Primary sclerosing cholangitis (PSC) remains a rare but potentially devastating chronic, cholestatic liver disease. PSC causes obstruction of intra- and/or extra-hepatic bile ducts by inflammation and fibrosis, leading to biliary obstruction, cirrhosis and portal hypertension with all associated sequelae. The most dreaded consequence of PSC is cholangiocarcinoma, occurring in 10-20% of patients with PSC, and with population-based estimates of a 398-fold increased risk of cholangiocarcinoma in patients with PSC compared to the general population. We use the 4-D approach to endoscopic evaluation and management of PSC based on currently available evidence. After laboratory testing with liver chemistries and high-quality cross-sectional imaging with MRCP, the first D is Dominant stricture diagnosis and evaluation. Second, Dilation of strictures found during ERCP is performed using balloon dilation to as many segments as possible. Third, Dysplasia and cholangiocarcinoma diagnosis is performed by separated brushings for conventional cytology and fluorescence in situ hybridization (FISH), and consideration for direct cholangioscopy with SpyGlass™. Fourth and finally, Dosing of antibiotics is critical to prevent peri-procedural cholangitis. The aim of this review article is to explore endo-scopic tools and techniques for the diagnosis and management of PSC and provide a practical approach for clinicians.
Primary sclerosing cholangitis (PSC) remains a rare chronic, cholestatic liver disease. PSC causes obstruction of intra- and/or extra-hepatic bile ducts by inflammation and fibrosis, eventually leading to biliary cirrhosis and portal hypertension with all associated sequelae.1-4 There is wide global variability in PSC prevalence with estimates in the United States ranging from 1 to 16 cases per 100,000 people.3 The association between PSC and inflammatory bowel disease (IBD), specifically ulcerative colitis (UC), is well described with nearly 5% of UC patients developing PSC and perhaps up to two-thirds of PSC patients developing IBD.3,5,6
The most dreaded consequence of PSC is cholangi-ocarcinoma, occurring in 10-20% of patients with PSC, and with population-based estimates of a 398-fold increased risk of cholangiocarcinoma in patients with PSC compared to the general population.7 Between 20 and 50% of cholangiocarcinoma cases are diagnosed within 1 year of PSC diagnosis, with an incidence of 0.5-1% per year, with risks further magnified in those with concomitant IBD, specifically UC as opposed to Crohn's Disease, and increasing with advancement of patient age at PSC diagnosis.8-10 While there is no approved medical therapy for PSC, endoscopic intervention is the primary management strategy in the setting of cholangitis, concern for biliary obstruction manifesting with worsening liver chemistries or pruritus, or for diagnosis and therapy of severe stricturing dis-ease.11,12 The aim of this review article is to explore endoscopic tools and techniques for the diagnosis and management of PSC and provide a practical approach for clinicians.
Imaging and Endoscopy in The Diagnosis of PscClassically, PSC is described on cholangiography as having areas of focal stricturing in addition to saccular dilation of the bile ducts, forming the “beaded” appearance often seen on imaging, with “pruning” of small intrahepat-ic duct branches at more chronic stages.11-13Cholangiog-raphy can be performed using magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), or via percutaneous trans-hepatic cholangiography (PTC) performed by interventional radiology. MRCP is the least invasive approach providing a three-dimensional reconstruction of the biliary tree without the procedural risks of ERCP or percutaneous approaches, and is the currently recommended primary modality for diagnosis of PSC.11,12,14-16 MRCP protocols may vary between institutions, and minimum test performance benchmarks should be met to standardize results.16 A meta-analysis of 6 studies of 456 patients comparing MRCP to ERCP or PTC as the gold standard for diagnosis of PSC demonstrated an 86% sensitivity and 94% specificity for MRCP.17
ERCP should be viewed as a primary therapeutic modality, and only reserved for diagnostic purposes in patients who are unable to undergo MRCP or in whom MRCP may be equivocal despite a high clinical index of suspicion.12,13,16 This approach favoring first MRCP followed by ERCP only in select situations was also found to be most cost-effective.18 Dominant strictures, identified at thetimeofERCP,aredefinedasastenosisof≤ 1.5mm inthecommonbileductor≤ 1.0mminthehepaticducts within 2 cm of the hepatic confluence, and often underlie the basis for endoscopic intervention.11,12,19 Dominant strictures develop in 45-58% of patients with PSC19-21 and can be found early at the time of diagnosis or later in the course of the disease. The clinical impact of dominant strictures cannot be overlooked. In a cohort of 128 patients with PSC, the presence of a dominant stricture had a marked negative effect on mean survival (13.7 years vs. 23 years), largely attributed to a 26% risk of developing cholangiocarcinoma, with 50% of cholangiocarcinoma cases presenting within 4 months of PSC diagnosis.22
Indications for Endoscopic InterventionThere are multiple indications for endoscopic intervention in PSC. These include jaundice, pruritus, clinical evidence of cholangitis and rising liver chemistries, suspicious for biliary obstruction.11-13,23-25 ERCP may also be undertaken for further diagnostic evaluation of a dominant stricture seen on imaging, as well as provide potential therapeutic options.2
Diagnostic Modalities to Evaluate Biliary Strictures During ErcpERCP affords additional diagnostic interventions in the evaluation of PSC-associated biliary strictures including cholangiography performed during the ERCP, biliary brushings for cytology and fluorescence in situ hybridization (FISH), as well as introduction of a small endoscope into the biliary tree to perform direct cholangioscopy. Charatcharoenwitthayal, et al. in a cohort of 230 patients with PSC of whom 23 developed cholangiocarcinoma, evaluated serum Cancer antigen 19-9 (CA 19-9) levels, imaging, stricture cytology and advanced molecular markers for test performance in diagnosing cholangiocarcinoma.26 They determined that a cutoff of serum CA 19-9 of 20 U/ mL resulted in a sensitivity of 78%, specificity 67%, 23% PPV, and 96% NPV. When serum CA 19-9 was added to ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI), sensitivity increased to range from 91-100%, however specificity decreased to 37-62%, and positive predictive value (PPV) remained low at 22-24%. Conventional biliary brush cytology performed during ERCP had low sensitivity (50%), with reasonable specificity 97%, PPV 86%, and negative predictive value (NPV) 83%. When aneusomy detection by FISH was added, there was a marked improvement in sensitivity to 86%, while specificity, PPV, and NPV remained largely unchanged (83%, 80%, and 88% respectively).
Recently, in a study of 261 asymptomatic PSC patients undergoing screening for biliary dysplasia, CA 19-9 was shown to have no prognostic value for biliary dysplasia or cholangiocarcinoma.27 CA 19-9 as noted is neither sensitive nor specific for cholangiocarcinoma, and may be elevated in a multitude of conditions both benign and malignant which cause biliary obstruction.11,12,28 Therefore, an elevated CA 19-9 alone should be interpreted with care, and while it may prompt additional investigation, should not be considered a harbinger of malignancy in and of itself.
A 2014 systematic review and meta-analysis by Trikundanathan, et al. of 11 studies of 747 PSC patients with histopathologic correlation showed a low sensitivity for brush cytology diagnosis of cholangiocarcinoma at 43%, with good specificity of 97%.29 Limited performance results of brush cytology alone were confirmed by Sangfelt, et al. in 70 PSC patients with strictures and by Levy, et al. in 86 PSC patients with indeterminate biliary strictures.30,31 Due to the poor yield of conventional brush cytology, FISH has been added to improve diagnostic yield of cholangiocarcinoma. When interpreting the results of FISH testing, presence of polysomy may be associated with cholangiocarcinoma, whereas trisomy and tetrasomy imply similar outcomes to a negative FISH test.
Patients with serial polysomy found on multiple brush-ings during subsequent ERCPs are likely at an even higher risk of cholangiocarcinoma, with a PPV of 70%.32-34 Additional studies have confirmed the diagnostic benefit of adding FISH to traditional brush cytology.31
The ideal diagnostic modality seems to be a combination of methods to improve diagnostic accuracy. Nanda, et al. compared use of brush cytology, intraductal biopsy with forceps, and FISH for diagnosis of cholangiocarcinoma in 50 patients undergoing ERCP.35 They found the 3 test combination had markedly improved test performance (sensitivity 82%, specificity 100%, PPV 100%, NPV 87%) compared to each modality alone with sensitivity of brush cytology at 27%, forceps biopsy at 50%, and FISH at 59%.
The use of direct visualization cholangioscopy has been examined for characterization of dominant strictures in 53 PSC patients, of whom 12 had cholangiocarcinoma and 41 had benign dominant strictures.34The investigators compared ERCP with endoscopic brush cytology to cholangioscopy. Cholangioscopy had significantly better sensitivity (92% vs. 66%), specificity (93% vs. 51%), accuracy (93% vs. 55%), PPV (79% vs. 29%), and NPV (97% vs. 84%). In a similar study by Awadallah, et al., cholangiosco-py not only provided additional benefit in characterizing PSC-associated biliary strictures, but also discovered choledocholithiasis, which was missed in 30% of patients on cholangiography, with added benefit of providing lithotripsy when needed during cholangioscopy.36 Interestingly, both studies were conducted with use of a prior generation of cholangioscopy equipment with newer digitized generations having improved optics, image quality, and maneuverability by the operator.
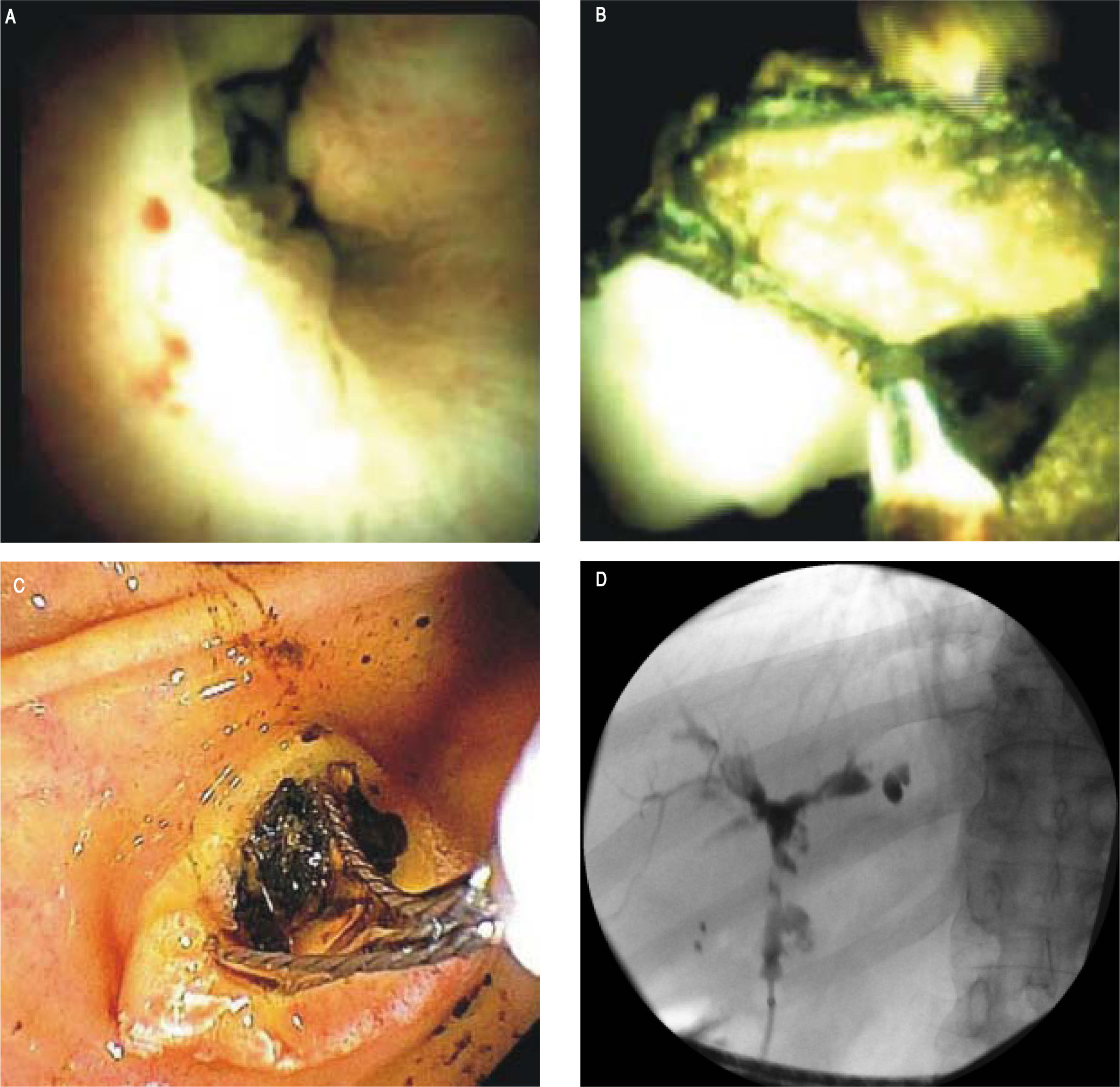
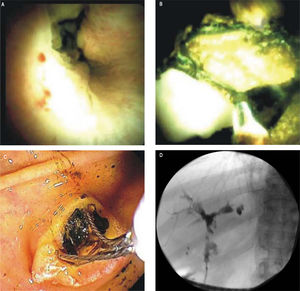
The newer generation cholangioscope, SpyGlass DS™ (Boston Scientific, Marlborough, Massachusetts), is a single operator, through-the-scope device with ability to take targeted biopsies through a small forceps (Spybites), with improved overall diagnostic accuracy compared to ERCP-based tissue sampling for evaluation of biliary stric-tures37,38 (Figure 1). In a prospective multicenter study from Japan, SpyGlass™ obtained adequate tissue for histo-logic examination in 80% (60/75) of patients, with 73.7% (53/75 patients) accuracy for diagnosis of indeterminate bile duct lesions.39 Small studies of SpyGlass™ in PSC patients showed modest improvements in diagnostic performance.40,41 In a study of 19 PSC and 10 non-PSC patients with biliary strictures, SpyGlass™ had improved diagnostic yield compared to conventional brush cytology with significantly more tissue obtained (p = 0.021) in both PSC and non-PSC patients.42 Further clinical performance evaluation of SpyGlass™ in larger studies of PSC patients is still needed. While cholangioscopy allows for direct visualization of larger ducts in the biliary tree with targeted biopsies, technical considerations include challenges in cannulating a stenosed duct with now a wider device than, for example, a sphincterotome, increased risks of cholangitis, and challenges with tissue yield due to the small caliber forceps used.43
Representative SpyGlass™ image showing PSC-associated common bile duct stricture (A).Representative SpyGlass™ image in the common bile duct (B) and endoscopic image at the level of the ampulla (C) demonstrating choledocholithiasis. Corresponding cholangiogram performed during ERCP showing PSC-related changes in the biliary tree with strictures and beading involving predominantly the left biliary system (D).
Endoscopic ultrasound (EUS) has been employed for an expanding number of indications in diagnostic and therapeutic endoscopy. EUS should not, however, routinely be employed for diagnosis of PSC.12 EUS can be employed in a variety of clinical scenarios to complement cross-sectional imaging and guide potential ERCP. EUS can be helpful if MRCP findings are unclear, the patient is unable or unwilling to undergo imaging, other causes of biliary obstruction are suspected, such as choledocholithiasis which is common in PSC, or for further imaging evaluation of a mass lesion. EUS with fine needle aspiration should not be performed for potential cholangiocarcino-mas in the proximal bile duct or hilum as this may negate transplant candidacy at many centers, due to concern for seeding of the needle tract. EUS with fine needle aspiration is useful for tissue acquisition when cholangiocarci-noma is already advanced and the patient is not a candidate for transplant, i.e. sampling of potential lymph node me-tastases.
Therapeutic Intervention Techniques With ErcpOnce a dominant stricture has been diagnosed either prior to ERCP or during ERCP, there are multiple therapeutic options available to the endoscopist including dilation and stenting, each with their own benefits and drawbacks. Both balloon dilation and endoscopic stenting are effective treatments of dominant strictures; however, only balloon dilation appears to positively impact survival compared to predicted Mayo Risk Score.19,44-49 Stenting was originally shown to be effective in a retrospective study of 25 PSC patients by van Milligen de Wit, et al. in 1996, in which stenting for a median of 3 months results in improvement of symptoms in 76% of patients and a significant decrease in bilirubin and liver chemistries, while precipitating 32 episodes of jaundice/cholangi-tis due to stent obstruction. There is not specific data on the optimal balloon dilation regimen for dominant strictures in PSC.12 In a non-randomized, uncontrolled, study of 63 PSC patients followed for a median of 34 months, Baluyut, et al. reported on the benefits of endoscopic intervention primarily consisting of balloon dilation of dominant strictures, showing a significantly improved actual overall 5-year survival compared to the projected 5-year survival based on initial Mayo Risk Score (83% vs. 65%; p = 0.027).46 These findings were again shown in a single center, retrospective study of 117 PSC patients followed for a mean of 8 years, 106 of which underwent ERCP and 84 of which had endoscopic interventions for dominant strictures or worsening clinical status.45 They reported a 7.3% procedural complication rate without any attributed mortality, which was outweighed by a significantly improved 3- and 4-year survival compared to their predicted survival by the Mayo Risk Score for PSC.
Balloon dilation is safe and associated with fewer complications than stenting. Gotthardt, et al. followed 171 PSC patients for up to 20 years, with 500 balloon dilations performed over that period for dominant strictures with good clinical efficacy, and a low complication rate (2.2% pancreatitis, 1.4% cholangitis, 0.2% perforation, and no deaths), with associated 81% 5-year transplant free surviv-al.44 Kaya, et al. compared endoscopic balloon dilation to balloon dilation combined with biliary stenting either en-doscopically or percutaneously performed for 71 PSC-as-sociated biliary strictures, and showed a significantly increased complication rate and cholangitis rate in the stent group with no significant differences in improvement of cholestasis amongst groups.48 Notably, the complications in the stented group were significantly driven by the percutaneously placed stents as opposed to endo-scopically placed stents, including perforation. Most recently, the European multicenter randomized trial (DILSTENT; Clinicaltrials.gov Identifier: NCT01398917) that compared balloon dilation to short-term biliary stent-ing was stopped early at the time of interim analysis given no differences in overall outcomes, but a significantly higher complication rate in the stented group.12 Balloon dilation should be performed in all accessible strictures at the time of ERCP. Anecdotal experience suggests improved outcomes with increasing the number of segments that undergo balloon dilation.
Routine biliary stenting as primary therapy for dominant strictures is not recommended by the most recent guidelines from the American Association for the Study of Liver Disease and the American College of Gastroenterol-ogy.11,13 Stenting is used based on endoscopist preference and expertise. When needed for severe strictures, short-duration (median 9-11 days) endoscopic biliary stent placement was shown to be beneficial to improve cholestasis, with a moderate complication rate of 7%.47,50 Short-duration stenting of 1-2 weeks had similar efficacy compared to standard-duration of 8-12 weeks, while longer duration overall had increased complications and need for repeat ERCPs, as stents may rapidly clog in PSC pa-tients.12,47-49 Of note, there is no published data on the use of Soehendra dilators in PSC-associated strictures nor are Soehendra dilators mentioned in the therapeutic algorithms of any of the major gastroenterology or hepatology society guidelines.11-13 When endoscopic approaches are unsuccessful, percutaneous approaches can be undertaken to achieve biliary access and decompression; however, percutaneous approaches carry increased morbidity.2,11 Lastly, performance of biliary sphincterotomy is not routinely recommended but should be considered in patients with complex, difficult biliary cannulations, with risks and benefits weighed in each patient on a case-by-case basis.12
Complications From ErcpThe risk of ERCP-related adverse events in PSC patients remains relatively low.51-53While bacterial cholangi-tis may be the initial presentation of PSC, either due to choledocholithiasis or to a stricture causing biliary obstruction, endoscopic instrumentation of the biliary tree itself may also precipitate bacterial cholangitis.54 In a study of 981 non-PSC patients and 168 PSC patients undergoing ERCP, a significantly increased risk of post-procedural cholangitis was noted in PSC patients (4% vs. 0.2%) despite antibiotic prophylaxis.55 Rates of post-procedural cholangitis range from approximately 0.6% to 8%.12,45,46,51,52,56 This is most likely due to incomplete drainage in smaller ducts of the liver causing seeding of intestinal flora into the liver during ERCP, and emphasizes the need for prophylactic antibiotics for PSC patients undergoing ERCP as well as a short course of prophylactic oral antibiotics for 3-5 days thereafter.12,57 Use of SpyGlass™ without antibiotic prophylaxis carried an 8.8% bacteremia rate and a 7.0% cholangitis rate in prospective study of 60 patients without known PSC undergoing ERCP, again solidifying the need for antibiotic prophylaxis in PSC where these risks are elevated.58,59 Aspiration of bile prior to injection of contrast media and balloon dilation with avoidance of biliary stenting when possible may further reduce risk of cholangitis.53 Performance of biliary sphincterotomy may increase peri-procedural risk of adverse events, perhaps related to bleeding due to thrombo-cytopenia and less likely perforation.52 However, specifically in PSC patients who may require multiple ERCPs, biliary sphincterotomy may be protective against post-ERCP pancreatitis development due to potential complexity of subsequent biliary cannulations.51
Post-ERCP pancreatitis is known to occur with estimates of up to 10% depending on patient and procedural risk factors, but had similar incidence in PSC and non-PSC patients.55,60 In general, risk is substantially reduced by having the ERCP done by an experienced endoscopist with a large PSC practice. Multiple peri-procedural strategies have shown benefit in risk reduction including routine use of rectal indomethacin, intravenous fluid hydration ideally with lactated ringers solution, and pancreatic duct stent placement.61-66 The use of each of these modalities will need to be considered on a case-by-case basis if cirrhosis is present as significant thrombocytope-nia may be a contraindication to indomethacin usage, and marked ascites may preclude aggressive intravenous fluid administration; however, when possible, these interventions should be performed to reduce post-ERCP pancreatitis risk, which can be potentially devastating.
Emerging Modalities for Evaluation of Biliary StricturesSeveral new technologies are currently being investigated for their role in the diagnosis and management of PSC and associated biliary strictures, including probe-based confocal laser endomicroscopy (CLE), positron emission tomography (PET) scanning, and intraductalul-trasound. Probe-based CLE has been suggested for evaluation of PSC-associated strictures, though data remains limited. Heif, et al. in a small study of 15 PSC patients with 21 dominant strictures undergoing ERCP with probe-based CLE, showed 100% sensitivity, 61.1% specificity, 22.2% PPV, and 100% NPV for detection of neoplasia. In this study, tissue sampling alone, had 0% sensitivity, 94.4% specificity, 0% PPV, and 89.5% NPV.67 Probe-based CLE may also be useful in differentiating PSC from non-PSC associated inflammatory strictures.68
Complimentary use of PET scanning with brush cytology for early identification of high-grade dysplasia and cholangiocarcinoma in those with dominant strictures was advocated by Sangfelt, et al. who showed improved performance when adding PET scanning compared to brush cytology alone.30 Additional studies revealed some potential utility of PET scanning in diagnosis of cholangiocarci-noma, with potential benefit compared to traditional CT or MRI.69-71 Widespread feasibility and performance of PET scanning in this area remain to be seen.
Intraductal ultrasound for evaluation of strictures was proposed approximately 10 years ago with some promising initial data with sensitivity of up to 86% for identification of malignancy but has not caught on in routine clinical practice to date.31,72 In the future, much like the use of FISH, or next generation DNA sequencing in the evaluation of pancreatic cyst fluid, additional genetic and molecular markers may be developed to improve identification of cholangiocarcinoma in PSC.
Our Approach to Endoscopic Evaluation and Management of PSCWe use the 4-D approach (Table 1) to the endoscopic evaluation and management of PSC based on currently available evidence. After laboratory testing with liver chemistries and high-quality cross-sectional imaging with MRCP, the first D is Dominant stricture diagnosis and evaluation. If a new dominant stricture is discovered, then ERCP is performed. Second, Dilation of strictures found during ERCP is performed using balloon dilation to as many segments as possible. Third, Dysplasia and cholan-giocarcinoma diagnosis is performed by separated brush-ings for conventional cytology and FISH and consideration for direct cholangioscopy with SpyGlass™. Unless contraindicated, administration of rectal in-domethacin and intravenous fluid administration with lac-tatedringers solution is undertaken to minimize risk of post-ERCP pancreatitis. If a native papilla is encountered, endoscopic sphincterotomy is performed in the vast majority of cases during first ERCP to facilitate future biliary access, cholangioscopy, and reduce risk of distal choledo-cholithiasis leading to biliary obstruction. Direct cholan-gioscopy is performed on a case-by-case basis only if feasible, with small biopsies taken of any visualized strictures, and usually will focus on distal ducts as there may be an increased chance of complications with more proximal ducts. If adequate drainage cannot be achieved or if significant intra-procedural bleeding occurs, a biliary plastic stent may be placed at the discretion of the endoscopist. When stents are placed, an effort is made to remove them after a short period of time, usually 2 weeks post-ERCP. Fourth and finally, Dosing of antibiotics is critical to prevent peri-procedural cholangitis. Pre-procedure prophylactic antibiotics are administered intravenously, followed by a prophylactic 5 day course of oral antibiotics for those without overt cholangitis.
The 4-D Approach to the Endoscopic Evaluation and Management PSC.
| The 4-D Approach | |
|---|---|
| 1 | Dominant Stricture diagnosis and evaluation. |
| 2 | Dilation of strictures found during ERCP. |
| 3 | Dysplasia and cholangiocarcinoma diagnosis by separated brushings for conventional cytology and FISH and consideration for direct cholangioscopy with SpyGlass™. |
| 4 | Dosing of antibiotics to prevent peri-procedural cholangitis. |
In conclusion, there are multiple tools in our diagnostic armamentarium for the evaluation and management of PSC and its complications. As technologies continue to evolve, current standards of care such as balloon dilation and brushings of dominant strictures may be replaced by newer modalities offering better diagnostic accuracy. Ultimately, patient-centered outcomes of transplant- and malignancy-free survival will be the most important markers of the quality of care provided to our patients.
Abbreviations- •
CA 19-9: cancer antigen 19-9.
- •
CT: computed tomography.
- •
CLE: confocal laser endomicroscopy.
- •
ERCP: endoscopic retrogradecholangiopancreatography.
- •
EUS: endoscopic ultrasound.
- •
FISH: fluorescence in situ hybridization.
- •
IBD: inflammatory bowel disease.
- •
MRCP: magnetic resonancecholangiopancreatography.
- •
MRI: magnetic resonance imaging.
- •
NPV: negative predictive value.
- •
PTC: percutaneous trans-hepatic cholangiography.
- •
PPV: positive predictive value.
- •
PET: positron emission tomography.
- •
PSC: primary sclerosing cholangitis.
- •
UC: ulcerative colitis.
In consideration of the FundaciónClínicaMédica Sur (FCMS) taking action to review and edit my submission, the undersigned authors, jointly and severally, hereby transfer, convey, and assign all right, title, and interest therein, including any and all copyrights in all forms and media now or hereafter known, to the FCMS. The authors retain the nonexclusive right to use all or part of the Article in future works of their own in a noncompeting way, provided proper copyright credit is given to the Foundation. Should the FCMS not publish the aforesaid submission, the FCMS agrees to release its rights therein (Note: material prepared by employees of the federal government in their official duties may not be copyrightable). No guarantee is made that the Article will be published.
Authorship ResponsibilityI, the undersigned author, certify that I have participated sufficiently in the intellectual content, the analysis of data, if applicable, and the writing of the Article, to take public responsibility for it. I have reviewed the final version of the Article, believe it represents valid work, and approve it for publication. As an author of this Article, I certify that none of the material in the manuscript has been published previously, is included in another manuscript, or is currently under consideration for publication elsewhere. I also certify that the Article has not been accepted for publication elsewhere, nor have I assigned any right or interest in the Article to any third party.
Financial DisclosureI, the undersigned author, certify that I have no commercial associations (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements) that might pose a conflict of interest in connection with the submitted Article, except as disclosed on a separate attachment. All funding sources supporting the work and all institutional or corporate affiliations of mine are acknowledged in a footnote.
Institutional Review Board/Animal Care Committee ApprovalNot applicable; Clinical Review.
Conflict of Interest and Financial DisclosureThe authors report no relevant conflicts of interest nor financial disclosures.