Aim: To make a preliminary determination of hepatitis C virus (HCV) genotype prevalence and possible genotype associations with risk factors and severity of associated liver disease in patients from Yucatan, Mexico. Methods: Sera from 54 patients with positive anti-HCV and HCV RNA were genotyped using reverse transcription-polymerase chain reaction. Risk factors were evaluated using a questionnaire. The evaluations also included serum ALT levels and liver biopsies in some participants. Results: HCV genotype 1 was detected in 37%, genotype 2 in 33.3%, genotype 3 in 16.7% and mixed genotypes in 7.4%. Subtype 2b was the most frequent (33.3%), followed by 1b (18.5%), 3a (16.7%) and 1a (14.8%). Surgeries (53.7%) and transfusion (38.9%) were the main risk factors. Liver biopsies were available in 24 (44.4%) patients. Severe liver disease was present in 6 (54.5%) of the patients with genotype 1 and in none of those with genotype 2. A statistically significant association was observed between patients with a family history of liver disease and genotype 2 (P = 0.021). Liver damage severity increased with longer duration of infection (P = 0.007). No statistically significant association was observed between severe liver damage and the different genotypes. Conclusion: Subtype 2b was the most prevalent. This contrasts with the studies done in different states of Mexico in that this subtype was not identified or had prevalence approximately 2 times less than reported here.
Abbreviations:
Hepatitis C virus (HCV)
Reverse transcription-polymerase chain reaction (RT-PCR)
Hepatitis C virus antibodies (anti-HCV)
Alanine amino transferase (ALT)
Histological activity index (HAI)
5´untranslated region (5´-UTR)
Standard deviation (SD)
IntroductionHepatitis C virus (HCV) infection is a serious worldwide public health concern. Approximately 170 million people are infected with HCV, although many ignore their condition.1 Prevalence varies by country with rates as high as in Egypt (19 to 60%)2 and as low as in Poland (0.5%).3 Infection rates can also vary between different groups in the same country.2,4 Infection with HCV is a serious public health problem because most cases are clinically asymptomatic but have serious consequences: 80% of those infected develop chronic hepatitis; 15 to 20% progress to cirrhosis; and 5% develop hepatocellular carcinoma.5,6
The hepatitis C virus (HCV) is divided into six major genotypes (1 to 6) based on genetic sequence, and then subdivided into over 50 alphabetically-designated subtypes. Genotype prevalence varies geographically. Some have a broad worldwide distribution (e.g. 1, 2 and 3), while others are restricted to certain geographical regions (e.g. 4, 5 and 6).7 The influence of genotypes in HCV progression is still controversial. Some researchers have associated the presence of genotype 3 with significant hepatic steatosis and fibrosis,5 while others have reported no significant differences in genotype prevalence in the progression of liver fibrosis.6 A number of studies have shown that different genotypes are mainly associated with patient age and HCV transmission route.9-11 Genotype association with disease evolution may not be completely understood, but there is evidence that HCV genotype is a predictive marker of antiviral therapy response.11,12 For example, patients with HCV subtype 1b exhibit a poor response to alpha interferon treatment.12
Data on genotypes is important to HCV therapy, but is also vital to epidemiological research. Most of the research on HCV infection in Mexico is based on screening of donor blood for HCV antibodies.13 However, HCV genotype prevalence is not determined in many states, leading to a lack of data on any associations between predominant HCV genotypes and transmission route, age, sex and liver histology in patients with hepatic disease caused by HCV. To begin addressing this shortcoming, the present study objective was to do a preliminary determination of HCV genotype prevalence and possible genotype associations with risk factors and severity of associated liver disease in HCV-infected patients in the state of Yucatan, Mexico.
MethodsPatients selectionThe 54 patients included in the study were referred to the Ignacio García Téllez National Medical Center in Merida, Yucatan, Mexico, between September 2005 and September 2006. All had repeatedly tested reactive for HCV antibodies (anti-HCV) (AxSYM HCV version 3.0) and had detectable serum HCV RNA. Although not all patients admitted to the hospital are tested for HIV and HBV, serum samples from each participant were also tested for hepatitis B surface antigen (HBsAg) (AxSYM HBsAg V2) and human immunodeficiency virus antibodies (anti-HIV) (AxSYM HIV 1/2 gO) using commercial Microparticle Enzyme Immunoassays (AxSYM Abbott, Weisbaden, Germany) and following manufacturer instructions. The patients were asked to fill out a detailed questionnaire providing epidemiological data. These data included sex, age, presumed source of infection (intravenous drug use, transfusion, surgery, sexual history, tattoos, acupuncture) and presumed length of infection. All participating patients were informed of the nature of the study and provided their written informed consent. This study was approved by the Institution Ethics Committee.
Biochemical testsAlanine amino transferase (ALT) levels in serum were determined with an automated Dimension RXL (Dade Behring Inc., Newark, USA) following manufacturer instructions.
HCV RNA detectionHCV RNA was extracted from blood samples using a commercial kit (QIAamp Viral RNA Mini Kit, QIAGEN GMBH, Hilden, Germany) following manufacturer instructions. A reverse transcription-polymerase chain reaction (RT-PCR) and nested PCR were used to test all the 54 serum samples for the presence of HCV RNA using a primer set that amplifies a highly conserved 5´ untranslated region (5´-UTR) of the HCV genome.14 Serum samples from patients negative for anti-HCV and RNA-free reagents were used as negative control. Detection limit for the nested RT-PCR assay was approximately 300 copies mL-1.15
HCV genotypingGenotyping was carried out by nested multiplex PCR of the core region using genotype specific primers, which allowed identification of at least six major types and a series of subtypes.16 When a double infection was observed, the nested PCR was repeated separately with each of the type-specific antisense primers. Samples with known genotypes (1a, 1b, 2a, 2b and 3a) were evaluated with this method and in all cases only genotype-specific bands were identified.
Histopathologic evaluationA hepatic biopsy was done for 24 of the studied patients. All the biopsies were read by the same pathologist. Liver disease severity was analyzed histopathologically and graded based on the histological activity index (HAI) and fibrosis stage according to Knodell et al.17
Statistical analysisThe continuous variables were expressed as the mean ± standard deviation (SD). A Chi-square test, Fisher’s exact test or ANOVA were used to compare results between groups, depending on the type of data analyzed. The independent value of the selected characteristic was determined with a logistic regression analysis. Statistical significance in all tests was P <0.05. Analyses were done with the SPSS software package (Version 14, SPSS Inc. Chicago, Illinois, USA).
ResultsThe cohort demographic was 27 males (50%) and 27 females (i.e. 27:27), with an average age of 50.2 ± 13.1 years and a range of 23-75 years. The most prevalent HCV genotype among the 54 evaluated patients was genotype 1, which was detected in 20 (37%) patients. Genotype 2 was found in 18 (33.3%) patients, genotype 3 in 9 (16.7%) patients, and mixed genotypes were present in 4 (7.4%) patients. Of these mixed infections, three included genotypes 1 and 2 and one included genotypes 2 and 3. Subtype 2b was the most frequent (33.3%), followed by 1b (18.5%), 3a (16.7%), 1a (14.8%) and 1a/1b (3.7%). The genotype in 3 (5.6%) patients could not be identified, and no cases with genotypes 4, 5 or 6 were found. No statistically significant association was observed between age or sex and the different genotypes. All patients were anti-HIV and HBsAg negative.
Mean ALT level in HCV infected patients was 48.3 ± 29.8 U/L (range 21-146). Abnormal ALT levels (normal range: 30-65 U/L) were found in only 7 (13%) patients. Genotype 3 patients had higher a mean ALT concentration than patients with genotype 2 (F3,52 = 3.15, P = 0.032). The logistic regression analysis showed a negative and significant relationship between ALT levels and genotype 2 (regression coefficient = 0.03, standard error = 0.017, odds ratio (95% CI) = 0.968 (0.935-1.002), P = 0.013).
At least one HCV infection-associated risk factor was identified in 32 (59.3%) of the evaluated patients. The two main risk factors were having had surgery (53.7%) or a blood transfusion (38.9%). Average infection duration among patients with a probable infection date (taking into account the first date of exposure to risk) was 20.5 ± 11.2 years (range 4-45). No statistically significant association was found between infection source or duration and any of the identified genotypes.
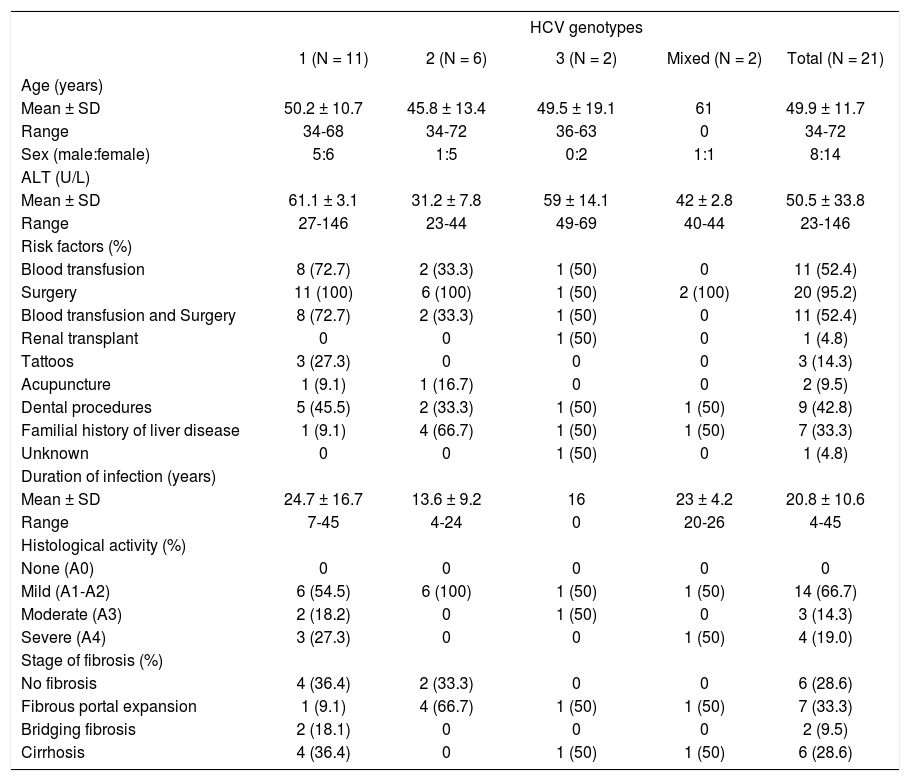
Liver biopsy was available in 24 (44.4%) patients, and in three of these HCV genotype could not be identified. Activity grading was done based on the inflammatory activities in the periportal and lobular regions, and the portal tract: A0 = no histological activity, A1 = minimal lesion, A2 = mild activity, A3 = moderate activity, A4 = severe activity. Severe liver disease was present in 6 (54.5%) of the patients with genotype 1 and in none of those with genotype 2 (Table I).
Clinical and histological characteristics in 21 HCV infected patients.
| HCV genotypes | |||||
|---|---|---|---|---|---|
| 1 (N = 11) | 2 (N = 6) | 3 (N = 2) | Mixed (N = 2) | Total (N = 21) | |
| Age (years) | |||||
| Mean ± SD | 50.2 ± 10.7 | 45.8 ± 13.4 | 49.5 ± 19.1 | 61 | 49.9 ± 11.7 |
| Range | 34-68 | 34-72 | 36-63 | 0 | 34-72 |
| Sex (male:female) | 5:6 | 1:5 | 0:2 | 1:1 | 8:14 |
| ALT (U/L) | |||||
| Mean ± SD | 61.1 ± 3.1 | 31.2 ± 7.8 | 59 ± 14.1 | 42 ± 2.8 | 50.5 ± 33.8 |
| Range | 27-146 | 23-44 | 49-69 | 40-44 | 23-146 |
| Risk factors (%) | |||||
| Blood transfusion | 8 (72.7) | 2 (33.3) | 1 (50) | 0 | 11 (52.4) |
| Surgery | 11 (100) | 6 (100) | 1 (50) | 2 (100) | 20 (95.2) |
| Blood transfusion and Surgery | 8 (72.7) | 2 (33.3) | 1 (50) | 0 | 11 (52.4) |
| Renal transplant | 0 | 0 | 1 (50) | 0 | 1 (4.8) |
| Tattoos | 3 (27.3) | 0 | 0 | 0 | 3 (14.3) |
| Acupuncture | 1 (9.1) | 1 (16.7) | 0 | 0 | 2 (9.5) |
| Dental procedures | 5 (45.5) | 2 (33.3) | 1 (50) | 1 (50) | 9 (42.8) |
| Familial history of liver disease | 1 (9.1) | 4 (66.7) | 1 (50) | 1 (50) | 7 (33.3) |
| Unknown | 0 | 0 | 1 (50) | 0 | 1 (4.8) |
| Duration of infection (years) | |||||
| Mean ± SD | 24.7 ± 16.7 | 13.6 ± 9.2 | 16 | 23 ± 4.2 | 20.8 ± 10.6 |
| Range | 7-45 | 4-24 | 0 | 20-26 | 4-45 |
| Histological activity (%) | |||||
| None (A0) | 0 | 0 | 0 | 0 | 0 |
| Mild (A1-A2) | 6 (54.5) | 6 (100) | 1 (50) | 1 (50) | 14 (66.7) |
| Moderate (A3) | 2 (18.2) | 0 | 1 (50) | 0 | 3 (14.3) |
| Severe (A4) | 3 (27.3) | 0 | 0 | 1 (50) | 4 (19.0) |
| Stage of fibrosis (%) | |||||
| No fibrosis | 4 (36.4) | 2 (33.3) | 0 | 0 | 6 (28.6) |
| Fibrous portal expansion | 1 (9.1) | 4 (66.7) | 1 (50) | 1 (50) | 7 (33.3) |
| Bridging fibrosis | 2 (18.1) | 0 | 0 | 0 | 2 (9.5) |
| Cirrhosis | 4 (36.4) | 0 | 1 (50) | 1 (50) | 6 (28.6) |
A statistically-significant association was observed between patients with a family history of liver disease and genotype 2 (P = 0.021). No significant association was found between liver disease severity and age, sex or ALT levels. Generally, no significant differences were observed in severe liver damage frequency between the identified genotypes, although an association between mild liver disease and genotype 2 was observed (P = 0.033).
With the exception of patients with tattoos, no significant difference was observed between source of infection and liver disease severity. Patients with tattoos had more severe than mild liver damage (P = 0.031). The logistic regression analysis showed that liver disease severity increased with duration of the disease (regression coefficient = 0.142, standard error = 0.067, odds ratio (95% CI) = 1.153 (1.003-1.325), P = 0.007).
DiscussionHepatitis C virus infection is considered a worldwide public health problem.1 Very few reports exist on HCV genotype distribution in Mexico. Analysis of the HCV genotypes within a population is a useful epidemiological tool for the study of the evolution of HCV infection in different geographical regions. In addition, it is important because it provides information as to strain variation and potential association with disease severity.
The HCV genotype distribution pattern observed here is similar to that in the United States with the exception of subtype 4a,18 which was not observed in the present study. The present data coincide with other studies showing that genotype 1 is the most common of the HCV genotypes in Mexico.19-21 However, subtype 2b was the most prevalent in the patients studied here (33% of cases). This contrasts with the studies mentioned above in that this subtype was not identified or had prevalence approximately 2 times less than reported here. Patients with genotype 2 respond better to antiviral treatment than those with genotype 1,18 making this higher prevalence important to consider when developing treatments.
Different researchers have stated that genotype 3 is associated with intravenous drug users,22 but those patients with genotype 3 in the present study had not been exposed to that risk factor. Eleven (20%) of the patients in the present study had a family history of liver disease. Further study is needed including family members of HCV-seropositive patients to determine if they become infected with HCV.
Blood transfusion has been reported as the primary cause of HCV infection in Mexico (64.2%), followed by surgery (12.6%).21 The present data, however, showed surgery to be the primary cause for HCV infection in Yucatan, followed by blood transfusion. As reported elsewhere, no association was found between age or transmission route and HCV genotype.18
Although chronic hepatitis C is usually associated with high ALT levels, some chronic hepatitis C patients have persistently normal ALT values.23,24 These persistently normal levels do not appear to be linked to different genotype prevalences, though a tendency towards genotype 2 has been reported.25 This coincides with the present results in which 87% (n = 47) of the patients (n = 54) had normal ALT levels and a negative and significant relationship was found between ALT levels and genotype 2. High ALT levels have been reported as not being associated with the progression of hepatic fibrosis,24,26which agrees with the present data.
The influence of viral genotype on the progression of hepatic fibrosis is still unclear. Some researchers have reported that the presence of genotypes 1 and 3 are associated with liver damage progression,8,27 whereas others have reported that HCV genotype has no influence on liver disease severity.26,28,29 No association was identified here between the different genotypes and severe liver disease. Fibrosis progression was found to be influenced by increased duration of HCV infection, which coincides with other reports.28
Given the low relative number of patients in the present study, it must be treated as preliminary. Future studies need to include larger numbers of patients to more accurately determine the influence of viral and host factors on the prognosis of HCV-related liver disease. These, along with continued molecular epidemiological studies of HCV infection, are essential to designing control measures and treatment modes for HCV, for which no vaccination yet exists.
AcknowledgementsThe authors thank the Ignacio García Téllez National Medical Center and the Faculty of Chemistry, UADY, for the use of their facilities in this research.