tool in the Brazilian cultural context.
Liver transplantation (LTx) is the preferred treatment modality for patients with end-stage liver disease.1 Approximately 2,500 LTx procedures are performed every year in Latin America.2 In Brazil, 1,700 LTx were performed in 2013.3 With improved outcomes the population of liver transplant recipients is growing worldwide.4 However, it should be taken into account that patients who undergo LTx require permanent care, largely due to clinical complications that usually occur after the proce-dure.5 As survival and other clinical aspects related to LTx have improved substantially, the focus of care has shifted to also include quality of life (QoL),6,7 defined by the World Health Organization (WHO) as “state of complete physical, mental, and social well-being and not merely the absence of disease and infirmity”, and, particularly, on health-related quality of life (HRQoL),8 which describes the repercussions of disease and its treatment on the lifestyle, mental balance, and well-being of patients, according to their own judgments and perceptions.
Systematic reviews and meta-analysis of studies that evaluated HRQoL in liver transplant recipients by means of generic questionnaires have shown that, in general, LTx improves HRQoL.9–11 However, until recently, no specific instrument was available for assessment of HRQoL in liver transplant recipients. To bridge this gap, the U.S. group of Saab, et al. developed the post-Liver Transplant Quality of Life (pLTQ) questionnaire.12 The pLTQ is relatively fast and easy to administer.
The applicability of HRQoL measurement instruments is limited by the validity and reliability of each questionnaire in different populations.11,13 When developed in countries other than the country in which they will be employed, additional validation through a standardized process (translation into the local language and adaptation to the cultural context of the country in question) is essential.13,14 The objective of the present study was to cross-culturally adapt and validate the pLTQ in Brazilian Portuguese for use in LTx recipients.
Material and MethodsPatientsBetween September 2012 and January 2013, a convenience sample of LTx recipients aged >18 years and treated at the outpatient clinic of the Liver Transplantation Group at Complexo Hospitalar da Santa Casa de Misericórdia de Porto Alegre, Brazil, was invited to take part in the study. Patients were excluded from analysis if they had undergone combined liver-kidney transplantation, were receiving treatment for hepatitis C virus infection, or had cognitive limitations that prevented understanding of questionnaire items.
QuestionnairesTwo questionnaires were administered to the population of interest: the pLTQ12 (Appendix 1) and the previously validated Brazilian Portuguese version of the WHOQOL-Bref.15 The pLTQ assesses specific factors that can affect the lives of patients who have undergone LTx, including symptoms, mood, limitation of the activities of daily living, energy level, and transplant-related care. It comprises 32 items, grouped into eight domains: Emotional (four items), Worry (seven items), Medications (four items), Physical Function (six items), Healthcare (four items), Graft Rejection Concern (two items), Financial (two items), and Pain (three items). Items are scored on a seven-point Likert-type scale, where 1 corresponds to “always” and 7 to “never”. The pLTQ yields individual scores for each domain as well as a multidimensional total score.12 The WHOQOL-Bref16 is a generic QoL assessment questionnaire developed by WHO that comprises 26 items across four domains: Physical Health, Psychological Health, Social Relationships, and Environment.
The questionnaires were administered by means of an interview (test), by two previously trained investigators, when patients attended the clinic for their regularly scheduled appointments. The instruments were readministered (retest) after, on average, 1 month. Alongside pLTQ administration, patients underwent a clinical assessment and completed a Global Rating of Change (GRC) survey. The GRC was designed as a self-assessment of changes in health condition between the first and second administrations of the questionnaire, and consisted of a single question answered on a five-point Likert-type scale.17 For analysis, the patients were divided into two subgroups: “change” (reported improvement or deterioration between the two visits) and “no change” (did not experience change). This enabled assessment of the sensitivity to change and reproducibility of the pLTQ.
Translation and validation processThe study consisted of two stages: translation of the pLTQ and assessment of its psychometric properties.
ValidationThe validation process was conducted in accordance with internationally recommended standard, in the following sequence of stages:18
- •
Initial translation: the items of the original English-language version of the pLTQ were translated (conceptually, not strictly literally) into Brazilian Portuguese by two independent, bilingual health professionals, both of whom were aware of the objective of the study. Differences between the versions produced by each translator were discussed between them and with one of the authors of the original questionnaire to reach a consensus version.
- •
Back-translation of the consensus version into English by two independent, bilingual translators, both of whom were aware of the objective of the study and neither of whom had been involved in the initial translation. The resulting version was compared with the original and discussed by the translators, with modifications made as necessary to achieve better equivalence.
- •
Assessment and approval of the resulting versions (Portuguese and English) by the authors of the original instrument.
- •
Administration of the questionnaire to 10 subjects drawn from the target population, to identify potential barriers to understanding, and implementation of final adjustments in response to the subjects’ suggestions.
This led to the inclusion of a parenthetical explanation to improve understanding of one item and to a change in the wording of another (replacing the term irritadiço, “cranky”, with irritado, “upset”). The questionnaire was administered by means of an interview, and patients had no issues choosing their responses.
Assessment of psychometric propertiesAssessment of psychometric properties (field validation) was carried out after administration of the resulting version of the pLTQ to LTx recipients receiving outpatient follow-up. The psychometric properties of interest were validity and reliability. The former was evaluated by measurement of convergent validity, assessing whether the pLTQ domains correlated well with the equivalent WHOQOL-Bref domains. The validity of the pLTQ was evaluated by measuring specific correlations (item-total and item-domain). Reliability was assessed by determining:
- •
Internal consistency, by means of Cronbach’s alpha (α) coefficient).
- •
Sensitivity to change (i.e., whether the instrument was able to identify differences in QoL between interviews), by measuring the effect size (ES).18
- •
Reproducibility (i.e., whether the instrument produces similar results, assuming there has been no change in patient condition), by measuring the intraclass correlation coefficient (ICC),17,19 and
- •
Ceiling and floor effects.18
Optimally, these effects should be below 15-20%.18 Internal consistency was evaluated in the sample as a whole, but sensitivity and reproducibility were dependent on GRC results. In the “change” subgroup, effect sizes were calculated, whereas in the “no change” subgroup, ICC was calculated instead.
Clinical variables of interestData were collected on the following variables: socioeconomic status, severity of liver disease on the day of LTx, time elapsed between LTx and first interview, and presence of comorbidities. Socioeconomic status was evaluated by means of the Brazilian Economic Classification Criterion,20 2009 version, which identifies five economic classes (A, B, C, D, E) according to a combination of the following elements: educational attainment of the head of household, ownership of certain material goods, and employing a domestic worker on a monthly basis. The Brazil-validated version of the Model for End-Stage Liver Disease (MELD) severity score21 was used to assess the severity of liver disease at the time of transplantation. The MELD score was calculated using the United Network for Organ Sharing (UNOS) formula.22 Time (in months) elapsed between transplantation and administration of the questionnaire was stratified as follows: 0 to 6 months; 6 to 12 months; or > 12 months.
The presence or absence of comorbidities was recorded, with particular emphasis on diagnoses of diabetes mel-litus, hypertension, osteoporosis, and obesity.
Statistical analysisThe sample size was calculated as 160 participants, taking into account the inclusion of five participants per item of the instrument. The statistical significance level was set at 5%.
Categorical variables were expressed as absolute and relative frequencies, and continuous variables as mean and standard deviation or median and interquartile range as appropriate.
Convergent validity was measured by assessing whether the pLTQ domains correlated well with the equivalent WHOQOL-Bref domains. This was done by means of Spearman correlation coefficients (r), with values > 0.3 deemed acceptable. Reliability was tested by means of internal consistency and ceiling and floor effects, which were considered substantial if > 20%. In assessing the internal consistency of the instrument, Cronbach’s a values ≥ 0.5 were deemed adequate. Reproducibility, as assessed by the ICC between interviews in the “no change” subgroup, was considered acceptable if ICC ≥ 0.60. Sensitivity to change was estimated by calculating the ES of differences between the two interviews in respondents allocated to the “change” subgroup on the basis of GRC results. Effect sizes are classified as small (ES = 0.2), medium (ES = 0.5), or large (ES = 0.8).
Ethical aspectsAfter written authorization for cross-cultural adaptation and validation had been obtained from the main author of the pLTQ instrument, the study project was submitted to and approved by the Research Ethics Committee of Irmandade da Santa Casa de Misericórdia de Porto Alegre. All respondents provided written informed consent for participation at the time of the initial interview.
ResultsOverall, 164 patients were eligible for inclusion; of these, four were excluded due to refusal to participate.
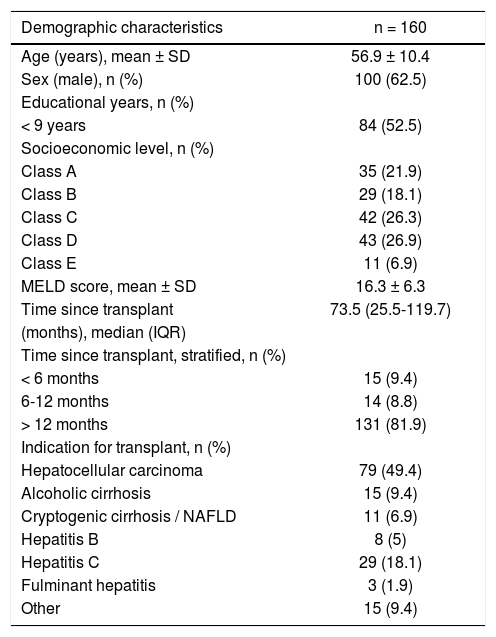
The general characteristics of the 160 patients included in the study are shown in table 1.
Demographic characteristics of liver transplant recipients.
| Demographic characteristics | n = 160 |
|---|---|
| Age (years), mean ± SD | 56.9 ± 10.4 |
| Sex (male), n (%) | 100 (62.5) |
| Educational years, n (%) | |
| < 9 years | 84 (52.5) |
| Socioeconomic level, n (%) | |
| Class A | 35 (21.9) |
| Class B | 29 (18.1) |
| Class C | 42 (26.3) |
| Class D | 43 (26.9) |
| Class E | 11 (6.9) |
| MELD score, mean ± SD | 16.3 ± 6.3 |
| Time since transplant | 73.5 (25.5-119.7) |
| (months), median (IQR) | |
| Time since transplant, stratified, n (%) | |
| < 6 months | 15 (9.4) |
| 6-12 months | 14 (8.8) |
| > 12 months | 131 (81.9) |
| Indication for transplant, n (%) | |
| Hepatocellular carcinoma | 79 (49.4) |
| Alcoholic cirrhosis | 15 (9.4) |
| Cryptogenic cirrhosis / NAFLD | 11 (6.9) |
| Hepatitis B | 8 (5) |
| Hepatitis C | 29 (18.1) |
| Fulminant hepatitis | 3 (1.9) |
| Other | 15 (9.4) |
SD: standard deviation. MELD: Model for End-stage Liver Disease. IQR: interquartile range. NAFLD: nonalcoholic fatty liver disease. Other: primary sclerosing cholangitis, autoimmune cirrhosis, polycystic liver disease, primary biliary cirrhosis, and liver metastases from neuroendocrine cancer.
Most patients (62.5%) were male, and the mean age was 56.7 years (SD, 10.4 years). Approximately 52% of patients had ≤ 9 years of formal schooling and 85% were in socioeconomic class C or D. Hepatocellular carcinoma (associated with hepatitis C virus infection in > 60% of patients) was the indication for LTx in half of all cases; in 82% of patients, LTx had been performed > 12 months before study inclusion.
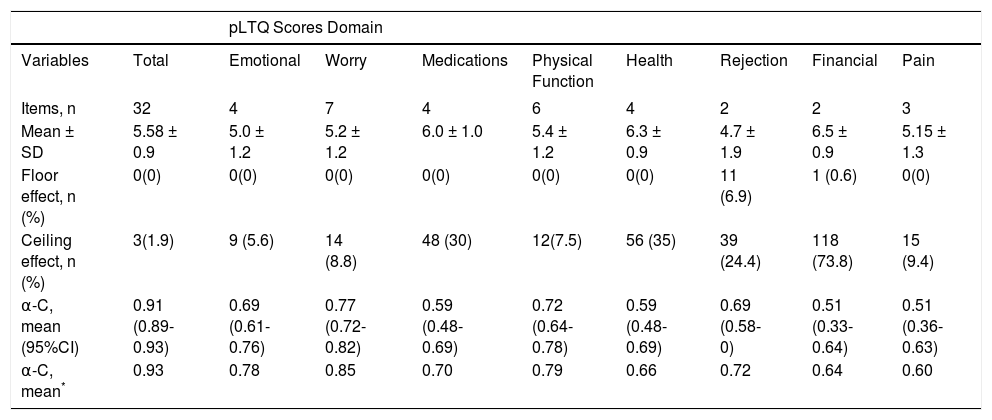
The results of the pLTQ in the study population are shown in table 2. The mean total score was 5.58 ± 0.9 points. The lowest mean score was observed in the Rejection domain (4.7 ± 1.9), and the highest, in the Financial domain (6.5 ± 0.9).
Characteristics of the post-Liver Transplant Quality of Life (pLTQ) questionnaire in a Brazilian population.
| pLTQ Scores Domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Total | Emotional | Worry | Medications | Physical Function | Health | Rejection | Financial | Pain |
| Items, n | 32 | 4 | 7 | 4 | 6 | 4 | 2 | 2 | 3 |
| Mean ± SD | 5.58 ± 0.9 | 5.0 ± 1.2 | 5.2 ± 1.2 | 6.0 ± 1.0 | 5.4 ± 1.2 | 6.3 ± 0.9 | 4.7 ± 1.9 | 6.5 ± 0.9 | 5.15 ± 1.3 |
| Floor effect, n (%) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 11 (6.9) | 1 (0.6) | 0(0) |
| Ceiling effect, n (%) | 3(1.9) | 9 (5.6) | 14 (8.8) | 48 (30) | 12(7.5) | 56 (35) | 39 (24.4) | 118 (73.8) | 15 (9.4) |
| α-C, mean (95%CI) | 0.91 (0.89-0.93) | 0.69 (0.61-0.76) | 0.77 (0.72-0.82) | 0.59 (0.48-0.69) | 0.72 (0.64-0.78) | 0.59 (0.48-0.69) | 0.69 (0.58-0) | 0.51 (0.33-0.64) | 0.51 (0.36-0.63) |
| α-C, mean* | 0.93 | 0.78 | 0.85 | 0.70 | 0.79 | 0.66 | 0.72 | 0.64 | 0.60 |
pLTQ: post-Liver Transplant Quality of Life Questionnaire. α-C: Cronbach’s alpha coefficient.
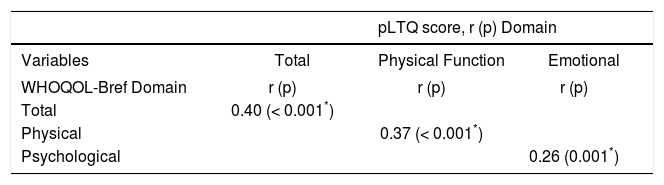
Correlations between WHOQOL-Bref and pLTQ scores are shown in table 3. Significant correlations were observed between WHOQOL-Bref and pLTQ scores, both global and domain-specific across their equivalent dimensions, which suggests adequate convergent validi-ty.21 Indication for transplant, MELD score on the day of LTx, time elapsed between LTx and initial interview, and presence of comorbidities in the postoperative period did not correlate with pLTQ scores.
Correlation between WHOQOL-Bref scores and post-Liver Transplant Quality of Life (pLTQ) questionnaire scores.
| pLTQ score, r (p) Domain | |||
|---|---|---|---|
| Variables | Total | Physical Function | Emotional |
| WHOQOL-Bref Domain | r (p) | r (p) | r (p) |
| Total | 0.40 (< 0.001*) | ||
| Physical | 0.37 (< 0.001*) | ||
| Psychological | 0.26 (0.001*) | ||
pLTQ: post-Liver Transplant Quality of Life. WHOQOL-Bref: World Health Organization Quality of Life assessment, abbreviated.
The pLTQ exhibited good reliability, with a Cron-bach’s a of 0.91 (0.89-0.93) for the total domain score, and all domains had Cronbach’s a coefficients ≥ 0.50. Repro-ducibility and sensitivity to change were also good, with ES = 0.90 (0.74-0.96) and ICC = 0.84 respectively. A floor effect was observed only in the Rejection and Financial domains. However, out of all domains, including the total score, three exhibited a ceiling effect. Cronbach’s a coefficients and floor/ceiling effects are shown in table 2.
DiscussionThe Brazilian Portuguese version of the pLTQ questionnaire developed in the present study demonstrated validity and reliability for assessment of HRQoL in adult liver transplant recipients in the Brazilian cultural context. The instrument exhibited adequate psychometric performance and total and domain values similar to those obtained with the original version of the instrument, which was validated in a similar population in the United States.
Despite the low socioeconomic level20 of the evaluated population, patients had no trouble understanding the instrument. With a mean response time of 14 min, the questionnaire can easily be administered in a waiting-room setting without taking up too much of the patient’s or the interviewer’s time.
We observed similarities between our findings and those of Saab, et al.12 The reliability of the translated version of the questionnaire as estimated by Cronbach’ a (0.91) was satisfactory and comparable to that of the original pLTQ (0.93).16 All domains exhibited acceptable ( ≥ 0.50) Cronbach’ a coefficients, which suggests that, as a whole, the translated version of the questionnaire can be considered a reliable tool for QoL assessment of liver transplant recipients in Brazil. The lowest scores (floor effect) were observed in the Rejection and Financial domains, both of which exhibited lower mean scores in the translated version than in the original instrument. The finding of lower Financial domain scores in our study may be attributed to the Brazilian health system; unlike in the United States, medications required for post-transplant care are provided free of charge by the Brazilian government. Therefore, in principle, patients would not have to worry about medication-related expenses. Both reproduc-ibility and sensitivity to change were within acceptable range (0.90 and 0.84 respectively).
Significant correlations were observed between the WHOQOL-Bref and the pLTQ, both in overall scores and in corresponding dimensions (Physical and Psychological), thus demonstrating the validity of the instrument in assessing similar aspects of general QoL. However, there are specific concerns pertaining to the liver transplant population that can affect patient QoL and cannot be captured by a generic QoL instrument; these include adverse effects of immunosuppressant drugs, rejection, and ability to afford post-transplant care and follow-up. Therefore, the pLTQ instrument is capable of capturing factors specific to liver transplant recipients, which usually are not addressed by generic questionnaires.16
Associations between pLTQ score and MELD score on the day of LTx were not significant, nor were potential associations with other variables of interest, such as time elapsed since transplantation, indication for transplant, and presence of comorbidities in the postoperative period. In our cohort, the mean MELD score on the day of LTx was 16.3; this appears to corroborate the findings of Rodrigue, et al.,23 who found that MELD scores greater than 25 (i.e., indicative of greater disease severity) have a relevant impact on post-LTx QoL.
In the immediate postoperative period of LTx, patients are susceptible to a series of complications, which are mostly associated with the surgical procedure itself, with psychological factors, and with immunosuppressive therapy. The ability to follow changes in the HRQoL of liver transplant recipients from the preoperative period through the immediate postoperative period and in the long term thereafter can contribute to the delivery of more comprehensive care to these patients.24 We expect this validated questionnaire will become an important instrument to clinicians and investigators alike involved in LTx in Brazil. Thus far, there was no disease-specific instrument for assessment and monitoring of HRQoL in liver transplant recipients in the country. This instrument should enable periodic assessment of this patient population and identification of those patients with the poorest QoL scores, potentially for delivery of distinct or additional support measures.
One limitation of this study is the fact that patients were recruited from a single transplant center in Southern Brazil. Nevertheless, this center has been operational since 1991, has performed over 1,000 liver transplants, and has a satisfactory record of patient and graft survival out-comes.21
ConclusionIn conclusion, the Brazilian Portuguese version of the pLTQ questionnaire developed in this study exhibited good psychometric properties. Consequently, the questionnaire was deemed adequate for and applicable to Brazilian liver transplant recipients, regardless of etiology or severity of underlying liver disease. Our findings support the use of the pLTQ instrument, which is now available in Brazilian Portuguese.
Abbreviations- •
ES: effect size.
- •
GRC: Global Rating of Change.
- •
HRQoL: Health-related quality of life.
- •
ICC: intraclass correlation coefficient.
- •
LTx: liver transplantation.
- •
MELD: Model for End-Stage Liver Disease.
- •
pLTQ: post-Liver Transplant Quality of Life.
The authors have no financial relationships relevant to this article to disclose.
Conflict of InterestThe authors have no conflicts of interest to disclose.
AcknowledgmentsThe authors thank the Liver Transplantation Group at Complexo Hospitalar da Irmandade Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, Brazil, for the collaboration in all stages of our research.
Appendix 1. Brazilian Portuguese version of the post-Liver Transplant Quality of Life (pLTQ) questionnaire.
Questionário sobre qualidade de vida após transplante de fígado
Este questionário foi concebido para descobrir como você vem se sentindo nas últimas quatro semanas. Você será perguntado sobre seus sintomas e humor em relação à sua condição de receptor de transplante de fígado. Também, será indagado em que medida ser um receptor de transplante de fígado afeta sua vida diária e seu nível de energia, e em que grau os cuidados posteriores ao transplante de fígado afetam a sua vida. Responda a todas as questões e selecione somente uma resposta para cada uma delas.
Escala:
- 1
Todo o tempo (diariamente).
- 2
Na maior parte do tempo (cerca de 5 vezes por semana).
- 3
Uma boa parte do tempo (de 2 a 4 vezes por semana).
- 4
Às vezes (uma vez por semana).
- 5
Poucas vezes (cerca de uma vez a cada duas semanas, ou duas vezes em 4 semanas).
- 6
Quase nunca (uma vez a cada período de 4 semanas).
- 7
Nunca.
- 1.
Nas últimas quatro semanas, com que frequência você se preocupou com ter perda de memória de curto e/ou longo prazo?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 2.
Nas últimas quatro semanas, com que frequência você se sentiu deprimido?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 3.
Nas últimas quatro semanas, com que frequência você se sentiu irritado?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 4.
Nas últimas quatro semanas, com que frequência você esteve mais preocupado do que o usual quanto ao seu atual estado de saúde?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 5.
Nas últimas quatro semanas, com que frequência você se sentiu ansioso?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 6.
Nas últimas quatro semanas, com que frequência você teve problemas em seguir as instruções para tomar as medicações de transplante?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 7.
Nas últimas quatro semanas, com que frequência você se sentiu incomodado por ter que fazer arranjos especiais em função de consultas médicas freqüentes?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 8.
Nas últimas quatro semanas, com que frequência você se sentiu afetado por ter que tirar sangue várias vezes?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 9.
Nas últimas quatro semanas, com que frequência você sentiu a necessidade de esclarecer tudo com o médico ou o coordenador do transplante?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 10.
Nas últimas quatro semanas, com que frequência você se incomodou por ter que tomar muitos remédios?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 11.
Nas últimas quatro semanas, com que frequência você se sentiu limitado em sua capacidade de realizar atividades diárias (tarefas caseiras, higiene pessoal etc.)?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 12.
Nas últimas quatro semanas, com que frequência você se sentiu afetado por ter menos força muscular?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 13.
Nas últimas quatro semanas, com que frequência você foi incomodado por mudanças em seus padrões de sono?
- 1
Todo o tempo do tempo
- 2
Na maior parte do tempo
- 3
Em boa parte
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 14.
Nas últimas quatro semanas, com que frequência você se sentiu afetado pela duração da recuperação da sua cirurgia de transplante?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 15.
Nas últimas quatro semanas, com que frequência você foi perturbado por medo de que sua doença volte a ocorrer (reincida)?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 16.
Nas últimas quatro semanas, com que frequência você se preocupou com talvez ter um tempo de vida menor?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 17.
Nas últimas quatro semanas, com que frequência você foi afetado pelo custo da sua medicação de transplante?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 18.
Nas últimas quatro semanas, com que frequência você teve problemas com as contas médicas das despesas de transplante?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 19.
Nas últimas quatro semanas, com que frequência você teve dores nas articulações ou nas costas?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 20.
Nas últimas quatro semanas, com que frequência você se sentiu afetado por ter que aprender a caminhar após a cirurgia?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 21.
Nas últimas quatro semanas, com que frequência você se sentiu afetado em relação à sua capacidade de dirigir?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 22.
Nas últimas quatro semanas, com que frequência você se sentiu afetado pela apreensão da sua família quanto à sua doença ou estado de saúde?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 23.
Nas últimas quatro semanas, com que frequência você se preocupou com ser um fardo para seus familiares?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 24.
Nas últimas quatro semanas, com que frequência você se sentiu afetado por ter de tomar mais conta da sua saúde?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 25.
Nas últimas quatro semanas, com que frequência você teve medo de que seu fígado transplantado seja rejeitado?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 26.
Nas últimas quatro semanas, com que frequência você se preocupou com desenvolver uma infecção devido a estar imunodeprimido (capacidade reduzida do seu corpo de combater infecções) como conseqüência de estar sob medicação antirrejeição?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 27.
Nas últimas quatro semanas, com que frequência você se sentiu afetado por desenvolver complicações em virtude de tomar sua medicação incorretamente ou es quecer de tomá-la?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 28.
Nas últimas quatro semanas, com que frequência você se preocupou com ter dificuldades para voltar ao trabalho?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 29.
Nas últimas quatro semanas, com que frequência você teve dor relacionada à sua cirurgia de transplante de fígado?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 30.
Nas últimas quatro semanas, com que frequência você foi incomodado por efeitos colaterais da sua medicação de transplante?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 31.
Nas últimas quatro semanas, com que frequência você se sentiu incomodado por longas esperas para consultas médicas?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1
- 32.
Nas últimas quatro semanas, com que frequência você sentiu dormências ou latejamentos?
- 1
Todo o tempo
- 2
Na maior parte do tempo
- 3
Em boa parte do tempo
- 4
Às vezes
- 5
Poucas vezes
- 6
Quase nunca
- 7
Nunca
- 1