Curcumin, an aromatic phytoextract from the turmeric (Curcuma longa) rhizome, has been used for centuries for a variety of purposes, not the least of which is medicinal. A growing body of evidence suggests that curcumin has a broad range of potentially therapeutic pharmacological properties, including anti-inflammatory, anti-fibrotic, and anti-neoplastic effects, among others. Clinical applications of curcumin have been hampered by quality control concerns and limited oral bioavailability, although novel formulations appear to have largely overcome these issues. Recent in vitro and in vivo studies have found that curcumin's cytoprotective and other biological activities may play a role in an array of benign and malignant hepatobiliary conditions, including but not limited to non-alcoholic fatty liver disease, cholestatic liver disease (e.g. primary sclerosing cholangitis), and cholangiocarcinoma. Here we provide an overview of fundamental principles, recent discoveries, and potential clinical hepatobiliary applications of this pleiotropic phytocompound.
Curcumin is a naturally-occurring phytocompound extracted from the turmeric rhizome Curcuma longa, a member of the ginger (Zingiberaceae) family. For centuries, it has been used as a dye, culinary spice, ceremonial substance, and traditional medicine in many cultures and countries, particularly in Southern Asia and the Indian subcontinent, where it grows naturally. A primary rationale for its use in traditional (e.g. Ayurvedic) medicine has been its putative anti-inflammatory and consistency prop-erties.1 More recently, curcumin has been appreciated in modern biomedical studies as having various potentially therapeutic properties, including anti-oxidant, anti-fibrot-ic, anti-senescent, and anti-neoplastic.2-8
Despite its long history and well-tolerated nature, cur-cumin has arguably not yet realized its full therapeutic potential in the prevention and treatment of human disease. This has in part been due to concerns regarding quality control (e.g. safety, purity, and other chemical attributes), poor oral bioavailability, and ostensibly a degree of skepticism toward traditional (i.e. alternative) medicine. However, several well-characterized, more bioavailable, novel formulations of curcumin have helped facilitate its growing study and emerging (though to date off-label) complimentary use in Western medicine. The potential applications thus far include, but are not limited to, prevention and treatment of hepatobiliary, digestive tract, cardiovascular, neurodegenerative, and dermatologic diseases as well as various systemic inflammatory and malignant conditions.1,2,8-10
The goals of this review are to:
- •
Provide a synopsis of the structural, biochemical, and pharmacological aspects of curcumin.
- •
Summarize the relevant in vitro and in vivo experimental data supporting its beneficial properties.
- •
Present recent findings and perspectives regarding the potential clinical role of curcumin as a pharmacothera-py in the context of hepatobiliary disease and chemo-prevention.
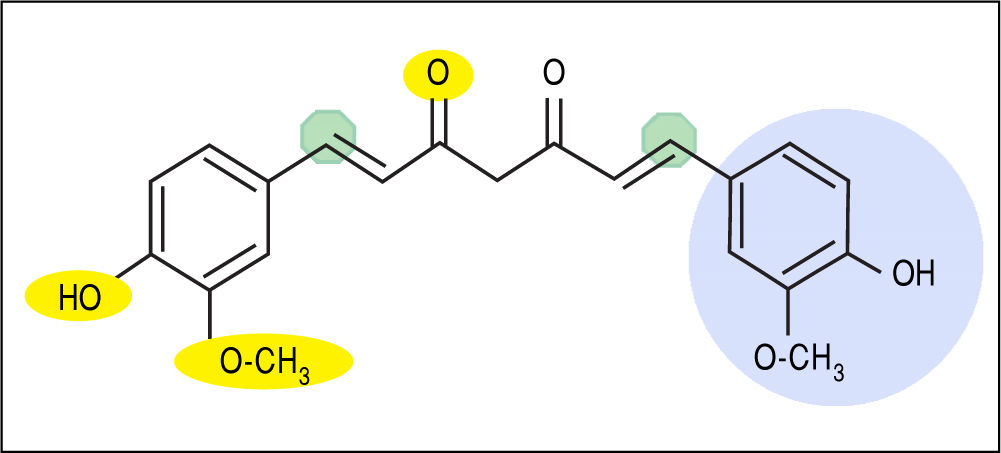
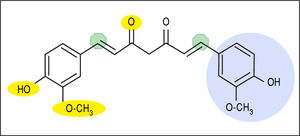
Curcumin [1,7-bis (4-hydroxy-3- methoxyphenyl)-1,6-heptadiene-3,5-dione] is a unique biphenolic molecule containing two ferulic acid residues joined by a methylene bridge (Figure 1). It has three key molecular functionalities:anaromatico-methoxyphenolicgroup,anα,β-un-saturatedβ-diketonemoiety,andasevencarbonlinker. Accordingly, there are several important physicochemical features associated with the biological activity and effects of curcumin. For example, the o-methoxyphenol group and methylenic hydrogen are responsible for the antioxi-dant activity of curcumin, and curcumin donates an electron/hydrogen atom to reactive oxygen species (ROS). Curcumin interacts with a number of biomolecules through non-covalent and covalent binding; the hydrogen bonding and hydrophobicity of curcumin, arising from the aromatic functional ends and keto-enol tautomerism of the central portion along, account for the non-covalent interactions.Theα,β-unsaturatedβ-diketonemoietiescova-lently interact with protein thiols through Michael reaction(i.e.Michaelnucleophilicaddition).Theβ-dike-to group chelates transition metals, thereby decreasing metal-induced toxicity and rendering some metal complexes with antioxidant activity as enzyme mimics. These nuanced molecular characteristics are reviewed in greater detail elsewhere.8,11 Notably, new analogues of curcumin have been and continue to be developed with chemical modifications to specific functional groups in order to potentiate or increase specificity of particular molecular interactions and downstream biological effects.11
Molecular structure of curcumin emphasizing key functional groups and chemical properties. Yellow ovals depict hydrogen bonding sites (not shown on right side of molecule), green octagons depict Michael acceptor sites, and light blue circle depicts hydrophobic moieties (not shown on left side of molecule). In addition, keto-enol tautomerism occurs between the central portion of the molecule (di-keto tautomer shown).
The clinical application of native curcumin has been limited due to its poor bioavailability (largely due to low solubility), physicochemical instability, and pharmacoki-netics (in particular rapid metabolism), as mentioned ear-lier;12 however, these issues can be largely mitigated by utilization an efficient delivery system. Therefore, various formulations of curcumin have been developed, including nanoparticles, liposomal encapsulation, and emulsions, in addition to more conventional delivery systems such as tablets and powders. These novel formulations have been found to improve curcumin's pharmacokinetics, especially its oral bioavailability, as well as its pharmacodynamics, with resultant enhanced biological activity.13-15
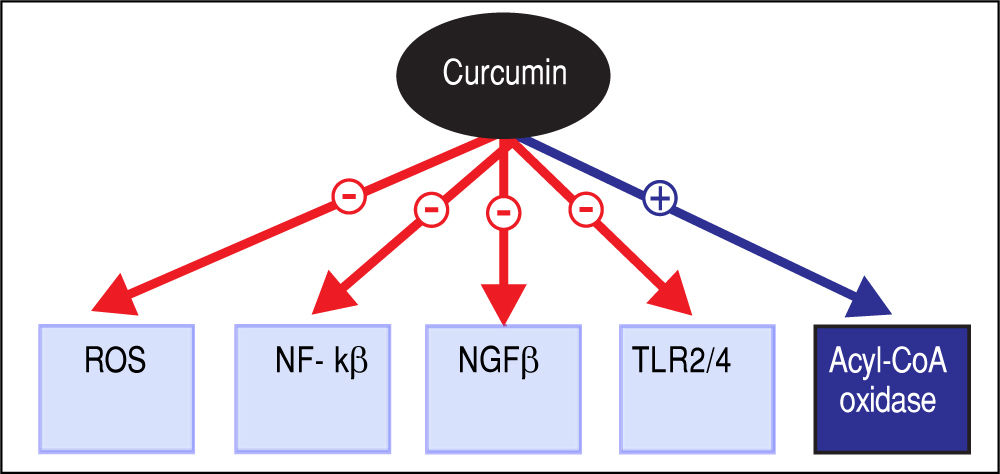
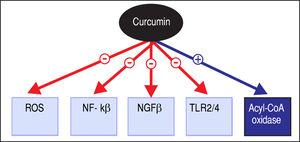
Pharmacotherapeutic Effects and General ApplicationsThe various potentially therapeutic pharmacological properties of curcumin have shown promise in effectuating several important cytological and biological effects; these include induction of cell apoptosis, inhibition of abnormal cell proliferation, and anti-angiogenic and antimicrobial activities, among others.16 The molecular mechanisms implicated are pleiotropic, including regulation of ROS production, LPS-mediated signaling, cell survival signaling pathways by nuclear factor kappa-light-chain-enhancerofactivatedBcells(NF-κB)andAkt,andcell survival-associated proteins such as Bcl-2 and Bcl-Xl, as illustrated in figure 2,17-22 and it is therefore not surprising that the use of curcumin has been investigated for a variety of indications.8,23
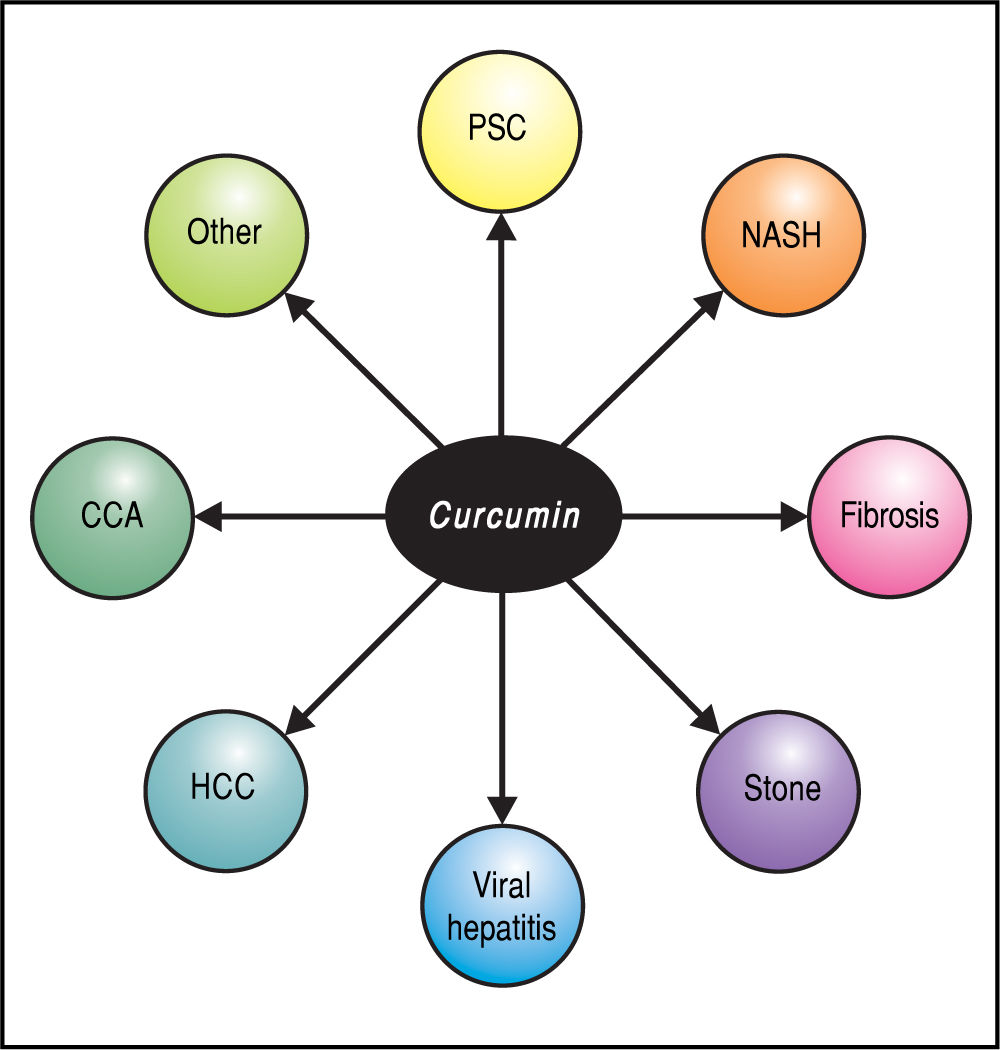
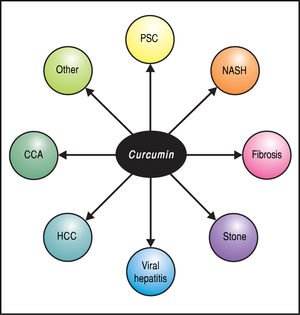
Although this review focuses on hepatobiliary applications (Figure 3), it is worth noting that curcumin has been examined in in vitro and animal models as well as in clinical studies of a wide array of human conditions, including benign or malignant neurodegenerative, cardiovascular, renal, and metabolic diseases as well as their associated com-plications.11 Two phase I clinical trials concluded that curcumin is safe and not toxic to humans, even at very high dosage.5,24,25 In a minority of patients, side-effects such as nausea, diarrhea, headache, somnolence, and contact dermatitis (with topical use) have been reported; these have typically been mild and self-limited. Considering the relatively low cost and favorable safety profile of curcumin, a therapeutic impact on these diseases with this agent, even if requiring long-term treatment or as an adjuvant, represents an exciting prospect.
Curcumin in Non-Malignant Chronic Hepatobiliary DiseasePreclinical dataA large body of preclinical literature has suggested pharmacotherapeutic properties of curcumin relevant to non-malignant (or pre-malignant) hepatobiliary disease. For example, in vitro studies using different cell lines such as human HSC-16 and Hep2G cells have shown significant antifibrotic effects through transforming growth factor-beta(TGFβ)signaling.22,26,27Inaddition,ithasalso been shown in vitro that curcumin has potent antioxidant effects through inducing the activity of glutathione, glutathione peroxidase, superoxide dismutase, and catalase as well as dose-dependent induction of HO-1. Moreover, curcumin can inhibit NF-kB activation, thereby preventing secretion of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-oc), interleukin 1(3), and interleukin 6.22,28
With respect to animal model data, there is a considerable body of evidence to support potential applications of curcumin in chronic, non-malignant liver disease. For example, using a model such as CCl4 or bile duct ligation-induced hepatic fibrosis, curcumin has been shown to significantly attenuate fibrogenesis.22,29,30 Similar effects were seen in the mdr2 knockout murine model of sclerosing cholangitis with 3% curcumin chow.31 Extending the mdr2 knockout model findings, our group recently found curcumin to inhibit cellular senescence and the senescence-associated secretory phenotype (unpublished data), which may be fundamentally linked to the pathogenesis of primary sclerosing cholangitis (PSC), in cultured human cholangiocyte models.32,33 Second, curcumin supplementation appears to reduce hepatocyte lipid accumulation attenuate nonalcoholic steatohepatitis (NASH) in rats,34,36 with similar findings in hamster35 and rabbit37 models. These benefits of curcumin appear to be mediated through mitochondrial protection and inhibition of apoptosis and are further supported by studies in a methionine and choline deficiency diet murine model demonstrating a protective role of curcumin against oxidative stress through reduced CYP2E1 and Prx1 expression and upregulated Prx6 expression.38 Collectively, these and other studies39,40 suggest that curcumin can decrease hepatic cholesterol, triglyceride, and free fatty acid accumulation and resultant hepatic injury and thus may have a promising role in treating or preventing NASH. Third, hepato-protective effects of curcumin have been evaluated and shown in non-NASH animal models. For example, curcumin appears to inhibit CYP2E1 activity,41 thus abrogating ROS production, and activate NF-E2-related factor 2 (Nrf2) translocation to the nucleus, thereby increasing expression of antioxidant enzymes.42,43 Fourth, the recently identified effect of inhibiting hepatitis C virus entry in a hepatoma cell line and in primary human hepatocytes44 as well as anti-hepatitis B virus activity in the HepG2 cell line45 suggest that curcumin may have preventive and/or therapeutic effects in viral hepatitis. Lastly, several studies have demonstrated significant anti-lithogenic effects of curcumin in murine experimental cholesterol cholelithiasis models, thus supporting a role in gallstone disease.46,47
These in vitro and in vivo data suggest that curcumin has therapeutic potential in benign chronic liver disease meriting further investigation and improved mechanistic understanding.
Clinical applicationsA small but growing number of clinical trials have examined the effects of curcumin on chronic liver diseases, including antituberculosis treatment-induced hepatotox-icity,48 gallstone disease (e.g. gallbladder hypocontractili-ty),49 and biliary dyskinesia.50 To date, however, curcumin has not become a mainstream or approved therapy for these indications. Of note, we recently received approval of an investigational new drug application to conduct a clinical trial of curcumin in patients with PSC based on promising pre-clinical findings; study enrollment is planned for later this year.
Curcumin in Malignant Hepatobiliary DiseasePreclinical dataPreclinical data, both in vitro and in vivo, suggest that cur-cumin has anti-neoplastic effects with regard to hepato-cellular carcinoma (HCC). Several in vitro studies using murine hepatoma51 or HepG2 cancer cell lines52 have demonstrated that its effect are likely through activation of ER stress and apoptosis or mediating MMP turnover53 as well as through NF-κB signaling.54In vivo, curcumin has similarly exhibited anti-properties in several animal models of hepatocellular carcinoma, as demonstrated in the N-bis-(2-hydroxypropyl) nitrosamine induced liver adenoma model,38 HepG2 xenografts,55-57 orthotopic implantation model of HCC CBO140C12 cells.58 Curcumin also has been shown to mitigate oxidative tissue damage during chemically induced hepatocarcinogenesis in N-nitrosodi-ethylamine -initiated and phenobarbital-promoted hepa-tocarcinogenesis in Wistar rats.59-61 Moreover, a recent breakthrough study revealed that curcumin not only inhibited the proliferation and invasion of HCC cell lines in vitro, but also drastically suppressed primary tumor growth and lung metastases in vivo. Furthermore, in combination with sorafenib, curcumin induced HCC cell apoptosis and cell cycle arrest by synergistically down-regulating the expression of MMP9 via NF-κB/P65 signaling pathway. This highlights the potential neo-adjuvant application of curcumin in the treatment of HCC.62
Curcumin may also have promise in the treatment of cholangiocarcinoma (CCA), a lethal malignancy for which effective pharmacotherapy remains lacking. Several in vitro studies using different CCA cell lines have shown anti-tu-morigenic effects on cholangiocarcinoma, which appear to be via suppressing proliferation and inducing apoptosis in malignant cells through modulating multiple cell signaling pathways.63,64 Similar effects were also observed in in vivo hamster65 and nude mouse xenograft models.66
Collectively, these data suggest that curcumin may have potential therapeutic implications in hepatobiliary malignancies.
Clinical applicationsAlthough there are no published clinical trials to date on malignant hepatobiliary disease with curcumin, the in vivo and in vitro data thus far may inform clinical studies of curcumin in treating such conditions.
Curcumin as a Hepatoprotective AgentPreclinical dataNumerous in vivo studies have found that curcumin has potent antioxidant and anti-inflammatory properties, which may account for its hepatoprotective effect. These properties may be mediated by inhibiting NF-κB signaling and by inhibiting production of nitric oxide and TNF-α by activated Kupffer cells, among other mechanisms, and as mentioned earlier.8,67,68 This has been shown in various models of hepatobiliary injury, including D-galactos-amine-, thioacetamide-, and CCl4-, iron-, and ethanol- induced acute liver injury.8,69,70 It is interesting to note that experimental liver steatosis induced by TNF-α injection in mice also showed significant attenuation after curcu-min treatment as indicated by decreased oxidative stress and neutrophil infiltration and improved hepatic his-topathological features.71 Curcumin also has been shown to decrease hepatocyte lipid storage in various animal models.34,35,39,40,72
Clinical applicationsThere are only limited data, much of which is related to pharmacokinetic and toxicity evaluation, with regard to clinical studies assessing the hepatoprotective (i.e. primary preventive) properties of curcumin.73 Given the progress made to pharmacologically improve the bioavail-ability of curcumin and its demonstrable safety profile, preliminary clinical application has been thus far successful in healthy human volunteers using a nanoparticle formulation for anti-cancer (preventive or therapeutic) purposes.74 The findings appear promising but require further study.
Future DirectionsWith the development of novel drug delivery systems and structural modifications to improve the oral bioavail-ability of curcumin, it is likely that pre-clinical research investigating its mechanisms of action in prevention and treatment of disease and potential applications to clinical medicine will continue to expand. Given its low negligible toxicity and relatively low cost, it would reason to believe that its role in human disease, be it preventive or for treatment of benign or malignant disorders, will draw increasing medical and pharmaceutical attention. Further study with well-designed clinical trials are needed to ascertain appropriate indications, dispel potential positive publication bias, and determine optimal formulations, doses, and other regimen-related factors. In addition, epidemiological data from regions of high dietary curcumin intake (e.g. India) may shed additional light on chemopre-ventive properties; for example, it is suggested that may in part be related to high curcumin consumption the low incidence of colorectal and small intestinal cancer in India,75 but data regarding hepatobiliary disease are lacking.
ConclusionsCurcumin is a phytocompound which has been used as a vital traditional medicinally for centuries. Pharmacological advances, including significantly improved oral bioavailability, and curcumin's favorable adverse effect profile and low cost have helped garner growing interest in its molecular properties and biological effects as well as its possible applications in the prevention and treatment of hepatobiliary and other (e.g. pancreatobiliary) diseases. The data thus far regarding its potential role as an adjunctive or primary pharmacologic agent appears promising. Future research is expected and eagerly awaited in this regard and to better and gauge clinical efficacy and utility elucidate mechanisms of action.
Abbreviations- •
CCA: cholangiocarcinoma.
- •
HCC: hepatocellular carcinoma.
- •
NASH: nonalcoholic steatohepatitis.
- •
NF-kB:nuclear factor kappa-light-chain-enhancer of activated B cells.
- •
PSC: primary sclerosing cholangitis.
- •
ROS: reactive oxygen species.
- •
TNF-oc tumor necrosis factor alpha.
- •
TGF0: transforming growth factor-beta.
None.