Endometriosis is characterized by the presence of endometrial-like tissue and stroma in extra-uterine locations. Hepatic endometriosis (HE) is one of the rarest forms of extrapelvic endometriosis. We aimed to summarize the existing evidence on HE with special consideration to natural history, diagnosis and surgical treatment.
Three electronic databases were systematically searched for articles published up to March 2019. All appropriate observational studies and case reports addressing cases of women with HE were considered eligible for inclusion.
A total of 27 studies which comprised 32 patients with HE were included. Mean age of patients was 39.7 years. Ten (62.5%) were nulliparous and 24 (75%) were women of reproductive age. Eleven patients (36.7%) had a history of pelvic endometriosis of various sites. Abdominal pain was the primary symptom in 28 patients (87.5%). Preoperative diagnosis of endometriosis was available for 5 patients and 6 underwent a preoperative diagnostic procedure. Cyst resection, minor and major liver resections were performed in 14/31, 9/31 and 8/31 patients, respectively.
Preoperative diagnosis of HE is challenging due to variable radiologic features and clinical symptomatology. Nonetheless, it should be considered in the differential diagnosis of a liver mass especially in premenopausal women with a history of endometriosis. The type of resection of the endometriotic lesion is based on the extent and the location of the disease and presented with favourable outcomes concerning morbidity, symptom relief and recurrence.
Endometriosis is a chronic estrogen dependent disorder characterized by the presence of endometrial-like tissue and stroma in extra-uterine locations. It most frequently is encountered in women of reproductive age (6–10%) while approximately 2.5% of postmenopausal women are diagnosed with the disease [1,2]. The clinical symptoms that have been associated with endometriosis include chronic pelvic pain (71–87%), infertility (21–47%), dyspareunia, dysmenorrhea and bowel symptoms (constipation, dyschezia and diarrhea) [3]. However, no symptoms are reported in a considerable proportion of patients. Despite being considered as a benign entity, symptomatic endometriosis has been associated with significant morbidity and poor quality of life.
The most common locations of endometriosis include the ovaries, the uterine ligaments, the fallopian tubes and the pelvic peritoneum [4]. Extrapelvic endometrial tissue deposits have been described in many areas of female human body, whereas structures that are close to the uterus, such as the small and large bowel, female genitourinary system, and thorax, are more frequently affected than other distant locations [5]. Other rare locations of extrapelvic endometriosis include operative skin scars, the kidneys and the central nervous system [6–8]. Hepatic endometriosis (HE) is one of the rarest forms of extrapelvic endometriosis and was first reported by Finkel et al. in 1986 [9]. Several case reports of HE have been published thereafter describing surgical treatment and clinicopathological characteristics of these lesions. Atypical clinical and radiological findings make diagnosis of HE challenging whilst confirmation of diagnosis can only be obtained by preoperative biopsy or via surgical resection.
The objective of the present systematic review was to summarize the existing evidence on HE with special consideration to the natural history, the diagnosis and the surgical treatment of the disease.
2Materials and methods2.1Study designAll appropriate observational studies and case reports addressing cases of women who were diagnosed with hepatic endometrial cyst (pre- or intraoperatively) were considered eligible for inclusion in the present systematic review. Reviews and animal studies were excluded from analysis and tabulation. Only studies in English language were included. Patients who received conservative treatment for HE other than surgery were excluded. APas and APr independently and meticulously searched the literature, excluded overlaps, and tabulated the selected indices in structured forms.
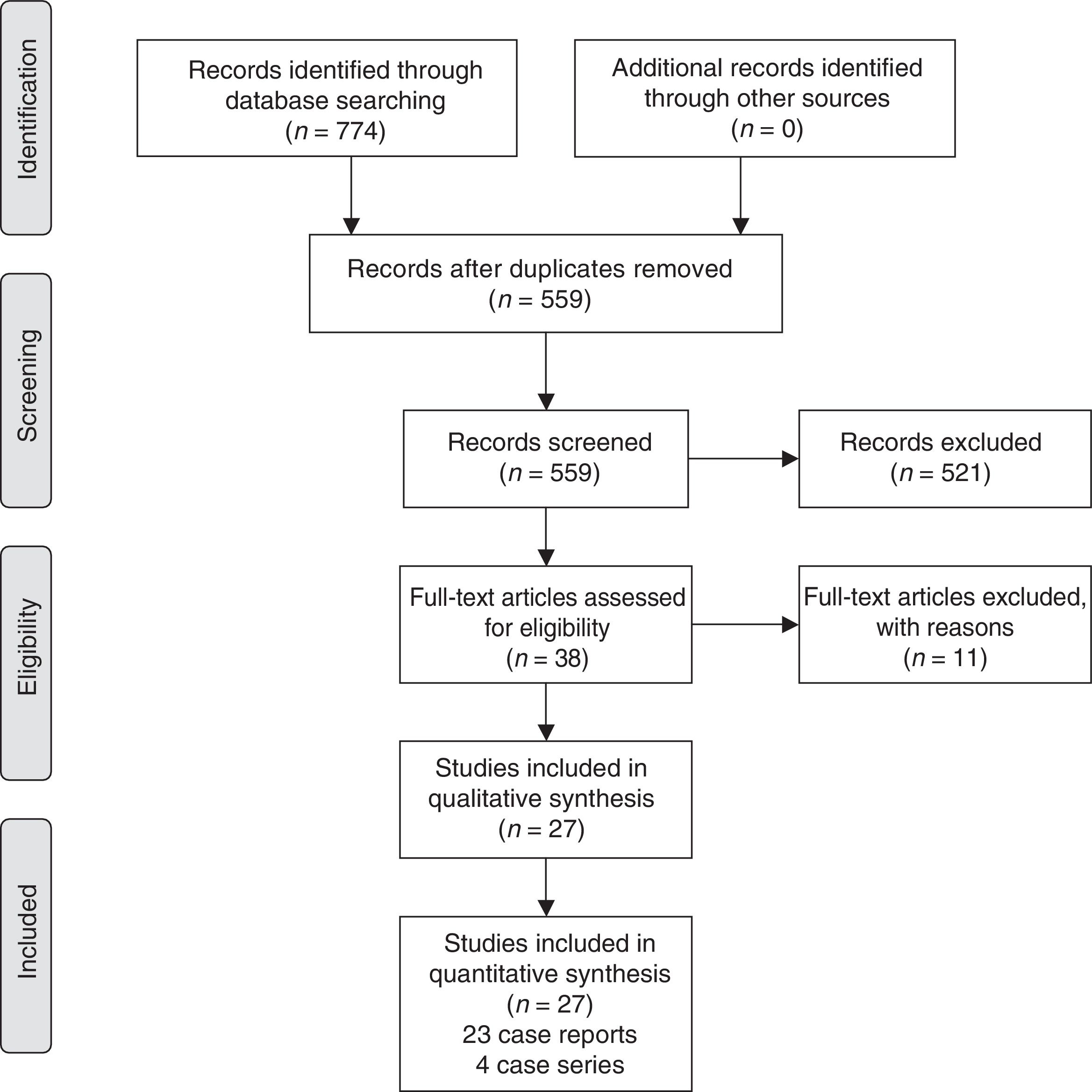
2.2Search strategy and data collectionWe systematically searched the literature for articles published up to March 2019 using PubMed (1966–2019), Scopus (2004–2019), and Google Scholar (2004–2019) databases along with the references of the articles which were retrieved in full text. The following key words were used for the search: “endometriosis”, “liver endometriosis”, “hepatic endometriosis”, “endometrial liver cyst”, “endometrial hepatic cyst”, “extrapelvic endometriosis”, “distant endometriosis”. A minimum number of search keywords were utilized in an attempt to assess an eligible number that could be easily searched while simultaneously minimizing the potential loss of articles. Articles that fulfilled or were deemed to fulfill the inclusion criteria were retrieved; all articles which described cases of women aged >18 years who were surgically managed for liver lesions diagnosed pre- or postoperatively as endometriosis were included. Studies including cases of women who did not underwent surgery for the treatment of the hepatic lesion were excluded. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram schematically presents the stages of article selection (Fig. 1).
Our search strategy included the MeSH terms:
- •
(“liver”[MeSH Terms] OR “liver”[All Fields]) AND (“endometriosis”[MeSH Terms] OR “endometriosis”[All Fields])
- •
hepatic[All Fields] AND (“endometriosis”[MeSH Terms] OR “endometriosis”[All Fields])
- •
endometrial[All Fields] AND (“liver”[MeSH Terms] OR “liver”[All Fields]) AND (“cysts”[MeSH Terms] OR “cysts”[All Fields] OR “cyst”[All Fields])
- •
endometrial[All Fields] AND hepatic[All Fields] AND (“cysts”[MeSH Terms] OR “cysts”[All Fields] OR “cyst”[All Fields])
- •
extrapelvic[All Fields] AND (“endometriosis”[MeSH Terms] OR “endometriosis”[All Fields])
Data on patient characteristics included age, parity, menopausal status, abnormal liver function parameters, prior endometriosis and surgical history, primary signs and symptoms and outcomes of clinical examination and imaging of the recruited patients. Concerning the main findings of the study, indication for surgery and type of surgery were appraised. Moreover, intraoperative and postoperative outcomes were as well evaluated and encompassed length of hospital stay, complications and histopathological outcomes of the excised specimens, if available.
2.3DefinitionsMinor liver resection is defined as resection of less than 3 liver segments while major liver resection is defined as resections of at least 3 liver segments [10]. Cyst resection is defined as the non-anatomic resection of liver parenchyma surrounding the endometriotic cystic lesion.
2.4Quality assessmentCase reports and case series are related to elevated bias due to the nature of those types of studies [11]. Nonetheless, in case when data on a certain condition is limited evidence from those studies is considered of clinical importance. We evaluated the quality of the enrolled studies by adopting a quality assessment tool for case reports and case series proposed by Murad et al. More specifically, the methodological quality of the studies was assessed based on the criteria including the domains of ascertainment, causality, selection and reporting. The sum of the scores derived from eight critical questions referred to the domains was used to evaluate the quality of each study as well as the reviewer's judgement on the presence of the most important domains according to certain clinical case.
3Results3.1Excluded studiesA total of 11 were excluded from the present review. Among them, 4 were excluded due to insufficient data [12–15]. Additionally, in 4 cases the patients received conservative HE treatment with pharmaceutical and thus they were not included [16–19]. The study by Finkel et al. was excluded as it was a part of an already included study [9]. Two studies described cases of HE with concomitant presence of adenosarcoma in the lesion [20,21].
3.2Main characteristics of included studiesA total of 27 studies (23 case reports and 4 case series) which comprised 32 patients who were diagnosed with a hepatic endometriotic lesion were included in the present systematic review [22–48].
Based on the type of the included clinical cases we considered the score of 5 points as the highest that could be assessed when excluding the three questions from the quality assessment tool that attributed to cases of adverse drug events. The scores of each study are presented in Table 1. A mean score of 3.7 (SD±0.98) was calculated whereas the overall judgement on the quality of the recruited studies was that they were of moderate quality.
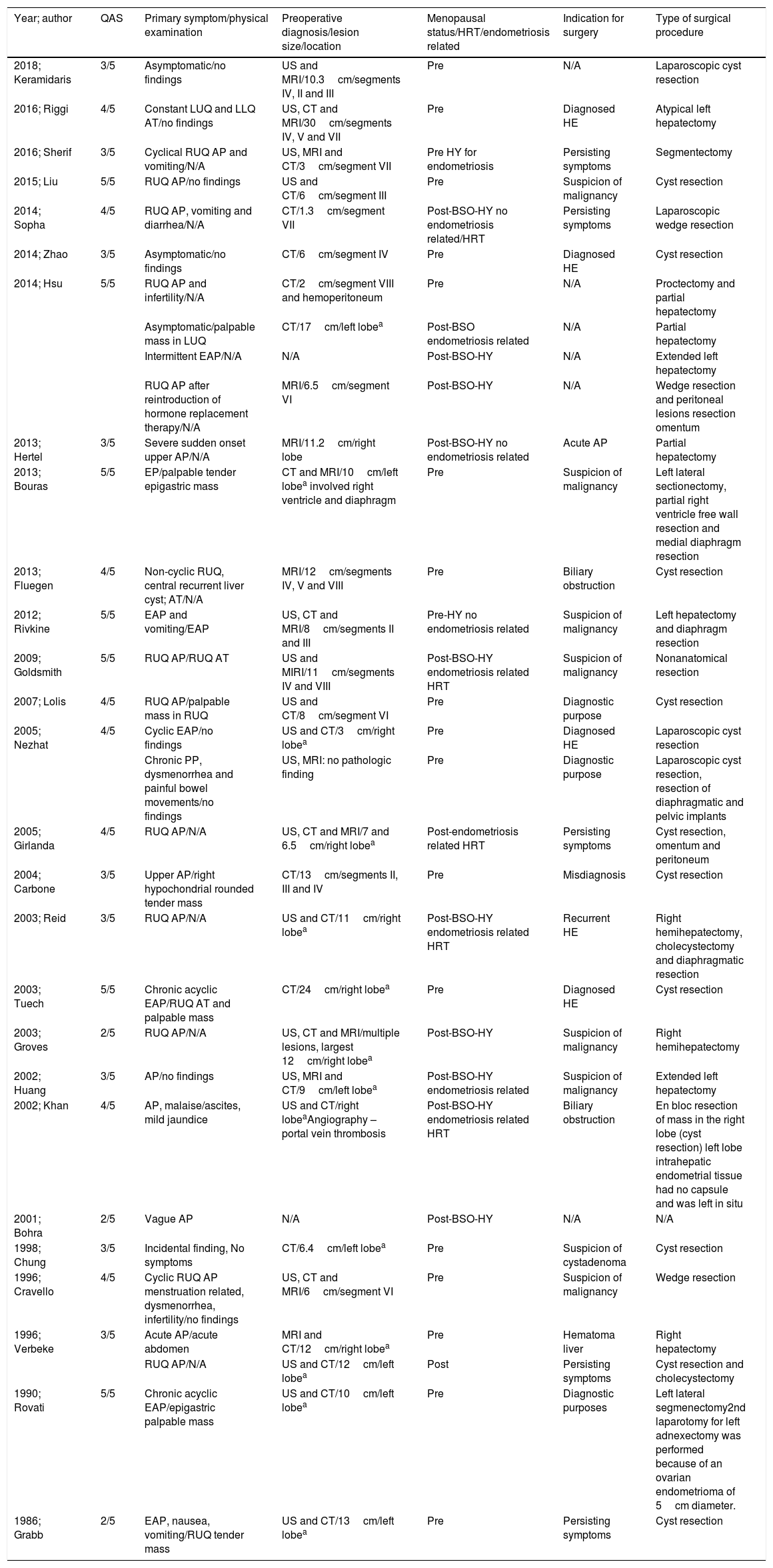
Main characteristics of the included patients.
| Year; author | QAS | Primary symptom/physical examination | Preoperative diagnosis/lesion size/location | Menopausal status/HRT/endometriosis related | Indication for surgery | Type of surgical procedure |
|---|---|---|---|---|---|---|
| 2018; Keramidaris | 3/5 | Asymptomatic/no findings | US and MRI/10.3cm/segments IV, II and III | Pre | N/A | Laparoscopic cyst resection |
| 2016; Riggi | 4/5 | Constant LUQ and LLQ AT/no findings | US, CT and MRI/30cm/segments IV, V and VII | Pre | Diagnosed HE | Atypical left hepatectomy |
| 2016; Sherif | 3/5 | Cyclical RUQ AP and vomiting/N/A | US, MRI and CT/3cm/segment VII | Pre HY for endometriosis | Persisting symptoms | Segmentectomy |
| 2015; Liu | 5/5 | RUQ AP/no findings | US and CT/6cm/segment III | Pre | Suspicion of malignancy | Cyst resection |
| 2014; Sopha | 4/5 | RUQ AP, vomiting and diarrhea/N/A | CT/1.3cm/segment VII | Post-BSO-HY no endometriosis related/HRT | Persisting symptoms | Laparoscopic wedge resection |
| 2014; Zhao | 3/5 | Asymptomatic/no findings | CT/6cm/segment IV | Pre | Diagnosed HE | Cyst resection |
| 2014; Hsu | 5/5 | RUQ AP and infertility/N/A | CT/2cm/segment VIII and hemoperitoneum | Pre | N/A | Proctectomy and partial hepatectomy |
| Asymptomatic/palpable mass in LUQ | CT/17cm/left lobea | Post-BSO endometriosis related | N/A | Partial hepatectomy | ||
| Intermittent EAP/N/A | N/A | Post-BSO-HY | N/A | Extended left hepatectomy | ||
| RUQ AP after reintroduction of hormone replacement therapy/N/A | MRI/6.5cm/segment VI | Post-BSO-HY | N/A | Wedge resection and peritoneal lesions resection omentum | ||
| 2013; Hertel | 3/5 | Severe sudden onset upper AP/N/A | MRI/11.2cm/right lobe | Post-BSO-HY no endometriosis related | Acute AP | Partial hepatectomy |
| 2013; Bouras | 5/5 | EP/palpable tender epigastric mass | CT and MRI/10cm/left lobea involved right ventricle and diaphragm | Pre | Suspicion of malignancy | Left lateral sectionectomy, partial right ventricle free wall resection and medial diaphragm resection |
| 2013; Fluegen | 4/5 | Non-cyclic RUQ, central recurrent liver cyst; AT/N/A | MRI/12cm/segments IV, V and VIII | Pre | Biliary obstruction | Cyst resection |
| 2012; Rivkine | 5/5 | EAP and vomiting/EAP | US, CT and MRI/8cm/segments II and III | Pre-HY no endometriosis related | Suspicion of malignancy | Left hepatectomy and diaphragm resection |
| 2009; Goldsmith | 5/5 | RUQ AP/RUQ AT | US and MIRI/11cm/segments IV and VIII | Post-BSO-HY endometriosis related HRT | Suspicion of malignancy | Nonanatomical resection |
| 2007; Lolis | 4/5 | RUQ AP/palpable mass in RUQ | US and CT/8cm/segment VI | Pre | Diagnostic purpose | Cyst resection |
| 2005; Nezhat | 4/5 | Cyclic EAP/no findings | US and CT/3cm/right lobea | Pre | Diagnosed HE | Laparoscopic cyst resection |
| Chronic PP, dysmenorrhea and painful bowel movements/no findings | US, MRI: no pathologic finding | Pre | Diagnostic purpose | Laparoscopic cyst resection, resection of diaphragmatic and pelvic implants | ||
| 2005; Girlanda | 4/5 | RUQ AP/N/A | US, CT and MRI/7 and 6.5cm/right lobea | Post-endometriosis related HRT | Persisting symptoms | Cyst resection, omentum and peritoneum |
| 2004; Carbone | 3/5 | Upper AP/right hypochondrial rounded tender mass | CT/13cm/segments II, III and IV | Pre | Misdiagnosis | Cyst resection |
| 2003; Reid | 3/5 | RUQ AP/N/A | US and CT/11cm/right lobea | Post-BSO-HY endometriosis related HRT | Recurrent HE | Right hemihepatectomy, cholecystectomy and diaphragmatic resection |
| 2003; Tuech | 5/5 | Chronic acyclic EAP/RUQ AT and palpable mass | CT/24cm/right lobea | Pre | Diagnosed HE | Cyst resection |
| 2003; Groves | 2/5 | RUQ AP/N/A | US, CT and MRI/multiple lesions, largest 12cm/right lobea | Post-BSO-HY | Suspicion of malignancy | Right hemihepatectomy |
| 2002; Huang | 3/5 | AP/no findings | US, MRI and CT/9cm/left lobea | Post-BSO-HY endometriosis related | Suspicion of malignancy | Extended left hepatectomy |
| 2002; Khan | 4/5 | AP, malaise/ascites, mild jaundice | US and CT/right lobeaAngiography – portal vein thrombosis | Post-BSO-HY endometriosis related HRT | Biliary obstruction | En bloc resection of mass in the right lobe (cyst resection) left lobe intrahepatic endometrial tissue had no capsule and was left in situ |
| 2001; Bohra | 2/5 | Vague AP | N/A | Post-BSO-HY | N/A | N/A |
| 1998; Chung | 3/5 | Incidental finding, No symptoms | CT/6.4cm/left lobea | Pre | Suspicion of cystadenoma | Cyst resection |
| 1996; Cravello | 4/5 | Cyclic RUQ AP menstruation related, dysmenorrhea, infertility/no findings | US, CT and MRI/6cm/segment VI | Pre | Suspicion of malignancy | Wedge resection |
| 1996; Verbeke | 3/5 | Acute AP/acute abdomen | MRI and CT/12cm/right lobea | Pre | Hematoma liver | Right hepatectomy |
| RUQ AP/N/A | US and CT/12cm/left lobea | Post | Persisting symptoms | Cyst resection and cholecystectomy | ||
| 1990; Rovati | 5/5 | Chronic acyclic EAP/epigastric palpable mass | US and CT/10cm/left lobea | Pre | Diagnostic purposes | Left lateral segmenectomy2nd laparotomy for left adnexectomy was performed because of an ovarian endometrioma of 5cm diameter. |
| 1986; Grabb | 2/5 | EAP, nausea, vomiting/RUQ tender mass | US and CT/13cm/left lobea | Pre | Persisting symptoms | Cyst resection |
QAS: quality assessment score, AP: abdominal pain, AT: abdominal tenderness, PP: pelvic pain, EAP: epigastric abdominal pain, RUQ: right upper quadrant, LUQ: left upper quadrant, LLQ: left low quadrant, BSO-HY: bilateral salpingo-oophorectomy plus hysterectomy, HE: hepatic endometriosis, IO: intraoperative, HY: hysterectomy.
Table 1 depicts the main characteristics of the included patients. The mean age of the included patients was 39.7 years (SD±8.95 years) while parity status was available for 16 (50%) patients. Among them, 10 (62.5%) were nulliparous whereas the remaining 6 (37.5%) have given birth to one or more children. Despite the fact that 24 (75%) women were of reproductive age (18–45 years), the proportion of premenopausal women was 19 (59.4%) while the remaining 13 (40.6%) were postmenopausal. A total of 12 (37.5%) women with age range from 31 to 56 had undergone bilateral salpingoophorectomy with hysterectomy (BSO-HY) or without and were considered surgical menopausal. Among them, 6 of them had undergone the aforementioned procedures due to endometriosis. Hormone replacement therapy (HRT) was administered in 4 of them and in one who underwent no endometriosis related BSO-HY. Eleven out of 30 patients (36.7%) had a history of pelvic endometriosis of various sites such as ovarian, fallopian, cervical, peritoneal, pouch of Douglas or colonic endometriosis. Sixteen out of 32 patients (50%) had a history of prior gynecologic or obstetric surgery for various indications which included cesarean section, endometriosis related hysterectomy, oophorectomy for teratoma, hysterectomy for uterine leiomyoma, endometrioma and pelvic endometriosis excision.
3.3Disease-related characteristicsAbdominal pain (AP) was the primary symptom in 28 patients (87.5%) whereas 4 (12.1%) were asymptomatic and were incidentally diagnosed with a liver mass. Among patients with AP, 13 (44.8%) women described right upper guardant (RUQ) AP, 7 (21.9%) complained for epigastric pain (EP), one (3.4%) had left AP whereas had diffuse AP. Abdominal pain was cyclic-menstruation related in 3 cases. Nausea and vomiting was described by 4 (12.5%) women. Clinical examination revealed a palpable mass in 7 women. In one patient ascites and mild jaundice were detected [42]. Imaging outcomes were reported for all except two women, included Ultrasound (US), Computed Tomography (CT), Magnetic resonance imaging (MRI) or a combination of them and are presented in Table 1. Abnormal liver function was present in 3 out of 24 (12.5%) patients. Preoperative diagnosis of endometriosis through either biopsy, imaging or patients’ history was available for 5 patients. Six patients underwent a preoperative diagnostic procedure (biopsy or fine needle aspiration – FNA). In 2 of them a preoperative percutaneous biopsy of the mass revealed endometrial tissue [24,42]. In the remaining cases, atypical findings such as necrotic and inflammatory tissue, hemangioma and epithelial cells suspicious of malignancy were detected.
As shown in Table 1, indication for surgery was available for 26 cases. Among them, 5 cases underwent surgery due to endometriosis either recurrent (n=1) or primary (n=4) which was preoperatively diagnosed. In 11 cases a misdiagnosis led to surgery. More specifically, differential diagnosis in these patients included malignancy or other lesions such as liver cystadenoma or hematoma. Eight patients were led to surgery due to symptomatic disease; two for biliary obstruction and 6 due to abdominal pain and/or vomiting. Finally, diagnostic purpose was the main indication of surgery in 2 cases.
3.4Operative outcomesAll patients underwent surgery for the treatment of their liver mass. Data with regard to the type of surgery was available for 31 patients. Among them, 14 (45.2%) underwent cyst resection while the remaining 17 had more extended liver resections which included minor or major hepatectomies (9/31 and 8/31, respectively). Intraoperatively, gross examination of the affected liver revealed cystic lesions filled with fluid which ranged in color from clear to chocolate colored. Intraoperative diagnosis of endometriosis with frozen section histology was made for 5 cases. Furthermore, in 8 women, an additional procedure for the excision of lesions in the surrounding structures or in the pelvic was performed as shown in Table 1. Four (12.5%) patients underwent laparoscopic surgical procedures whereas the remaining 28 (87.5%) underwent open surgery. Histological examination confirmed the diagnosis of endometriosis and revealed the characteristic endometriotic features which included the presence of endometrial stroma and glands. In cases in which immunochemistry was performed ER and PR positivity was recognized as well as CK7 positive staining in the examined specimens. The length of hospital stay ranged from 1 to 13 days, based on the type and the approach of the procedures. No postoperative complications were reported except one case bile leak [42]. The same patient was readmitted 18 months postoperatively when two liver masses in the left lobe were found and aspired with complete remission of the symptoms and no complication reported for the following 4 years. Finally, one patient in the study by Hsu et al. showed pelvic endometriosis recurrence within 18 years postoperatively [28]. No postoperative deaths were reported from the included studies.
4DiscussionExtrapelvic endometriosis is defined as the presence of ectopic endometrial tissue in structures outside the pelvis such as the lungs, the brain, the urinary system and the gastrointestinal tract. Presence of endometriosis in extrapelvic sites is uncommon with a prevalence that is not easily estimated due to lack of population based epidemiological trials [6]. Additionally, the pathogenesis of extrapelvic endometriosis still remains ill-determined; a plethora of theories have been proposed concerning the pathogenetic pathway of extrapelvic endometriosis. Among them, metaplasia of the peritoneum due to chronic inflammation and implantation of endometrial tissue in extrapelvic sites through fallopian tubes during retrograde menstruation are the most prevalent [49]. Furthermore, a mechanism of lymphatic or haematogenous spread in distant structures similar to this of metastatic malignancies has also been advocated [6,50]. The present study indicates that approximately half of the included patients had a history of previous gynaecologic surgery and thus one can hypothesize that a previous gynaecologic surgery could play a role in dissemination of endometrial cells in the peritoneal cavity.
Preoperative diagnosis of HE can be challenging due to atypical clinical findings and radiological features; a limited number of the examined cases in the present study reported a clear preoperative endometriosis diagnosis even in cases in which a preoperative diagnostic procedure was performed. Additionally, the diagnosis was even more challenging to establish due to the lack of previous history of pelvic endometriosis in more than half of the included women or the manifestation of non-specific endometriosis-related symptoms in a significant proportion of the included patients. In that setting, despite the fact that almost in all the included cases the primary symptom was AP, association of pain with menses (cyclical onset of symptoms and pain) was only reported in 2 cases. To that end, the specific symptoms that can be attributed to HE still remain ill-determined. Additionally, despite the fact that almost all the included patients preoperatively underwent more than one diagnostic imaging procedure including US, CT and MRI along, which mainly described a hepatic cystic lesion without special characteristics with biopsy in some cases, preoperative recognition of the liver mass as endometriotic was only successful in only 5 patients (16%). The differential diagnosis in most cases included hematoma, haemangioma, abscess, metastasis or echinococcal cyst, even in cases of a past endometriosis history. Notably, 4 cases had preoperative radiological features which demonstrated a malignant condition [30,32,33,41].
On the other hand, malignant transformation of endometriosis is relatively rare and accounts for less than 1% of cases with the vast majority of them arising in the ovary [21]. Accordingly, with regard to malignancy arising from hepatic endometriosis data is extremely scarce. To that end, 2 cases of adenosarcoma arising from hepatic endometriosis have been identified in a 52- and 54-year-old women who both underwent resection with no evidence of recurrence during the follow-up period [20,21].
One could argue that endometriosis as a hormone-dependent disease mostly affects women of reproductive age [51]. The current literature estimates an incidence of endometriosis in menopause which ranges from 2% to 5% [52]. The exact pathogenetic pathway still remains elusive; one theory on that suggests that postmenopausal endometriosis is due to the presence and activation of endometrial implants especially in women who received exogenous hormone replacement therapy and have a previous history of endometriosis [52]. Additionally, some cases of de novo postmenopausal endometriosis in patients without history of endometriosis have been recorded but whether those cases are de novo or previous asymptomatic undiagnosed endometriosis is still under consideration [53]. According to the findings of the present study, a significant proportion of patients (approximately 40%) were postmenopausal. Four of them, were diagnosed with HE but reported no previous history of the disease. Menopause was surgically induced in 12 patients while 5 received hormone replacement therapy. This finding is in accordance with previous reports concerning the detection of extrapelvic endometriosis in older age compared to the pelvic form of the disease [6,54].
The vast majority of the included patients underwent open procedures, which ranged from simple cyst resection to major liver resection based on the location and size of the endometriotic lesions within the liver. However, there were also 4 patients who underwent cyst resection through a laparoscopic approach, indicating that it may be an efficient minimally invasive approach in selected cases. It should be also reminded that both minor and major laparoscopic liver resections for both benign and malignant hepatic lesions are currently acknowledged as standard procedures with improved short- and adequate long-term outcomes [55–57]. Regardless of the type of approach, cumulative morbidity and mortality rates were 3% and 0%, respectively, whereas recurrence was noted only in 1 patient. To that end, it is evident that liver resections for the treatment of HE are safe and efficient procedures when performed by surgeons with experience in the field.
The present review is to the best of our knowledge, the only review in literature, which presents a cumulative report of the natural history, characteristics and management of adult females with HE. A thorough search of the literature along with the fact that no date restrictions were imposed, eliminated the risk of potential loss of articles. The true prevalence of HE could not be precisely reached and data concerning its pathophysiology, clinical appearance and treatment is limited to case reports and small case series precluded further research due to its rare entity. Furthermore, the significant heterogeneity of the included studies along with the fact that some parameters were omitted by some studies was another limitation and precluded reaching to firm results.
In conclusion, preoperative diagnosis of HE can be challenging due to variable radiologic features and clinical symptomatology. Nonetheless, it should be considered in the differential diagnosis of a liver mass especially in premenopausal women with a history of endometriosis of other sites. The type of resection of the endometriotic lesion is based on the extent and the location of the disease and presented with favorable outcomes with regard to morbidity, mortality, symptom relief and recurrence.AbbreviationsHE
hepatic endometriosis
APabdominal pain
BSO-HYbilateral salpingoophorectomy with hysterectomy
HRThormone replacement therapy
RUQright upper guardant
EPepigastric pain
FundingNone.
Conflict of interestNone.