Evaluation of a liver nodule detected with ultrasound includes the recovery of a detailed medical history, a physical exam, appropriate contrast imaging examinations and, in selected cases, histopathology. In this setting, identification of liver disease accompanying a liver nodule helps distinction between benign nodules and metastatic malignant nodules from primary liver cancer, as recommended by scientific liver societies. Diagnostic algorithms for a liver nodule in patients with liver disease involve contrast CT scan, magnetic resonance imaging or contrast enhanced ultrasounds to show the typical neoplastic pattern of early arterial hyperenhancement wash-in followed by hypoenhancement in the late portal phase wash out. The flow charts developed by western societies utilize the discriminant criterion of tumor size i.e. the radiological diagnosis being endorsed in a nodule equal or greater than 1 cm whereas eastern societies rely on the recognition of a typical vascular pattern of the node, independently of size. Differential diagnosis should be obtained to differentiate liver related nodules like regenerative macronodules (more than 20% of the cases) and the less frequent intrahepatic cholangiocarcinoma (~2% of the cases) from liver disease unrelated nodules like hemangioma (~4%), neuroendocrine metastatic nodules and focal nodular hyperplasia. In patients without liver disease, the most common liver nodules in the liver are hemangioma (~1.5%), focal nodular hyperplasia (0.03%) and hepatocellular adenoma (up to 0.004% in long term users of oral contraceptives). Optimization of management of patients with a liver nodule requires establishment of a multidisciplinary clinic.
The liver is the site of both benign and primary and secondary malignant nodules. Following the widely application of non invasive, user friendly imaging techniques to investigate the liver, the number of patients harboring a small nodule in the liver has steadily being increasing. In a ultrasound (US) study involving 30.9 million imaging examinations (25.8 million person per year), from 1996 to 2010 the number of US abdominal examinations per 1,000 enrollees climbed from 134 to 230 whereas during the same period of time, computed tomography (CT) examinations increased from 52 to 149 and magnetic resonance (MRI) use from 17 to 65.1 As a consequence, the increase in imaging use during this period of time led to increased detection of both benign and malignant liver nodules, with important clinical benefits. This was even more so in the hepatocellular carcinoma (HCC) scenario where the widespread application of imaging techniques translated in both earlier presentation and application of curative treatments, and convincing evidence of a decreased mortality in patients with an early detected cancer.2 In a retrospective study of SEER 18 registry (covering 28% of USS activity) the incidence trends of HCC between 2000 and 2010 showed a significant increase of tumor ≤ 5 cm in size surpassing diagnosis of larger tumors.3 In parallel, both western and eastern liver societies released recommendations to optimize management of patients with a HCC with a focus on non invasive radiological criteria of diagnosis.2,4–6
Critical Components of Evaluating a Liver NodulePatients in whom a liver nodule has been detected by abdominal US, either within and outside a screening program, need to be carefully assessed with respect to their medical history, physical examination, radiological examinations and, whenever required, pathological assessment.6 Given that HCC is the commoner primary liver cancer, patients with a liver nodule should be investigated for the presence of chronic liver diseases that are associated with HCC. The American (AASLD), European (EASL) and Asian Pacific (APASL) liver societies have identified cirrhosis, chronic hepatitis B, chronic hepatitis C and non alcoholic fatty liver disease (NAFLD) as the patient populations who need to be targeted by periodic surveillance with abdominal US since they are at increased risk of developing HCC.2,4,5 For the sake of cost effectiveness, other patient populations have been identified to be worth of screening, namely chronic hepatitis B patients with active hepatitis, those with a family history of HCC, Asian males more than 40 years and females more than 50 years, and African American blacks more than 20 years. While EASL identified patients with bridging fibrosis due to hepatitis C (Metavir F3) as a HCC risk population in need of screening, not included in the recommendations of international societies are other patient populations worth of screening, like chronic hepatitis B patients with high propensity scores in the REACH B, GAG/HCC and Chinese University models, and hepatitis C patients with high scores in the model constructed by the NIH multicenter investigational group HALT-C. Finally, most cases of HCC associated to NAFLD developed in the context of advanced fibrosis and cirrhosis only, and the same holds true for liver cancer developing in patients with primary biliary cirrhosis.7
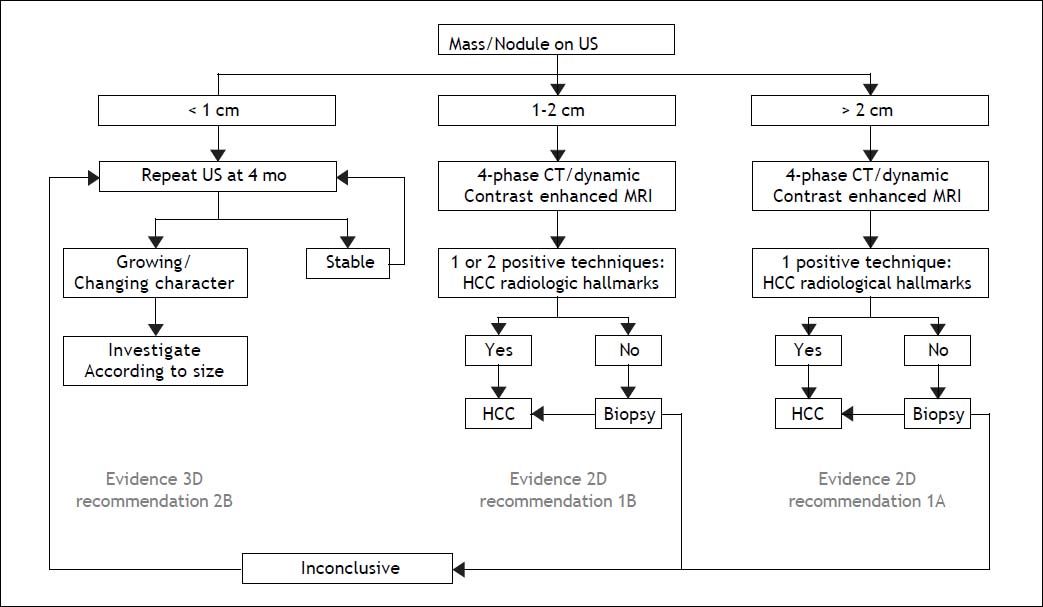
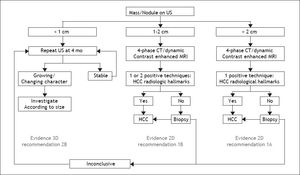
The Diagnosis of a Liver Nodule During ScreeningWhereas physical examinations is meant to identify signs and symptoms of chronic liver disease, diagnosis of a liver nodule substantially relies on radiological investigations. Diagnosis of HCC is obtained by contrast CT and/or gadolinium MRI through the identification of the typical vascular pattern of arterial hyperenhancement during the early contrast phase (wash-in) followed by hypoenhancement during the late and very late phase of investigation (wash-out). In nodules ≤ 2 cm a diagnosis of HCC can confidently be achieved following the application of one single technique in a sequential study, a strategy that proved to be superior to the original algorithm requiring concurrent positivity with two contrast radiological examinations. The advantage of using a single contrast imaging over two contrast imaging techniques, was both in terms of sparing histopathological examinations and lower per patient diagnosed cost.8 Contrast-enhanced ultrasound examination (CE-US) has been dismissed for the diagnosis of HCC by both AASLD and EASL, due to its inaccuracy in the diagnosis of intrahepatic cholangiocarcinoma (ICC), whereas it has been retained by APASL. An additional reason for the Western societies to abandon CE-US are the high rates of false negative results in cirrhotic patients with a small HCC that may amount to 33% of nodules lacking arterial hyperenhancement at CEUS, mainly as a consequence of the presence of a well differentiated tumor.9 Among the nuances in the strategy of radiological diagnosis of HCC between APASL and Western societies, are the APASL recommendations based on the vascular pattern of the nodule independently of tumor size, whereas western societies created algorithms based on the initial size of the nodule (Figure 1). The use of alfafetoprotein to discriminate benign nodules from HCC has been dismissed as well, given the high rates of false positive and false negative results with this assay in patients with underling chronic hepatitis or cirrhosis.4
EASL-EORTC: diagnostic algorithm and recall policy.2 * One imaging technique only recommended in centres of excellence.
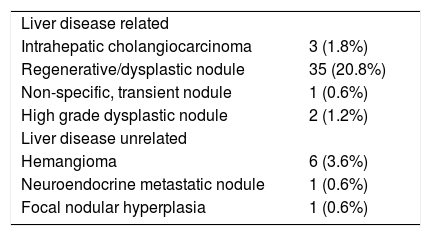
During screening approximately 1/3 of newly detected liver nodules are not HCC.9 A minority of them (< 5%) are not liver disease related, like hemangioma, neuroendocrine metastatic nodules and focal nodular hyperplasia. In these patients, the differential diagnosis with HCC can easily be obtained with radiological investigations. The vast majority of the non HCC nodules detected during screening are liver disease related, like regenerative macronodules, high degree dysplastic nodules, non specific, transient nodules and ICC (Table 1), where diagnosis can be obtained through histopathological examination with a fine needle biopsy (FNB). This may be challenging in cirrhotic patients with a high grade dysplastic nodule unless stromal invasion by tumor cells can histologically be identified.10 While this can easily be achieved in nodules examined in liver resected specimens, differential diagnosis may hardly be achieved in thin liver cores obtained by FNB. In the latter setting, differential diagnosis can be improved by immune stain with cancer specific proteins like GPC3 HSP70, GS, CHC, but the absolute specificity provided by the combined detection of at least two of these markers has been associated with a 60% sensitivity, only.11
The prevalence and diagnosis of non HCC nodules detected in cirrhotic patients under surveillance for HCC.9
| Liver disease related | |
| Intrahepatic cholangiocarcinoma | 3 (1.8%) |
| Regenerative/dysplastic nodule | 35 (20.8%) |
| Non-specific, transient nodule | 1 (0.6%) |
| High grade dysplastic nodule | 2 (1.2%) |
| Liver disease unrelated | |
| Hemangioma | 6 (3.6%) |
| Neuroendocrine metastatic nodule | 1 (0.6%) |
| Focal nodular hyperplasia | 1 (0.6%) |
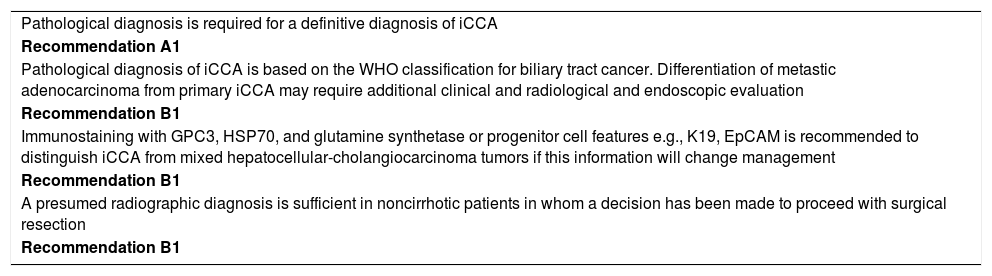
ICC is the second most common primary cancer of the liver, yet accounting for no more than 3-4% of all malignant primary hepatic tumors.2 The International of the Liver Cancer Association (ILCA) has recently released specific recommendations with respect to the management of ICC patients12 (Table 2). In patients with chronic liver disease, histopathological diagnosis is required for a definitive diagnosis of the tumor, using WHO classification for the cancer. In a set of patients, differentiation of metastatic ade-nocarcinoma from ICC may be difficult thereby requiring the application of additional radiological and endoscopic criteria. ICC needs also to be differentiated from the mixed forms of ICC/HCC tumors, whose natural history is closest to HCC than to ICC, particularly with respect to indications to liver transplantation.13 In patients without liver disease, a cholangiocarcinoma can safely be diagnosed with radiology.
ILCA Guidelines for the Diagnosis of Intrahepatic Cholangiocarcinoma.12
| Pathological diagnosis is required for a definitive diagnosis of iCCA |
| Recommendation A1 |
| Pathological diagnosis of iCCA is based on the WHO classification for biliary tract cancer. Differentiation of metastic adenocarcinoma from primary iCCA may require additional clinical and radiological and endoscopic evaluation |
| Recommendation B1 |
| Immunostaining with GPC3, HSP70, and glutamine synthetase or progenitor cell features e.g., K19, EpCAM is recommended to distinguish iCCA from mixed hepatocellular-cholangiocarcinoma tumors if this information will change management |
| Recommendation B1 |
| A presumed radiographic diagnosis is sufficient in noncirrhotic patients in whom a decision has been made to proceed with surgical resection |
| Recommendation B1 |
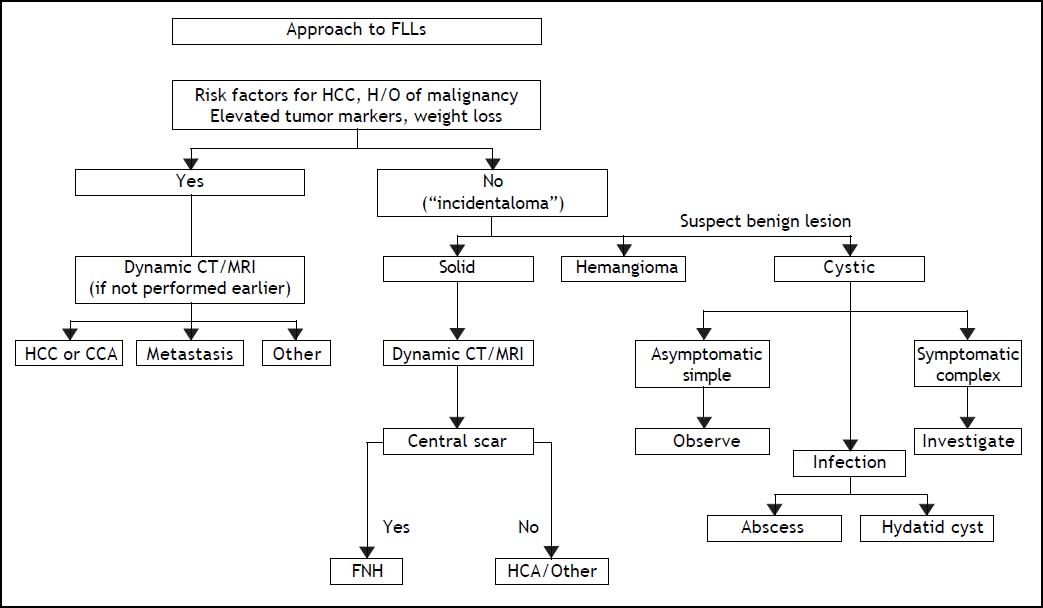
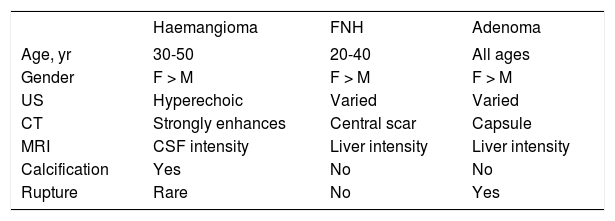
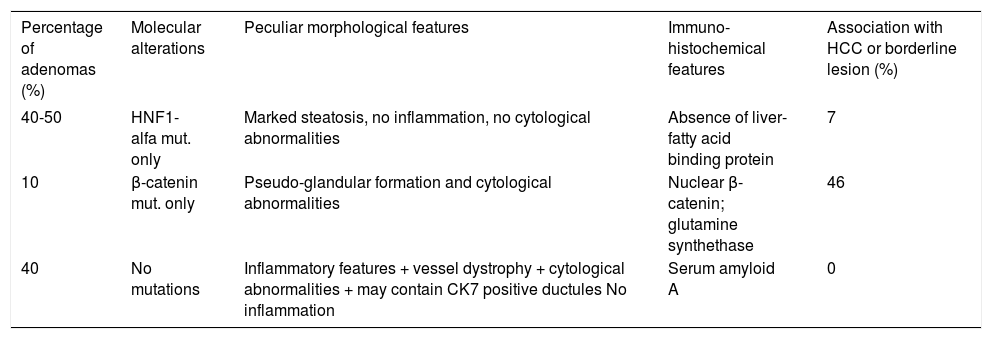
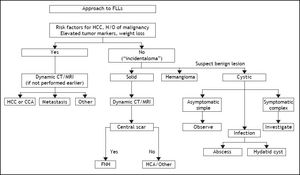
Benign and malignant tumors in the liver can be identified though radiological and /or histological investigations (Table 3). Liver nodules most commonly occurring in normal liver include hemangioma, focal nodule hyperplasia (FNH) and hepatocellular adenoma (HA). Hemangiomas have been found at a rate spanning from 0.4 to 21% in autopsy series and from 0.7 to 1.5% in clinical series.6 FNH is the second most common nodule in the liver with a prevalence rate between 0.3 and 3% in autopsy series and of 0.03% in clinical series.6 HA is a very rare tumor in males, whereas it can more frequently occur in females at a rate between 0.001 and 0.004%, with preference for long term users of oral contraceptives. Distinction between these nodules bears important clinical implications, given the tendency of HA to spontaneously bleed and, less frequently, to evolve into HCC. Differential diagnosis is made by clinical and radiological investigations whereas US guided FNB of the nodule should be avoided due to the bleeding risks of all these tumors (Table 4). In most patients, hemangioma and FNH can easily be identified at radiology as the former nodules may present with centripetal enhancement of the contrast whereas the latter nodules show the classical central scar at contrast radiology. In most patients, a differential diagnosis of FNH and HA from other nodules is eased following withdrawal from oral contraceptives and prospective monitoring with contrast imaging techniques. Patients not responding to these maneuvers can have a definitive diagnosis provided by nodule resection, an intervention which is deemed necessary since a HA exceeding 3-5cm in size is considered at risk of spontaneous rupture, bleeding and neoplastic transformation. In a systematic review of 157 studies published from 1970 to 2009, 4.2% of HA underwent HCC transformation, 4.5% of 1,462 resected nodules harbored a focal malignancy and 32% of the patients bled.14 HA evolving to HCC may be difficult to distinguish from a pre-existing, low grade HCC. In a landmark study investigating the genotype/phenotype pattern of HA, an association with a HCC or borderline neoplastic lesions were found in 45% of HAs with betacatenin mutations and in a minority (7-13%) of HAs characterized by either no molecular alterations or HFN1 alfa mutations only (Table 5).15 The recommendations released by the American College of Gastroenterology strongly advise histological diagnosis of benign liver nodules in patients with inconclusive imaging studies for whom diagnosis is required to make treatment decisions. MRI and contrast CT scan are the techniques of choice for the diagnosis of hemangioma, FNH and HA (Figure 2) whereas the use of serum markers of gastrointestinal neoplasia like alfafetoprotein, carcinoembrionic antigen and gastrointestinal cancer antigen, may result in rates of false positive results.
Classification of hepatic tumours.
| Origin | Benign | Malignant |
|---|---|---|
| Hepatocellular | Adenoma Focal nodular hyperplasia Regenerating nodules Nodular reg. hyperplasia | Hepatocellular carcinoma Fibrolamellar carcinoma Hepatoblastoma |
| Cholangiocellular | Bile duct adenoma Biliary cystadenoma | Cholangiocarcinoma Cystadenocarcinoma |
| Mesenchymal | Haemangioma Angiolipoma | Angiosarcoma Primary lymphoma |
| Heterotopic | Adrenal / pancreatic | Metastases |
Demographic and radiological characteristics of common benign liver nodules.
| Haemangioma | FNH | Adenoma | |
|---|---|---|---|
| Age, yr | 30-50 | 20-40 | All ages |
| Gender | F > M | F > M | F > M |
| US | Hyperechoic | Varied | Varied |
| CT | Strongly enhances | Central scar | Capsule |
| MRI | CSF intensity | Liver intensity | Liver intensity |
| Calcification | Yes | No | No |
| Rupture | Rare | No | Yes |
Genotype-phenotype correlations in hepatocellular adenomas.15
| Percentage of adenomas (%) | Molecular alterations | Peculiar morphological features | Immuno-histochemical features | Association with HCC or borderline lesion (%) |
|---|---|---|---|---|
| 40-50 | HNF1-alfa mut. only | Marked steatosis, no inflammation, no cytological abnormalities | Absence of liver-fatty acid binding protein | 7 |
| 10 | β-catenin mut. only | Pseudo-glandular formation and cytological abnormalities | Nuclear β-catenin; glutamine synthethase | 46 |
| 40 | No mutations | Inflammatory features + vessel dystrophy + cytological abnormalities + may contain CK7 positive ductules No inflammation | Serum amyloid A | 0 |
ACG Clinical Guideline: the diagnosis and management of focal liver lesions (FLLs).6
The widespread use of abdominal imaging techniques has led to increased recognition of liver nodules in asymptomatic individuals, thereby increasing the need for a standardized diagnostic approach. The recommendations released by liver societies and the American College of Gastroenterology for the diagnosis of malignant and benign liver nodules made management of patients with a liver nodule standardized, safe and cost effective. Further optimization of the care of patients with a liver nodule, however, requires the establishment of a multidisciplinary clinic. This has been clearly demonstrated to be the case when the outcome of patients with a HCC who were treated by a single expert was compared with the outcome of similar patients who in the same health care setting, were managed by a multidisciplinary team. In a study in the USA, the number of patients who received a treatment and their survival were significantly improved after establishment of multidisciplinary clinic compared to the time period when patients were managed by an expert alone (56 vs. 44% and 15.2 vs. 4.7 months). At the same time, the time to treatment was shorter in the former than in the latter patients (2.2 vs. 47 months).16