Lamotrigine is a non-aromatic antiepileptic drug. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome is a severe idiosyncratic reaction to drugs, especially anti-epileptic drugs. Associated clinical features include cutaneous eruption, fever, multiple peripheral lymphadenopathies, and potentially life-threatening damage of one or more organs. We report a case of DRESS syndrome induced by lamotrigine presenting with a hypersensitivity syndrome and fulminant hepatic failure requiring liver transplant. A 21-year old female patient presented an episode of seizure with loss of conscience. CT and EEG studies performed were normal. Treatment with lamotrigine was prescribed. In the course of 30 days, the patient developed skin lesions, pruritus, cholestatic hepatitis, and systemic symptoms-fever, lymphadenopathies, extensive exfoliative erythematous maculopapular rash, and jaundice. Serologic and laboratory tests showed no other causes responsible for the clinical spectrum. Hematologic tests revealed peripheral eosinophilia. Fulminant hepatic failure was diagnosed and an orthotopic liver transplant was performed. Histologic sections of the ex-planted liver demonstrated submassive hepatic necrosis, with the remnant portal spaces and lobules showing a mixed inflammatory infiltrate with lymphocytes and eosinophils. Lamotrigine treatment has been associated with multiorgan failure, DRESS syndrome, acute hepatic failure, and disseminated intravascular coagulation. In conclusion, we suggest that these potentially fatal side effects should be considered in any patient with clinical deterioration following administration of this drug.
Lamotrigine [6-(2,3-dicriloroprienyl)-1,2,4-triazine-3,5-diamine] is an antiepileptic drug, effective for a broad range of seizures in adults and children, which is structurally and pharmacologically unrelated to other antiepileptic medications.1
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, a type of hypersensitivity reaction, is a severe idiosyncratic reaction to drugs, especially to anti-epiletic drugs. Associated clinical features include cutaneous eruption, fever, multiple peripheral lymphade-nopathies, and potentially life-threatening damage of one or more organs.2
Less than 10 cases concerning severe hypersensitivy syndrome associated with lamotrigine have been reported in the literature, two of them with acute hepatic failure.3,4 We report a case or fulminant hepatic failure induced by lamotrigine: after presenting with a hypersentivity syndrome, rash and fever, the patient developed elevated liver function tests and clinical signs and symptoms of hepatic failure, requiring an orthotopic liver transplantation.
Case reportA 21 year-old female patient without prior pathological conditions presented a seizure assumed as a tonic convulsion, with loss of the conscience of 5 minutes. No further episodes occurred. CT and EEG studies were performed, with normal results. Treatment with lamotrigine was prescribed. On the 18th day she presented with fever and red throat. In the course of the following week she was treated with amoxicillin, later switched to erythro-mycin after allergic reactions (erithema) developed, and finally switched back to amoxicillin. During this time there was progression of the skin lesions and development of pruritus. Laboratory tests on the 38th day informed serum elevation of hepatic enzymes and bilirrubin, which were interpreted as cholestatic hepatitis. All previous medications were suspended and antihistamine therapy was administered. On the 43rd day she presented general symptoms: fever, lymphadenopathies and extensive descamative erythematous maculopapular rash with jaundice. In the following days laboratory tests showed further elevation of hepatic enzymes and bilirrubin and neurologic signs (flapping) developed. Coagulogram test showed: Quick 46%, after treatment with Vit K. Quick: 40%, Factor V: 23%. Serologic tests turned out negative for HIV, EBV, HCV, HBsAg, VDRL, HAV, Toxoplasma gondii, and autoantibodies. Blood tests revealed peripheral eosinophilia. Ceruloplasmin and • • 1-antitrypsin levels were normal. Liver function did not improve and an orthotopic liver transplant was performed.
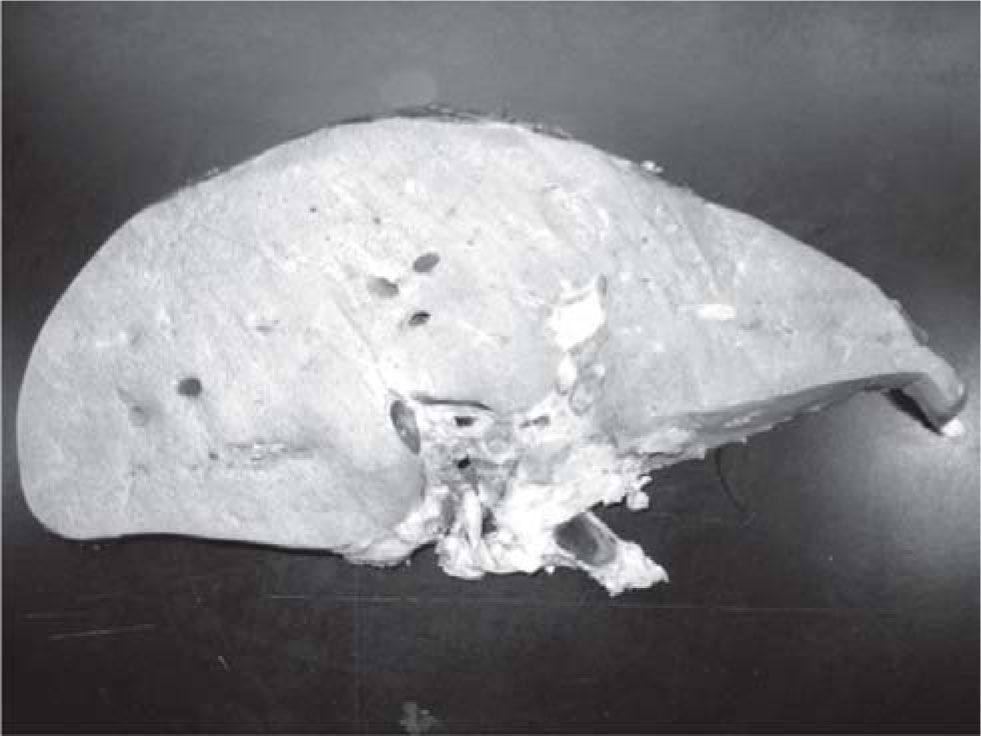
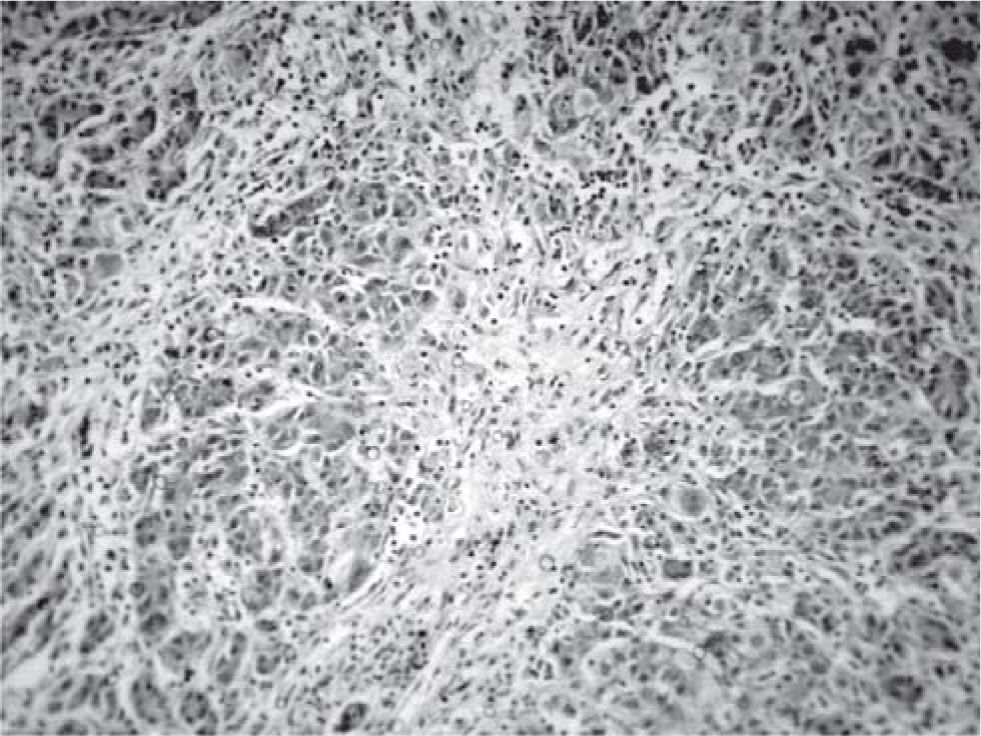
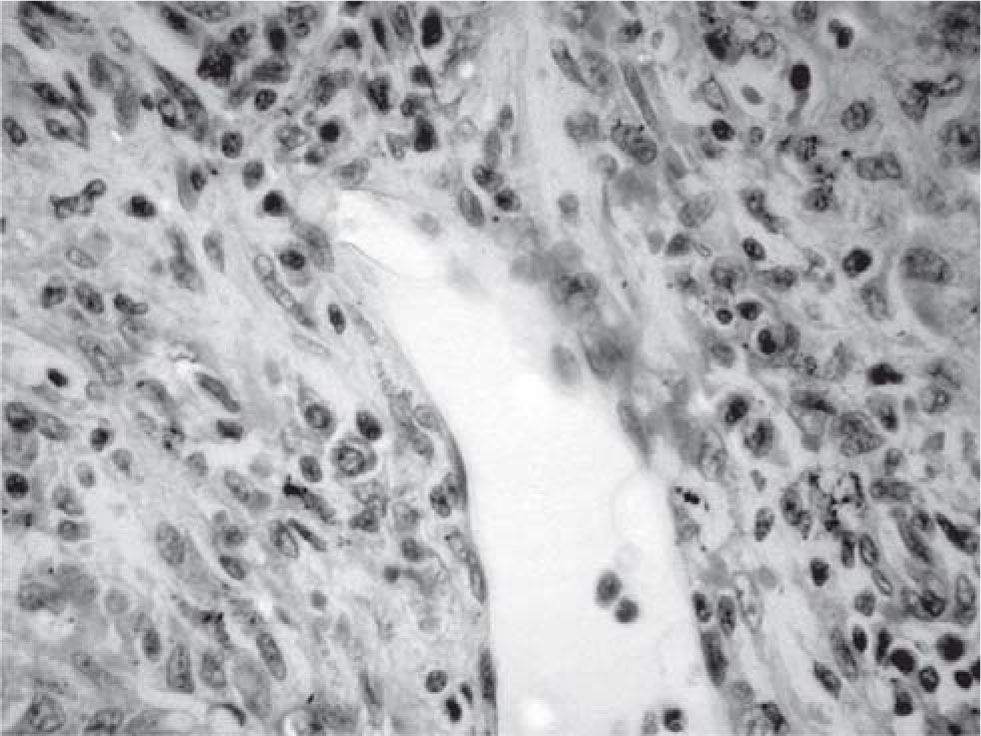
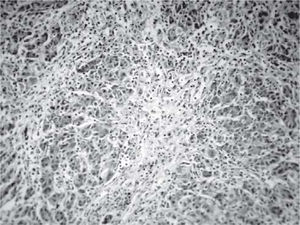
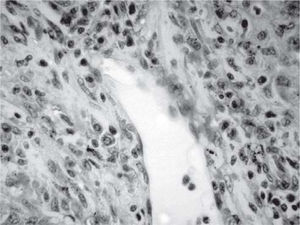
Results and discussionThe explanted liver had an abnormal appearance, with a diminished weight (700 g), a shrunken capsule and a greenish color on the cut surface (Figure 1). Tissue samples representative of each segment of the organ were obtained, along with samples from the hepatic hilum and suprahepatic veins. Routine stains were performed (HE, PAS, Masson trichrome, Gordon sweet reticulin, Perls). Microscopic analysis revealed extensive areas of confluent necrosis with collapse of the reticular frame, with a submassive extent approach of portal tracts and vascular elements (Figure 2). Minimal fibrosis was observed. Remnant portal spaces represented less then 10% of the cut surface; they presented marked duct reaction and neo-cholangiolos. A mixed inflammatory infiltrate was present in the portal spaces and lobules (Figure 3). Eosinophils, present in great numbers in the inflammatory reaction, suggested an immunoallergic mechanism rather than a direct toxic liver injury. Severe hepatocanalicular cholestasis and balonization coexisted. The vascular and neural elements of the hilum and the suprahepatic veins showed no significant alterations.
The hypersensitivity syndrome typically develops two to six weeks after the drug is first administered, later than most other serious skin reactions. This syndrome manifests as rash, fever, tender lymphadenopathies, hepatitis, and eosinophilia.2–4 The mechanism of the hypersensitivity syndrome is unknown. Several theories have been proposed. The reaction is either secondary to circulating antibodies or involves toxic metabolities. Associated infection by human herpes virus 6 may also play a role in its development.3
Hypersensitivity reactions to aromatic antiepileptic drugs appear to have an immune etiology, much like the lamotrigine-induced reaction: bioactivation, detoxification, covalent adduct formation, processing and presentation of antigen to the immune system, and consequent formation of antibody and T-cell immune effectors. Another theory involves toxic metabolites; the aromatic antiepileptic agents are metabolized by cytochrome P-450 to an arene oxide metabolite.3
These are normally detoxified by the enzime epoxide hydrolase, which may be lacking or mutated in persons that develop the syndrome. Lamotrigine exhibits first-order linear elimination. It is mainly metabolized by hepatic glucuronidation; the resulting metabolite has no pharmacologic activity and is excreted in urine. The average elimination half-life in adults is 20 to 35 hours.5
Our patient had symptoms and laboratory findings similar to those found in the anticonvulsant hypersensitivity syndrome. This suggests that there is a threshold concentration of lamotrigine above which the body’s ability to metabolize the drug may be overwhelmed, leading to a similar hypersensitivity reaction.6
The incidence of DRESS syndrome is estimated in 1/1,000-1/10,000 exposures. However, it is probably underestimated. No standard treatment has been proposed, but parenteral corticosteroid therapy was successful in three cases, leading to a rapidly favorable clinical course, although liver tests took longer to return to normal.7
Lamotrigine treatment has been associated with multi-organ failure, DRESS syndrome, acute hepatic failure, and disseminated intravascular coagulation.8,9 We suggest that these potentially fatal side effects should be considered in any patient when clinical deterioration follows treatment with this drug. Prompt recognition and withdrawal of the suspected agent is essential. The goal of research is to describe a susceptibility profile, identifying individuals who are at risk for these forms of drug toxicity.10 We recommend careful monitoring of liver function when lamotrigine is administered.