Background and aim. The etiology of non-alcoholic fatty liver disease (NAFLD) progression, and why some patients develop non-alcoholic steatohepatitis (NASH) vs. uncomplicated NAFLD, is not well understood. Obesity and NAFLD are thought to be associated with high circulating levels of leptin; however, the role of leptin in NASH has been controversial. Secondly, as ob/ob mice are known to have elevated circulating levels of TLR4-stimulating endotoxin secondary to increased intestinal permeability.
Material and methods. We evaluated the long-term effects of steatosis on the livers of aleptinemic (OB) mice and the role of TLR4 in the development of hepatic sequelae in these animals.
Results. At 20 weeks of age OB animals displayed grossly steatotic livers, but also features of early stage NASH including hepatocellular ballooning and numerous necroinflammatory foci with associated changes in serum aspartate aminotransferase (AST) and alanine transaminase (ALT). TLR4 KO did not affect the development of obesity or steatosis in ob/ob mice, but protected these animals from hepatitis and liver injury.
Conclusions. In conclusion, the data presented here indicate that steatohepatitis develops in the absence of leptin, and that TLR4 is integral to the development NASH secondary to hyperphagia.
Under homeostatic conditions, the protein hormone leptin, produced primarily by white adipose tissue, signals satiety through the arcuate nucleus of the hypothalamus. However, in the setting of metabolic syndrome this feedback response fails due to central leptin resistance.1 Initially, adipose tissue prevents other organ systems from exposure to excessive fatty acids through the hypertrophy and hyperplasia of adipocytes. However, as hypertrophic adipocytes become insulin resistant, the liver responds adaptively to uptake circulating free fatty acids (FFA), protecting other organ systems from the toxicity of long and very long chain fatty acids released from adipose tissue stores.2 The end result of this process is the development of nonalcoholic fatty liver disease (NAFLD), which may then progress to nonalcoholic steatohepatitis (NASH).
Little is known about the etiology of NAFLD progression and why some patients develop NASH vs. uncomplicated NAFLD. NAFLD is usually a benign condition while NASH can result in hepatocellular carcinoma, cirrhosis, and even death. Obesity and fatty liver disease are thought to be associated with high circulating levels of leptin. While it is generally accepted that leptin deficient mice suffer increased endotoxin sensitivity and an overall skewing of tissue macrophage populations from alternatively activated/anti-inflammatory (M2) to classically activated/pro-inflammatory (M1) phenotypes, it is unknown if this is due to the absence of leptin or the presence of steatosis and metabolic syndrome.
The roles of leptin in NAFLD and NASH have been studied in both animal models and clinical subjects. Leptin is elevated in the setting of NAFLD in human patients.3,4 Furthermore, studies in mice have suggested that leptin signaling may play a causal role in the development of steatohepatitis in response to endotoxin.5 Likewise, leptin was found to potentiate inflammatory cell infiltration, tumor necrosis factor alpha (TNF-α) expression, and hepatocellular damage in a CCl4 model of liver injury.6
Several studies have suggested that Leptin is a pleiotropic cytokine with significant pro-inflammatory effects. Leptin deficient animals display a deficit in TNF-α expression in response to endotoxin challenge and in fact leptin may enhance innate immune responses through p38 and JNK.7,8 Imajo, et al. implied that leptin promotes expression of the TLR4 co-receptor CD14, mediating the development of inflammation in fatty liver disease.5 Yet, observations within the clinical environment have been conflicting. Initial studies reported a correlation between leptin levels and the degree of hepatosteatosis and that leptin may be elevated in NASH as compared to other forms of hepatitis.3,9 However, serum leptin was not linked to necroinflammatory grade and future studies failed to confirm elevation of leptin within NASH patients as compared to controls.10,11 Furthermore, no correlation was found between leptin levels and fibrosis stage.12
We were curious what the long-term effects of steatosis would be within the livers of aleptinemic (OB) mice. It is often taken for granted that ob/ob mice do not develop steatohepatitis in the absence of an external stressor.13 Secondly, as OB mice are known to have elevated circulating levels of endotoxin secondary to increased intestinal permeability, we evaluated the role of TLR4 in the development of hepatic sequelae in these animals.14
Material and MethodsAnimal studiesTlr4lps-del mice on a C57BL/6 background (TLR4 KO) and wild type C57BL/6 mice purchased from Jackson Laboratories were used in these experiments. We also completed generation of a novel double knockout murine model: TLR4 KO, leptin deficient (OB) mice. Genotyping of the Lepob allele was performed using high resolution melt curve analysis.15 All mice were fed 8656 Teklad Sterilizable Rodent Diet (Harlan, USA) and were housed 3 to 4 per cage in a temperature-controlled room (22-25 °C) with a 12-h light-dark cycle and provided with water and food available ad libitum. All experiments were performed under clean conditions, were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee (MUSC IACUC), and were in accordance with “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health.
Collection of tissue and seraAt the time of sacrifice, mice were weighed and peripheral blood samples collected under isoflurane inhalation anesthesia by sterile cardiac puncture. Mice were then euthanized humanely by isoflurane and cervical dislocation. Livers were promptly dissected from the animal, gallbladders removed, and weighed. The median, right, and caudate lobes were immediately snap frozen in liquid nitrogen and stored at −80 °C until analysis. The left hepatic lobe was divided into sections for fixation in 10% buffered formalin and storage in RNAlater (Qiagen, USA).
Serum transaminase measurementWhole blood was allowed to clot at room temperature for 20 min followed by centrifugation at 3,500 g for 5 min at room temperature to separate serum. Serum aminotransferase activities were measured by standard enzymatic-colorimetric microplate assay according to the manufacturer’s instructions and expressed as IU/L (Pointe Scientific, USA).
Pathological analysis of steatohepatitisUpon the sacrifice of an animal, sections of liver tissue from the left lateral lobe were placed in 10% neutral-buffered formalin for fixation, embedded in paraffin, cut, placed on a glass slide, and heat-fixed. Hematoxylin and Eosin (H&E) staining was performed. Slides were read by an experienced liver pathologist and graded based on the semi-quantitative schema outlined by Kleiner, et al.16 In brief, NAFLD activity score was determined by the sum of:
- •
Steatosis.
- •
Lobular inflammation, and
- •
Hepatocellular ballooning scores.
Hepatocellular ballooning was identified as enlarged (1.5-2 X diameter) cells with rarefied cytoplasm. Fibrosis is not included in the activity score, as it is representative of stage, rather than grade, of NAFLD and subsequent NASH.17
Cytochemical stainingNeutrophils were identified by Leder stain (specific esterase or naphthol AS-D chloroacetate esterase, Sigma-Aldrich). In brief, four-micrometer thick sections of formalin-fixed, paraffin embedded (FFPE) liver were deparaffinized in histoclear (National Diagnostics, USA) and rehydrated in a graded alcohol series. Specific staining was performed, without further fixation, according to manufacturer’s protocol. Sections were counterstained with Gill’s hematoxylin, followed by were coverslipping with Aqua-Mount slide mounting media without allowing tissue to become dry (Thermo Scientific, USA). Positive cells were counted in 10 high-powered fields per section.
F4/80 immunohistochemistryFFPE sections were deparaffinized in histoclear, and rehydrated. Antigen retrieval was performed by incubation with proteinase K solution for 1 min at 37°C. After washing, endogenous peroxidases were blocked with Bloxall, followed by blocking with an endogenous streptavidin/biotin blocking kit (Vector Laboratories, USA). Sections were then incubated with 1.5% normal rat serum, followed by incubation with 1:150 primary antibody (rat anti-F4/80, clone CI:A3-1, Abcam, USA). Samples were then washed and incubated with biotinylated secondary antibody (Vector Laboratories, USA) for 30 min. After washing, sections were incubated with Vectastain ABC kit (Vector Laboratories, USA) for an additional 30 min. Immunoperoxidase staining was performed with the Diaminobenzidine (DAB) substrate kit (Vector Laboratories, USA), followed by washing and a counterstain with hematoxylin. Slides were then dehydrated through a graded alcohol series and xylene, and cover-slipped with Cytoseal 60 mounting medium (Thermo Scientific, USA). Positive cells were counted as a ratio of total cells in 10 high-powered fields (HPF) per section.
Collagen stainingFFPE sections were deparaffinized and rehydrated, followed by incubation for 30 min in a solution containing 0.1% sirius red (Direct red 80, Sigma) and saturated picric acid (picric acid 6.0 g, 200 mL H2O). Sections were then washed in 0.003 N HCl and coverslipped with Cytoseal 60. Stained slides were analyzed imaged under polarized light with an Olympus Bx50WI scope.
Quantitative real time RT-PCRTotal RNA was isolated from cells with RNeasy Lipid Tissue Mini Kit (Qiagen, USA) with on-column DNAse digestion using RNase-Free DNase Set to remove genomic DNA, according to manufacturer’s instructions. Quality of RNA was confirmed on a Nano Drop spectrophotometer (Thermo scientific, USA) and concentrations normalized in RNase free water. For qRT-PCR, RNA (100 ng) was reverse transcribed and amplified on a LightCycler 480 instrument (Roche, USA) via a one-step method, using the TaqMan one-step PCR master mix (Life Technologies, USA) and gene expression TaqMan-FAM-MGB primers/ probe assays (Adiponectin, Mm00456425_m1; HPRT, Mm00446968-m1; IL-1β, Mm00434228-m1; IL-6, Mm00446190_m1; IL-10, Mm00439616_m1; ICAM-1, Mm00516023-m1; IFN-γ, Mm00801778-m1; PPAR-γ, Mm00440945_m1; TGFβ1, Mm00441724-m1; TNF-α, Mm00443258-m1: Applied Biosystems, USA) in a 20 μl reaction volume according to the manufacturers protocol. Thermocycling parameters were as follows: Reverse transcription -50 °C 5 min; reverse transcriptase inactivation/denaturation − 95°C 20 seconds; amplification − (95°C 3 seconds, 60°C 30 seconds) x 40 cycles. Quantification of a given gene, expressed as relative mRNA level, was calculated after normalization to the housekeeping gene HPRT1 calculated relative to wild type (WT) control using the comparative ΔΔCt method.
Statistical analysisAll values here are expressed as mean ± standard error of the mean. Statistical significance was chosen a priori as α ≤ 0.05. For multiple comparisons of means, a one-way analysis of variance with Tukey-Kramer post hoc tests was used. Hypothesis testing was performed using GraphPad PRISM version 5 for Windows (GraphPad Software, USA).
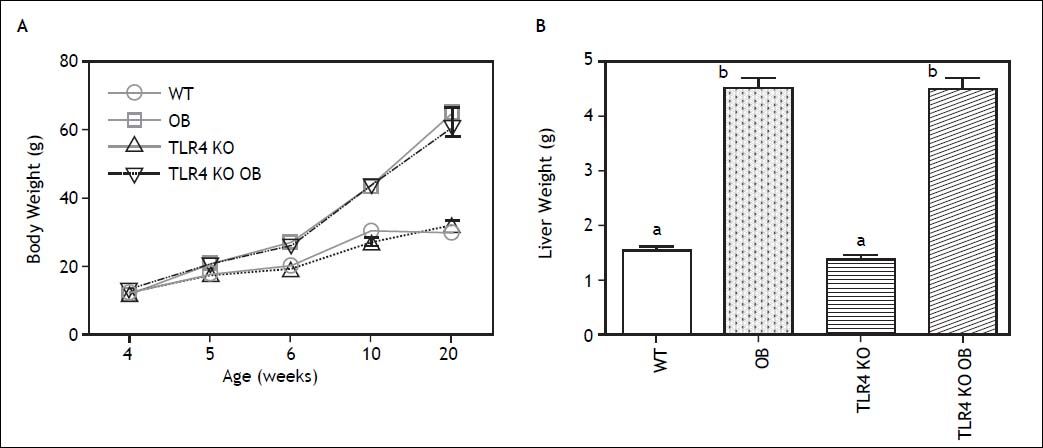
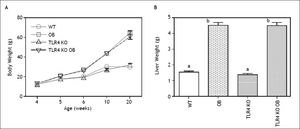
ResultsDevelopment of steatohepatitis in OB miceBody weight was initially followed in wild type and OB mice out to 25 weeks of age. Weight gain in these animals followed roughly logistic growth curves. Growth declined early in wild type animals, exiting the log phase after around 4-5 weeks and becoming stationary by 10 weeks. In contrast, OB mice continued within the log phase until nearly 7 weeks of age and did not reach a stationary phase until 20 weeks. Mean weights at 4, 5, 6, 10, and 20 weeks of age are shown in (Figure 1A). We thus chose to compare animals at this time point of 20 weeks. As expected, OB animals at this time point displayed grossly steatotic livers, several times the mass of WT animals (Figure 1B).
Animal and liver weights. A. Weights of wild type (WT), obese (OB), TLR4 knockout (TLR4 KO) and TLR4 knockout obese (TLR4 KO OB) animals. B. The masses of TLR4 KO OB mice livers were not significantly different from those of wild type OB animals. Means with different lettered subscripts within each group are significantly different from each other, p < 0.05, n = 5-7.
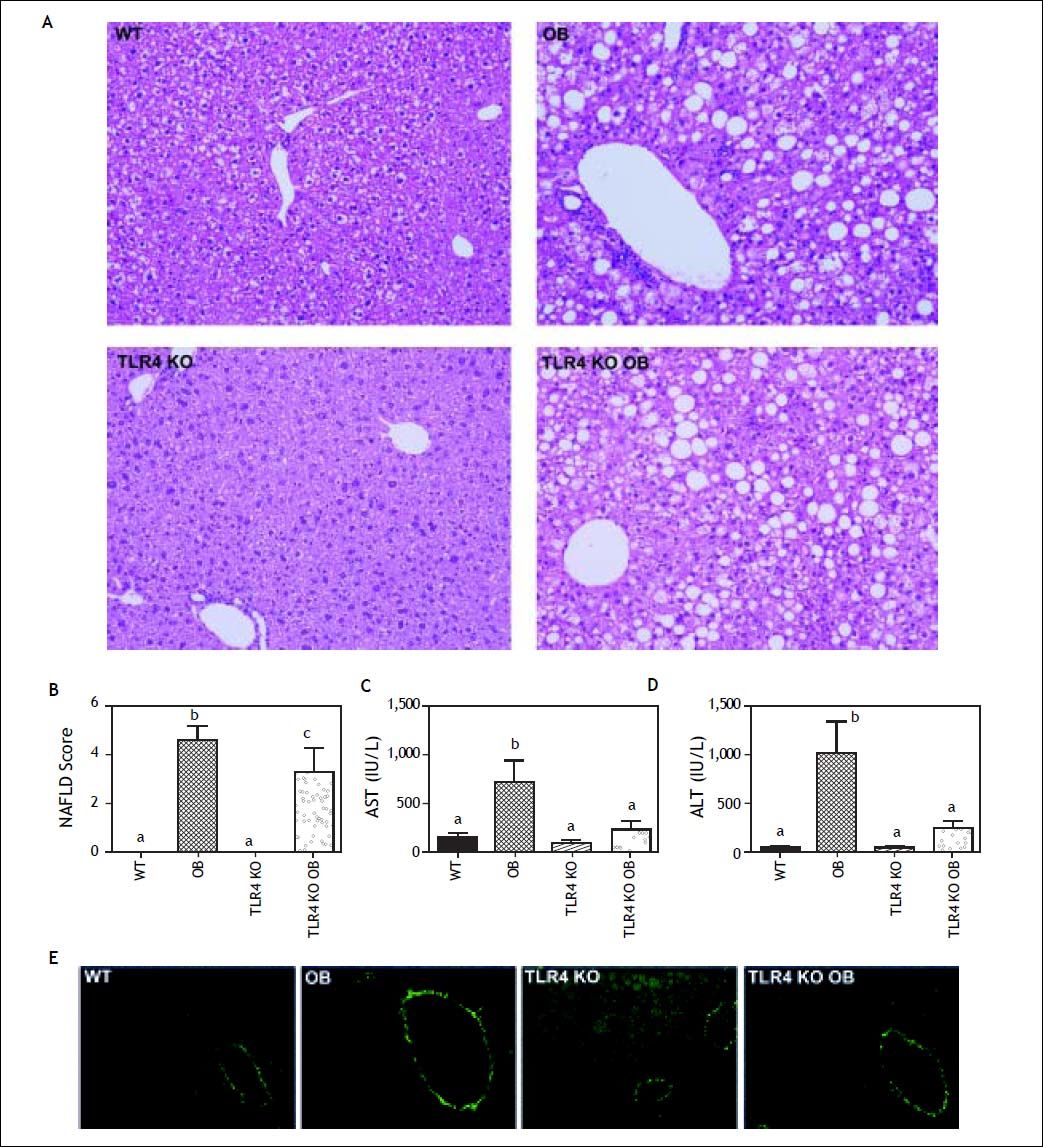
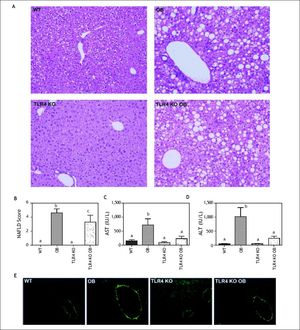
We were surprised to find that livers of OB mice displayed not only significant macrosteatosis, but also features of early stage nonalcoholic steatohepatitis including hepatocellular ballooning and numerous necroinflammatory foci (Figure 2A), which were reflected in NAFLD scores (Figure 2B). Furthermore, serum ALT and AST were elevated in these animals, indicating substantial and ongoing hepatocellular injury (Figures 2C and 2D). Fibrosis was absent in all groups as evidenced by Sirius red stained sections observed under plane-polarized light (Figure 2E).
Assessment of liver injury. A. Hematoxylin and eosin (H&E) stained sections from the livers of wild type (WT), obese (OB), TLR4 knockout (TLR4 KO) and TLR4 knockout obese (TLR4 KO OB) animals at 20 weeks. TLR4 KO OB mice had similar steatosis but decreased inflammatory foci and ballooned hepatocytes as compared to OB. B. TLR4 KO OB mouse livers had significantly lower NAFLD activity scores, as well as serum (C) aspartate aminotransferase (AST) and (D) alanine aminotransferase (ALT) levels as compared to wild type OB mice. E. Representative micrographs of liver sections stained with picrosirius red and acquired under plane-polarized light. Means with different lettered subscripts within each group are significantly different from each other, P < 0.05. Data are expressed as mean + SEM; n = 4-5.
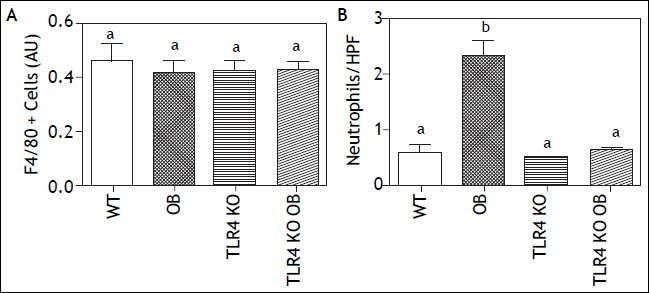
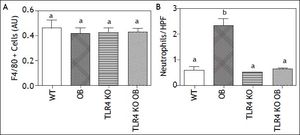
Histology revealed increased inflammatory cell infiltrate and hepatocellular ballooning, as compared to their lean counterparts. While numerous studies have reported infiltration of additional F4/80+ cells into the liver during the development of steatohepatitis, we did not find any difference in this population (Figure 3A).18,19 We did however find significantly increased neutrophil accumulation (Figure 3B).
F4/80+ Cell and Neutrophil Infiltration. A. Quantification of hepatic F4/ 80+ cells showed no significant differences in the number of monocytes/macrophages within the livers of wild type (WT), obese (OB), TLR4 knockout (TLR4 KO) and TLR4 knockout obese (TLR4 KO OB) animals. B. Quantification of hepatic neutrophil infiltration showing increased neutrophil infiltrate in livers of OB but not TLR4 KO OB mice Means with different lettered subscripts within each group are significantly different from each other, P < 0.05. Values as mean or mean number of cells per HPF + SEM, n = 4-5.
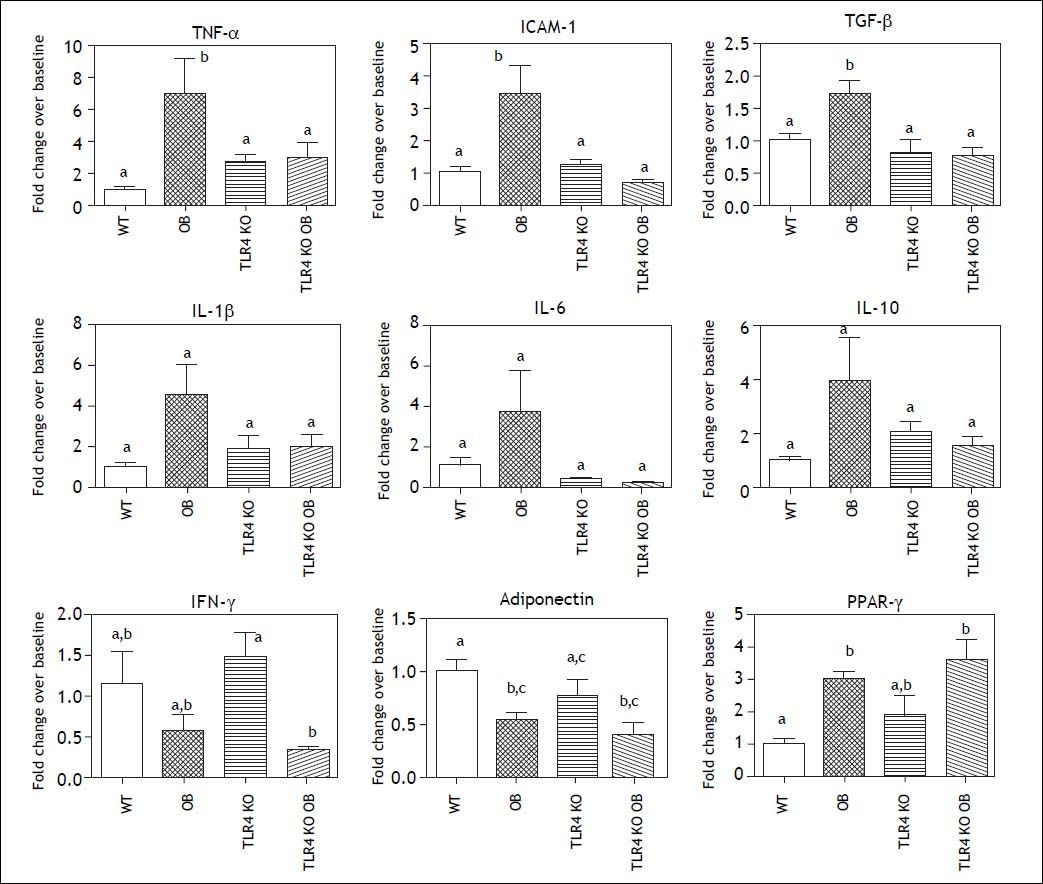
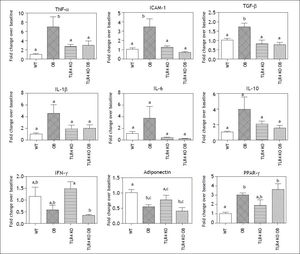
To gain further insight into the inflammatory milieu present within the livers of obese leptin deficient animals, we evaluated expression of several cytokines and other genes associated with NASH by RT-PCR (Figure 4). Expression of TNF-α, ICAM-1 and TGF-β was increased in the livers of OB mice as compared to lean counterparts. There was also a trend toward increased expression if IL-1β, IL-6, and IL-10. IFN-γ expression was lower in ob mice, though not significantly. Expression of the adipokine adiponectin was decreased in leptin deficient animals as compared to their lean counterparts, while PPAR-γ expression was increased.
Cytokine and NASH Associated Gene Expression. Hepatic mRNA expression of pro-inflammatory cytokines such as TNF-α, ICAM-1, and TGF-β was elevated in OB mice but not TLR4 KO OB. Expression of the adipokine adiponectin and PPAR-γ was changed during obesity but similar between the wild type and TLR4 KO groups. Means with different lettered subscripts within each group are significantly different from each other P < 0.05. Data are expressed as mean + SEM; n = 4-5.
OB mice display increased gut permeability contributing to metabolic endotoxemia and increased basal systemic inflammation.20
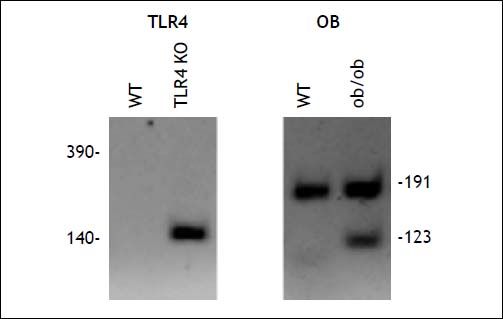
To address the role of the endotoxin receptor, TLR4, in the hepatic inflammation and injury incurred by older obese leptin knockout (OB) mice, we generated double knockout mice. As described in methods, double knockout mice homozygous for both the Lepob (OB) and Tlr4lps-del (TLR4 KO) alleles (TLR4 KO OB) were generated on a C57BL/6 background (Figure 5). These mice do not express functional leptin and are thus hyperphagic. Several studies have reported that TLR4 KO affects weight gain and/or the development of hepatic steatosis. We did not observe either of these phenomena, with TLR4 KO OB mice weighing consistently the same as their wild type OB counter parts at all time points (Figure 1A). Liver weights did not differ between TLR4 KO OB and OB animals at 20 weeks of age (Figure 1B).
TLR4 KO OB mice are protected from hyperphagic liver injuryIn contrast to OB mice, ALT or AST were not elevated in TLR4 KO OB mice (Figure 2C). Furthermore, TRL4 KO OB mice displayed fewer necroinflammatory foci and ballooned hepatocytes on histologic evaluation (Figure 2A), which was reflected in lower NAFLD activity scores (Figure 2B). These data suggest that hepatocellular injury, an integral component of NASH, was absent in TLR4 KO leptin deficient (OB) mice at 20 weeks.
TLR4 KO OB mice do not develop steatohepatitisTLR4 KO did not affect F4/80+ cell numbers within the livers of lean or obese animals (Figure 3A). However, the neutrophilic infiltrate present within OB mice was not present in the TLR4 KO OB animals (Figure 3B). Furthermore, mRNA expression of TNF-α, ICAM-1, and TGF-β was lower in TLR4 KO OB livers compared to OB (Figure 4). There was also no elevation in the expression if IL-1β, IL-6, and IL-10 in TLR4 KO OB samples. Expression levels of adiponectin, PPAR-γ, and IFN-γ were not significantly affected by a lack of TLR4.
DiscussionModels of NASH utilize dietary modifications (e.g. high fructose, fat or cholesterol, or deficiencies of essential nutrients such as choline) or genetic manipulations (e.g. Apolipoprotein E knockout). While the leptin deficient OB mouse is one of the oldest models of obesity, it has not been considered to develop hepatic inflammation in conjunction with steatosis without dietary manipulation or exposure to toxins. We found that at an advanced age of 20 weeks, there was significant inflammatory activity within the livers of OB mice fed a normal diet. This activity resembled that observed in dietary models of NASH at much earlier time points, including hepatocellular ballooning, neutrophil infiltration, expression of inflammatory mediators such as ICAM-1 and TNF-α, and hepatocellular injury as judged by AST and ALT. Together, these data indicate that:
- •
Leptin is not necessary for the development of inflammation in the steatotic liver,
- •
Hyperphagia and subsequent steatosis alone is sufficient for the development of hepatic inflammation and a NASH-like phenotype.
Increased numbers of F4/80+ cells within the liver have been observed in a number of models of NASH, and are thought to represent a population of pro-inflammatory cells unique from Kupffer cells (KC).21 While we did not observe this phenomenon in leptin deficient mice, we did find increased numbers of neutrophils as previously shown in clinical samples and a high dietary saturated fat model (Sutter, et al., Unpublished Data). These data suggest distinct, yet overlapping mechanisms for the development of steatohepatitis in the settings of leptin deficiency and dietary modification. It is possible that in dietary models, leptin resistance, rather than hyperleptinemia itself, may underlie the progression of NAFLD to NASH. This hypothesis is supported by recent evidence that leptin protects the liver from both alcohol and CCl4 mediated inflammation and injury.22,23
Numerous studies have suggested that leptin augments hepatic stellate cells (HSC) activation and the fibrogenic response.24,25 Indeed, we did not observe development of bridging fibrosis or even the peri-sinusoidal ‘chickenwire’ fibrotic changes characteristic of early stage NASH in our animals. Thus, while leptin is dispensable to inflammation and hepatocellular injury, it may be required for disease progression to cirrhosis. Interestingly, we observed increased TGF-β expression in OB animals, despite the absence of an overt fibrogenic response. This contrasts with other studies utilizing models of liver injury such as CCl4 in leptin deficient animals, which found decreased TGF-β expression in the absence of leptin.26
The effects of TLR4 KO on obesity have been assessed in diet induced obesity models with varying results. Reports range from increased, to unchanged, and even decreased obesity in the setting of TLR4 KO.27–29 We found that TLR4 KO OB animals become severely obese, but with body mass paralleling that of OB mice wild-type for the Tlr4lps-del allele at all time points, indicating that TLR4 KO does not affect the development of obesity in the OB model at the ages used in our experiments.
Likewise, reports differ as to the effect of TLR4 KO on the development of hepatic steatosis in varying models, but the majority of data suggests that functional TLR4 promotes the development of steatosis. We found no difference in the extent of hepatic steatosis between TLR4 KO OB and wild type OB animals, indicating that either steatosis secondary to hyperphagia alone is unaffected by the activity of TLR4, or that the activity of leptin is integral to the effects of TLR4 on the development of steatosis.
However, TLR4 KO OB animals did not display evidence of hepatitis as found in wild type OB animals. These data indicate that TLR4 is integral to the development steatohepatitis secondary to hyperphagia.
Abbreviations- •
ALT: Alanine transaminase.
- •
AST: serum aspartate aminotransferase.
- •
FFA: free fatty acids.
- •
FFPE: formalin-fixed, paraffin embedded.
- •
KO: knock out.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
NASH: non-alcoholic steatohepatitis.
- •
OB: aleptinemic.
- •
TLR4: Toll like receptor 4.
- •
TNF-α: tumor necrosis factor alpha.
This study was supported by NIH-R01 DK069369, Protection of Steatotic Livers from Primary Nonfunction. Awarded to Chavin KD.