Background and aims. Cytokeratin19 positive (CK19+) hepatocellular carcinoma (HCC) is thought to derive from liver progenitor cells (LPC). However, whether peritumoralductular reaction (DR) differs between CK19+ and CK19 negative (CK19−) HCC patients remains unclear.
Material and methods. One hundred and twenty HBV-related HCC patients were enrolled in this study. Clini-copathological variables were collected, and immunohistochemistry staining for CK19, proliferating cell nuclear antigen (PCNA), inter-leukin-6 (IL-6) and β-catenin were performed in tumor and peritumor liver tissues.
Results. CK19+ HCC patients had higher grade of peritumoral DR and proportion of proliferative DR than the CK19- group. The mean number or the proportion of cytoplasmic β-catenin+ DR was higher in the CK19+ group than in the CK19− group. Furthermore, there were more patients with nuclear β-catenin+ peritumoral DR in the CK19+ group as compared to the CK19- group.
Conclusion. Peritumoral DR was more abundant and proliferative in CK19+ HCC patients, with higher level of nuclear translocation of β-catenin. However, it is unclear whether peritumoral DR is the cause or result of poor prognosis in these patients.
Cytokeratin19 (CK19), a marker for biliary epithelial cells and liver progenitor cells (LPCs) in adult livers, is expressed in some hepatocellular carcinomas (HCC) and is a predictor for poor prognosis.1–3 Accumulating evidence indicates that CK19 positive (CK19+) HCC may be derived from bipotential LPCs, which can differentiate into either hepatocytes or cholangiocytes.2,4 Although the underlying mechanism is not completely elucidated, the inflammatory microenvironment or mutations of certain tumorigenic genes may lead to this transition.5,6
Ductular reaction (DR), a pattern of liver regeneration by cholangiocytes and/or LPCs, is often used to evaluate the regenerative LPCs in chronic liver diseases.7 Background DR and LPC activation were related to the recurrence of combined hepatocellular-cholangiocarcinoma after hepatectomy, indicating that these tumors may originate from LPC.8 In HCC, peritumoral DR was associated with a poor prognosis even after curative resection.9 However, the relationship between the peritumoral DR and HCC with or without CK19 expression remains unknown.
In the present study, a clinicopathological analysis in HCC patients who underwent hepatectomy was performed to investigate the differences in characteristics of peritumoral DR between CK19+ and CK19- HCC patients.
Material and MethodsPatients and specimensFrom January 2003 to December 2007, 120 HBV-related HCC patients, including 101 men and 19 women who received hepatectomy in Shanghai General Hospital, Shanghai Jiaotong University School of Medicine and Eastern Hepatobiliary Hospital, the Second Military Medical University were enrolled in this study, with the mean age of 47.03±9.95 years. Patients with metastasis at the time of diagnosis were excluded. Informed consent was obtained from all patients based on a protocol approved by the Ethics Committees at both study centers. The tumor stage was determined according to the 2009 UICC TNM classification system.10 Laboratory parameters including serum albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), alpha-feto-protein (AFP), and HBV-DNA were collected. The tumor and peritumoral (> 2 cm away from the tumor) tissues from all patients were paraffin-embedded.
Histological and immunohistochemistry (IHC) analysisLiver tissues were fixed in 4% formaldehyde, embedded in paraffin, and 4 μm sections were stained with hematoxylin-eosin. Inflammation and fibrosis in the peritumoral tissues were blindly scored by two experienced pathologists according to the Scheuer scoring system.11 For IHC, after a phosphate-buffered saline wash, sections were transferred into 10 mM sodium citrate buffer (pH 6.0), and antigen unmasking was performed in a microwave. After cooling down, sections were incubated in peroxidase blocking reagent (Dako) for 1 h and then stained overnight at 4 °C with the following primary antibodies: anti-CK19 (Dako, Germany), 1:200; anti-PCNA (Santa Cruz Biotechnology, USA), 1:200; anti-β-catenin (Dako, Germany), 1:100; and anti-IL-6, (Abcam, London, UK), 1:100. Sections were developed with diaminobenzidine for 5 min. DR grade was evaluated according to the following standard:
- •
0: no or minimal DR around a few portal tracts and septa.
- •
1: focal DR around most portal tracts/septa.
- •
2: continuous DR around < 30% of portal tracts/septa.
- •
3: continuous DR around 30–50% of portal tracts/septa; and
- •
4: continuous DR > 50% of portal tracts/septa.
For quantitative analysis, mean values of immunoreactive cells were obtained by randomly counting three fields in portal or septal regions at 200 x magnification. The proliferation index of DR (PI-DR) was calculated as the ratio between the number of PCNA+ cells and the total number of reactive ductular cells.CK19 positivity was defined as positive area > 5%.
Statistical analysisData were analyzed using the Statistical Package for the Social Sciences (SPSS Inc, USA) version 13.0 for Windows, and are presented as means ± standard deviations (SD). Student’s t-tests were used to compare the continuous quantitative data of weight loss. A two-tailed Wilcoxon signed-rank test was used to compare ranked variables. The correlation between the degree of DR and clinicopathological variables was determined by Spearman’s or Pearson’s correlation, as appropriate. P < 0.05 was considered to be statistically significant.
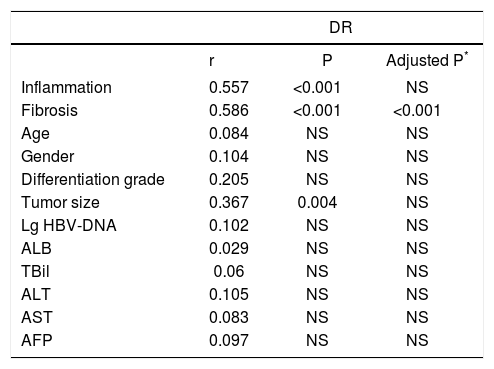
ResultsCorrelations between peritumoral DR grade and clinicopathological featuresAs shown in table 1, among the clinicopathological variables, inflammation and fibrosis stage in peritumoral liver tissue were significantly related to DR grade (both P < 0.001). The correlation analysis also indicated that the tumor size was positively correlated to DR grade. Based on the possibility that tumor size may affect inflammation in peritumoral areas, we adjusted age, gender and inflammation. After adjustment, the fibrosis stage was still significantly correlated to DR grade, while there was no correlation between DR grade and tumor size.
Correlation between peritumoral DR grade and clinicopathological features in HCC patients.
| DR | |||
|---|---|---|---|
| r | P | Adjusted P* | |
| Inflammation | 0.557 | <0.001 | NS |
| Fibrosis | 0.586 | <0.001 | <0.001 |
| Age | 0.084 | NS | NS |
| Gender | 0.104 | NS | NS |
| Differentiation grade | 0.205 | NS | NS |
| Tumor size | 0.367 | 0.004 | NS |
| Lg HBV-DNA | 0.102 | NS | NS |
| ALB | 0.029 | NS | NS |
| TBil | 0.06 | NS | NS |
| ALT | 0.105 | NS | NS |
| AST | 0.083 | NS | NS |
| AFP | 0.097 | NS | NS |
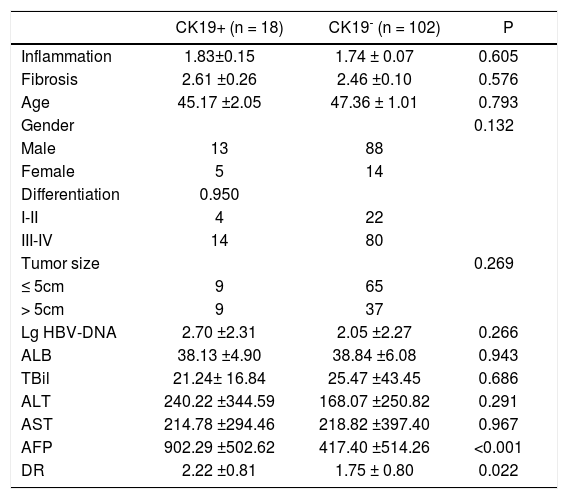
Among the 120 HCC patients, 18 (15%) had positive expression of CK19 in tumor cells. We compared the differences in clinicopathological parameters between CK19+ and CK19- groups. As shown in Table 2, the CK19+ group had significantly higher level of serum AFP (902.29 ± 502.62 vs. 417.40 ± 514.26, P < 0.001) and peritumoral DR grade (2.22 ± 0.81 vs. 1.75 ± 0.80, P = 0.022) than the CK19- group (Figure 1).
Comparison of clinicopathological features between CK19+ and CK19− HCC patients.
| CK19+ (n = 18) | CK19- (n = 102) | P | |
|---|---|---|---|
| Inflammation | 1.83±0.15 | 1.74 ± 0.07 | 0.605 |
| Fibrosis | 2.61 ±0.26 | 2.46 ±0.10 | 0.576 |
| Age | 45.17 ±2.05 | 47.36 ± 1.01 | 0.793 |
| Gender | 0.132 | ||
| Male | 13 | 88 | |
| Female | 5 | 14 | |
| Differentiation | 0.950 | ||
| I-II | 4 | 22 | |
| III-IV | 14 | 80 | |
| Tumor size | 0.269 | ||
| ≤ 5cm | 9 | 65 | |
| > 5cm | 9 | 37 | |
| Lg HBV-DNA | 2.70 ±2.31 | 2.05 ±2.27 | 0.266 |
| ALB | 38.13 ±4.90 | 38.84 ±6.08 | 0.943 |
| TBil | 21.24± 16.84 | 25.47 ±43.45 | 0.686 |
| ALT | 240.22 ±344.59 | 168.07 ±250.82 | 0.291 |
| AST | 214.78 ±294.46 | 218.82 ±397.40 | 0.967 |
| AFP | 902.29 ±502.62 | 417.40 ±514.26 | <0.001 |
| DR | 2.22 ±0.81 | 1.75 ± 0.80 | 0.022 |
ALB: albumin. ALT: alanine aminotransferase. AST: aspartate aminotransferase.TBil: total bilirubin. AFP: alpha fetoprotein.
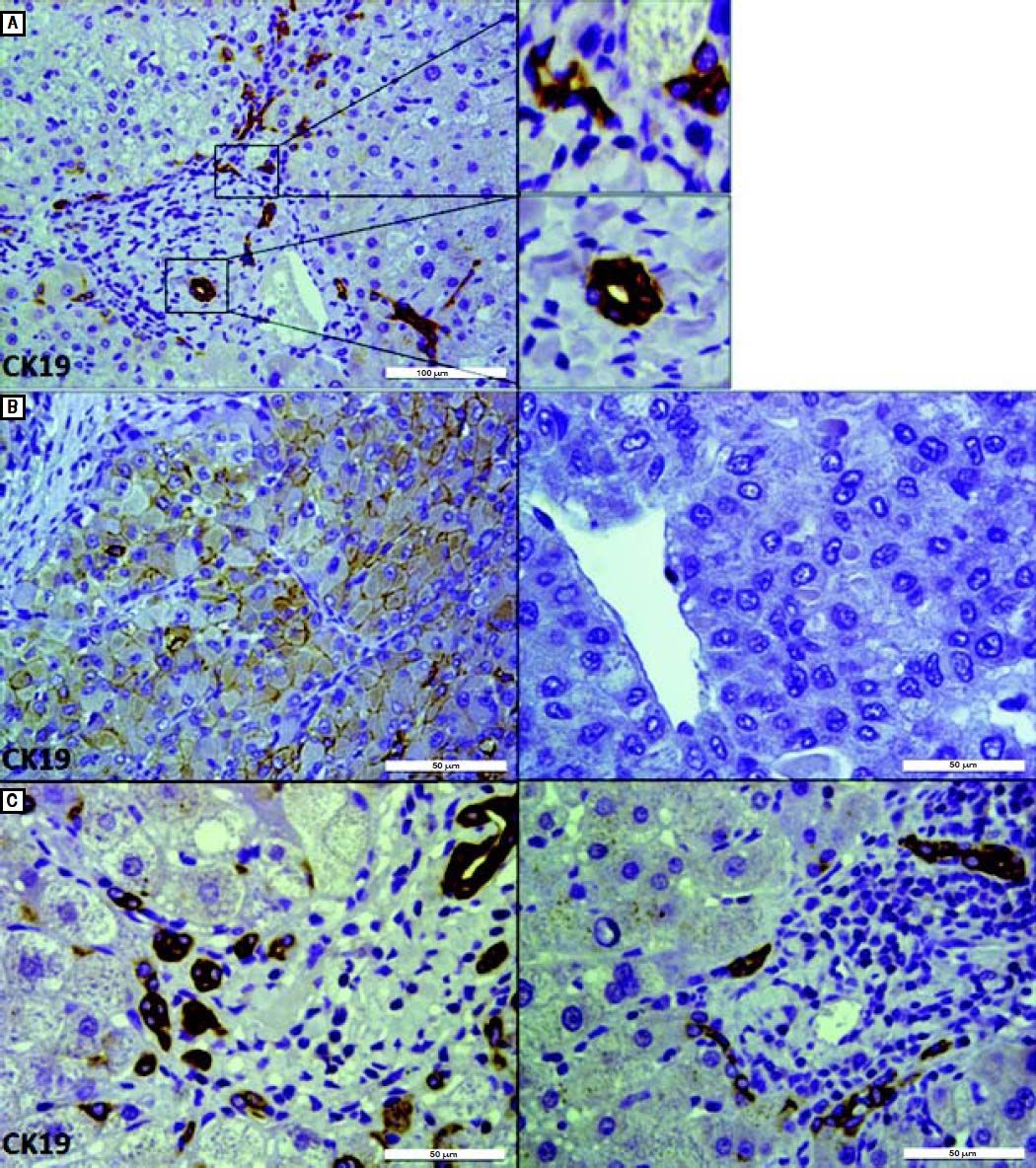
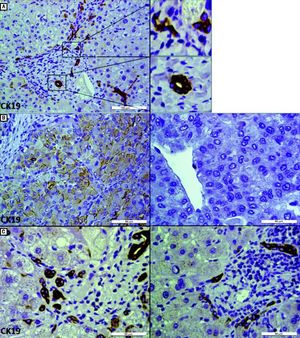
IHC staining for CK19 in tumor and peritumoral areas. A. CK19 staining in peritumoral area shows both a typical bile duct and DR in a port area (scale bar, 100 μm). In a high-power field of CK19 staining (right), DR is described as elongated and irregular structures that are lined by flattened cells and lack clear lumina(upper right). Typical bile duct has a recognizable lumen lined by cuboidal cells(lower right) (scale bar, 50 μm). B. IHC staining for CK19 differentiates CK19+ (left) and CK19- (right) HCC (scale bar, 50 μm). C. Representative images of IHC staining for CK19 show that the peritumoral DR in CK19 + HCC patients (left) was more than that in CK19− HCC patients (right), although the extent of local inflammation and fibrosis were similar (scale bar, 50 μm).
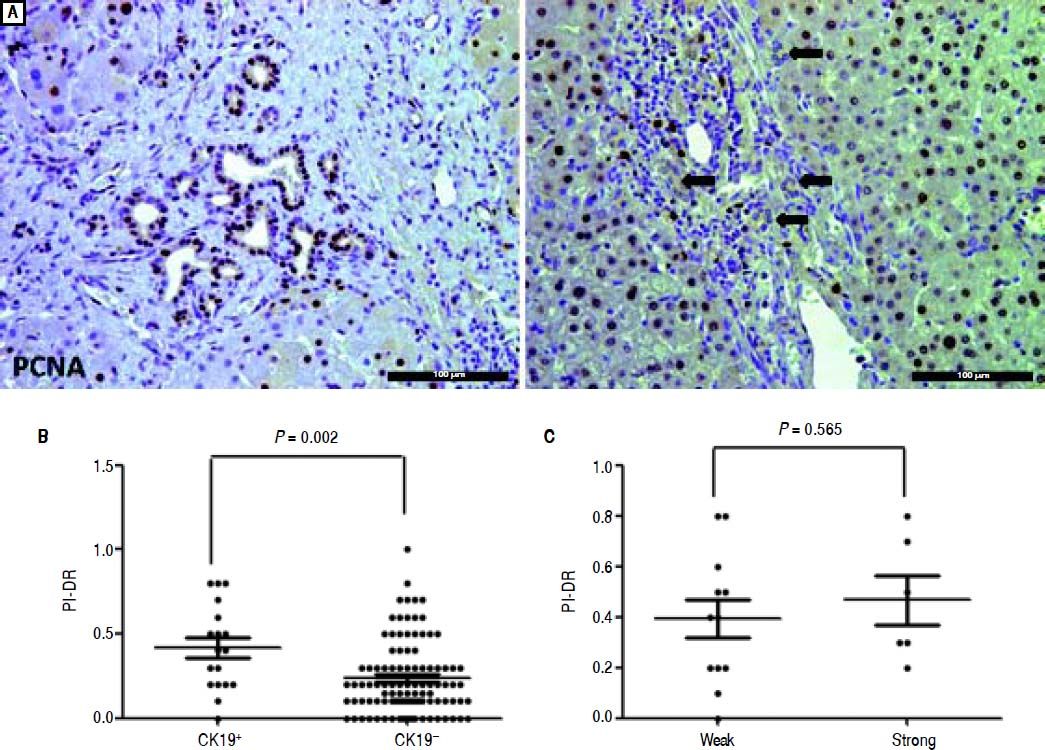
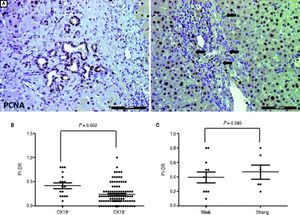
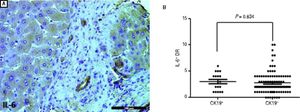
We used IHC staining for PCNA to examine the extent of PI-DR (Figure 2A). Peritumoral PI-DR in the CK19+ HCC group was higher than that in the CK19− group (0.42 ± 0.25 vs. 0.23 ± 0.20, P = 0.001) (Figure 2B). While PCNA-negative DR appeared in one patient in the CK19+ group, there were more patients with PCNA-negative DR in the CK19− group (6.56% vs. 18.63%). There was no relationship between the grade of CK19 expression and peritumoral PI-DR in CK19+ HCC patients (Figure 2C).
Comparison of proliferative peritumoral DR in CK19 + and CK19− HCC patients. A. IHC staining for PCNA shows peritumoral DR with high (left) or low (right) proHferation(scale bar, 100 μm). B. The scatter plot shows that the CK19 + group has high peritumoral PI-DR than the CK19− group. C. The scatter plot shows that peritumoral PI-DR is unrelated to the expression level of CK19 in tumors.
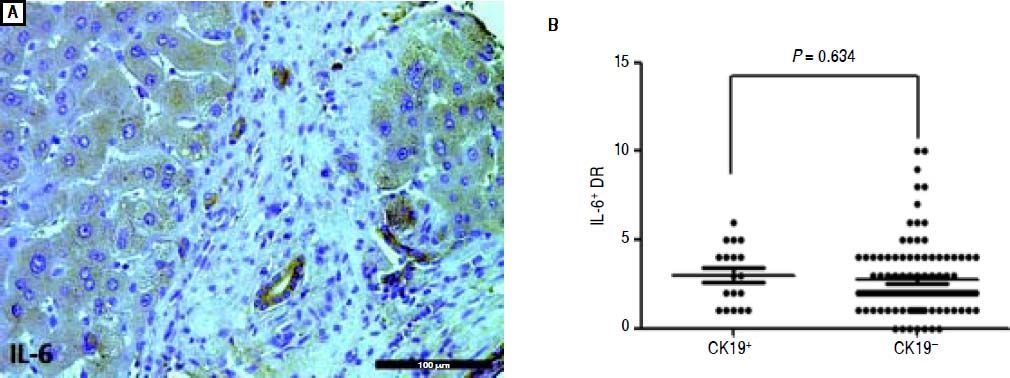
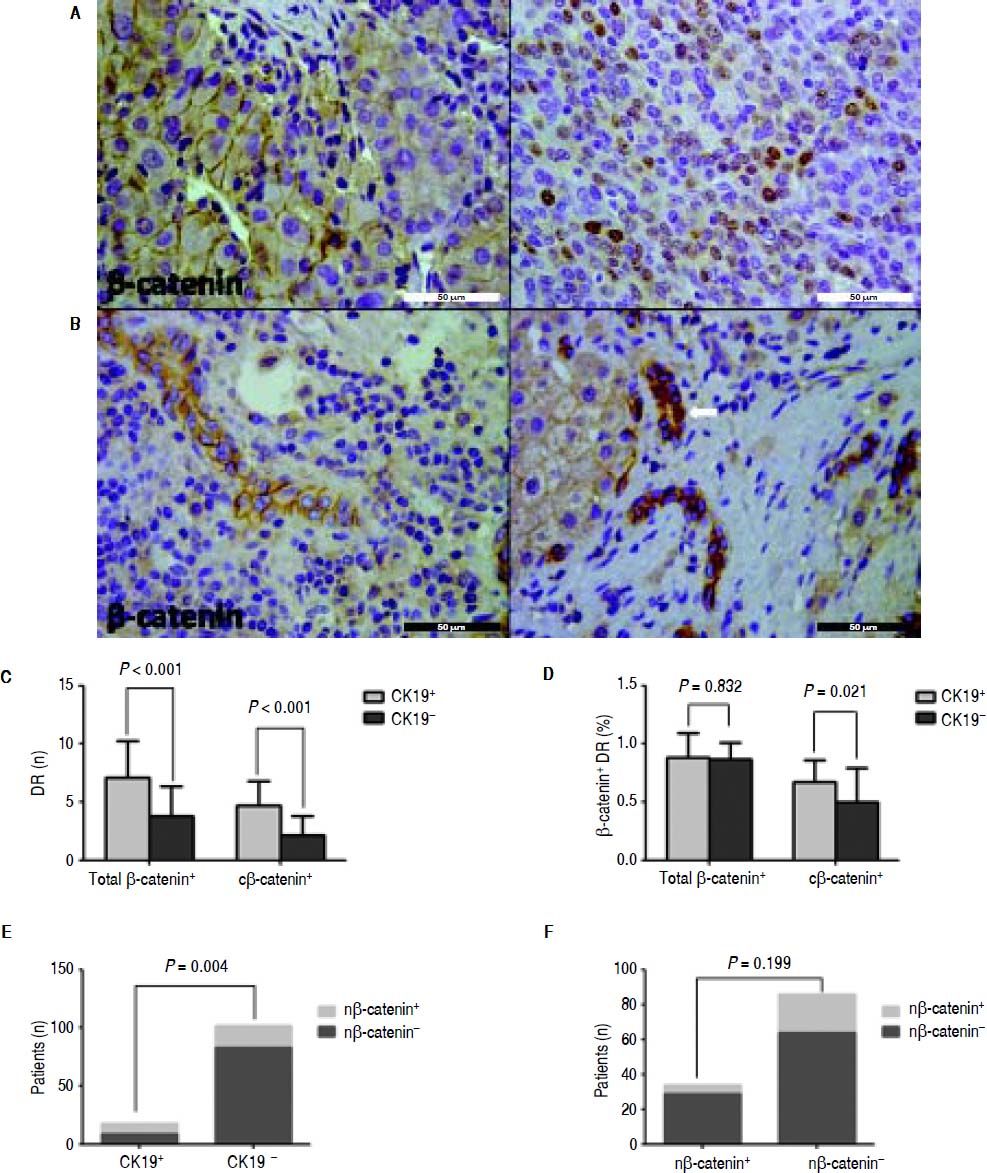
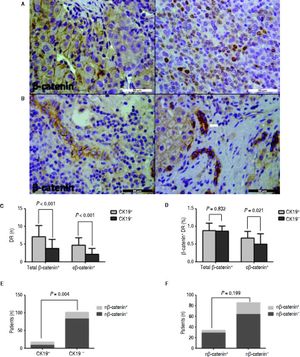
Almost all the peritumoral reactive bile ducts expressed IL-6, and the expression level was similar between the CK19+ and CK19- groups (Figure 3). In HCC, β-catenin was expressed on the membrane or the nucleus (Figure 4A). Most patients had ductular expression of β-catenin on the membrane and/or in the cytoplasm, but some patients also had scattered nuclear β-catenin+ cells in DR (Figure 4B). β-catenin+ DR was slightly higher in the CK19+ group regardless of the location (7.11 ± 3.14 vs. 4.78 ± 2.55, P = 0.06) (Figure 4C). The proportion of β -catenin+ DR was not significantly different between the two groups (0.88 ± 0.14 vs. 0.87 ± 0.21, P = 0.833) (Figure 4D). In terms of cytoplasmic expression of β-catenin, the mean number and the proportion were more in the CK19+ group than in the CK19- group (4.72 ± 2.11 vs. 2.12 ± 1.67, P < 0.001; 0.67 ± 0.19 vs. 0.50 ± 0.29, P = 0.021, respectively) (Figure 4C and 4D). Also, there were more patients with nuclear β-catenin+ and peritumoral DR in the CK19+ group than in the CK19- group (9/9 vs. 19/83, P = 0.004) (Figure 4E). Nuclear translocation of β-catenin in tumor cells was unrelated to the nuclear expression of peritumoral DR (Figure 4F).
IHC staining for β-catenin shows that peritumoral DR in the CK19 + group has more cytoplasmic and nuclear β-catenin expression than the CK19− group. A. Representative images of membranous (left) and cytoplasmic (right) expression of β-catenin in HCC (scale bar, 50 μm). B. Representative images of membranous β-catenin (left) or cytoplasmic and nuclear (arrow) expression of β-catenin (right) (scale bar, 50 μm). C. The total and cytoplasmic β-catenin (c β-catenin) positive peritumoral DR are more in the CK19 + group than in the CK19− group. D. Peritumoral DR in the CK19 + group has a higher proportion of total or cytoplasmic β-catenin expression than in the CK19− group. E. More patients in the CK19 + group had nuclear expression of β-catenin (n β-catenin). F. Nuclear translocation of β-catenin in tumor is not related to its nuclear translocation in peritumoral DR.
Clinicopathological studies have shown that DR is positively correlated to liver inflammation and fibrosis in chronic hepatitis C, nonalcoholic steatohepatitis and hereditary hemochromatosis.10–14 The present study showed that peritumoral DR was closely related to tumor size as well as liver inflammation and fibrosis in non-tumor area. However, tumor size may affect the inflammation in peritumoral area because after adjustment by inflammation, this correlation was no longer present.
LPC and HCC are closely related.1,4,9 However, whether peritumoral LPC could be a source of malignant tumor and contribute to the recurrence and/or metastasis is unclear. Among liver carcinomas, combined hepatocellular-cholangiocarcinoma is most likely to derive from LPC because of its dual characteristics of hepatocytes and cholangiocytes. Cai, et al. demonstrated that background proliferative LPCs/DRs marked with PCNA were strongly associated with multifocal occurrence and recurrence, and could be an independent prognostic factor for overall and disease-free survival.8 Therefore, peritumoral LPC could be a potential source of cancer cells. In addition, peritumoral DR is associated with a poor prognosis for HCC after resection.9,15 However, CK19+ HCC is strongly correlated with bipotential LPCs due to their expression of biliary markers. It is unclear whether the pattern of peritumoral DR differs between CK19+ and CK19- HCC patients.
In this study, 15% HCCs were CK19+, which is in agreement with previous reports.2,16 Univariate analysis showed that CK19+ patients had higher grade of peritumoral DR than CK19- patients although the inflammation or fibrosis was similar, which indicates that the peritumoral DR is more active in the CK19+ group. Because PI-DR may be a more accurate indicator for the activation of DR, we used it in this study. Our results showed that PI-DR was higher in the CK19+ group, but was unrelated to the extent of CK19 expression in tumors. This confirmed that the DRs adjacent to the CK19+ liver tumors were more proliferative.
Inflammatory microenvironment is related to the malignant transformation of LPC, and autocrine IL-6 signaling of liver cancer progenitor cells plays an important role in malignancy progression.6,17 However, the proportion of IL-6+ DR was comparable between the two groups in our study.
β-catenin, a downstream element of canonical Wnt signaling, plays a pivotal role in liver regeneration and carcinogenesis. Wnt/ β-catenin pathway activation alone fails to induce liver tumors; while in combination with other genetic alterations such as Ha-Ras, MET or Akt mutation in adult hepatocytes can lead to HCC.18 Recently, a study demonstrated that activation of β-catenin in LPC is sufficient to induce HCC.5 β-catenin may be present in several cellular compartments, such as the membrane, cytoplasm and nucleus, where it forms an active complex. When activated, β-catenin is accumulated in the cytoplasm followed by translocation into the nucleus, and serves as a transcription factor to activate downstream target genes. Intriguingly, a strong CK19 immunoreactivity significantly correlated with medium to high nuclear β-catenin expression.19 In the present study, we found that CK19+ HCC patients had higher cytoplasmic β-catenin expression in peritumoral LPC and higher percentage of peritumoral DR with β-catenin nuclear translocation, which suggests that the peritumoral DR in these patients was more likely related to HCC.
ConclusionIn conclusion, our study demonstrated that the peritumoral DR in CK19+ HCC patients were more abundant and proliferative, with more nuclear translocation of β-catenin. However, this is a correlation study based on the results from IHC staining and the molecular mechanism is not elucidated. Therefore, it is still unclear whether the peritumoral DR contributes to the poor prognosis of CK19+ HCC or is an accompanying phenomenon.
Competing InterestsThe authors declare that they have no competing interests.
Financial DisclosureThis study was supported by the Science and Technology Commission of Shanghai Municipality (No. 10411955300; 11DZ2292900; 12DZ1941603), and China foundation for hepatitis prevention and control (No. TQGP20120087).