Although hepatotoxicity accounts for 10% of adverse drug reactions, it remains poorly understood and underreported. This study aimed to summarize case reports of herb- and drug-induced liver injury in Brazil.
MethodologySystematic review in the following databases: PubMed, SciELO, Science Direct, CAPES, and gray literature.
ResultsTwenty-seven studies reporting 32 cases were identified. Brazilian cases were primarily detected in hospitals, and occurred mainly in young males suffering from chronic diseases. Drugs (n=29) were a more frequent cause of liver injury than herbs (n=3). Almost a third of these drugs were anticonvulsants, and 15 appear in the Brazilian List of Essential Medicines. In 50% of the cases, clinical manifestations started within 30 days of drug ingestion. Regarding the decline of liver enzymes, 50% of the cases reached normality after drug withdrawal. However, 7 deaths and 2 liver transplantations were reported. Only one study assessed causality using RUCAM.
ConclusionGiven the severe outcomes of DILI and HILI, early detection and management of hepatotoxicity to increase drug safety are necessary, as well as pharmacotherapeutic monitoring of patients with chronic diseases. Moreover, the application of the RUCAM algorithm in clinical practice has to be further disseminated.
Drug-induced liver injury (DILI) is difficult to diagnose not only due to its similarity to viral hepatitis and other liver diseases, but also due to the lack of a confirmatory laboratory test. This means that the detection of this type of injury has a strong reliance on anamnesis, and its diagnosis is often obtained by exclusion [1].
In the United States, hepatotoxicity occurs in 10% of all adverse drug effect cases, in more than 50% of acute jaundice cases, and in 13–30% of acute liver failure cases [2]. Judging by case reports, DILI can be considered a rare event, its frequency ranging from 1 in every 10,000 to 1 in every 100,000 adverse drug effect cases. However, since the number of patients involved in clinical studies is less than 10,000, pharmacovigilance studies are likely to detect hepatotoxicity [3,4].
In fact, the severity of hepatotoxicity has led regulatory agencies to withdraw several drugs from the market [5]. This goes to show the importance of pharmacovigilance studies and the need for proper reporting of adverse effects [6].
Direct toxicity is dose-dependent, reproducible, and has a predictable evolution. Idiosyncratic reactions manifest themselves after a period of latency, ranging from 5 to 90 days after drug ingestion. DILI is considered present when changes in hepatic markers are observed. It is classified as hepatocellular, cholestatic, or mixed, as indicated by the ratio between alanine aminotransferase (ALT) and alkaline phosphatase (AP) markers [7].
A recent literature review observed great discrepancies among Latin American case reports—between 2006 and 2012, Chile, Argentina, and Colombia were responsible for 91% of all published cases [8]. It is therefore reasonable to infer that in the remaining Latin American countries, DILI is considerably underreported. Uncoincidentally, although in 2010 the Brazilian Society of Hepatology warned that the number of DILI cases was growing, in this review only two Brazilian DILI research publications were identified [8]. In this context, there is an urgent need for alerting health managers about the importance of this topic, and for a proactive, countrywide scientific study of DILI [9].
The use of medicinal plants is customary in Brazil, usually lacking medical recommendation or prescription [10–12], since information on medicinal plants is rooted in traditional knowledge. Moreover, different studies point out to herb-induced hepatotoxicity as a common occurrence in several countries [13–15].
Considering the shortage of data on hepatotoxicity-inducing drugs and medicinal plants in the context of the Brazilian population, as well as the relevance of this topic, this study aims to identify the report profile of published Brazilian cases of drug- and herb-induced hepatic injury.
2MethodologyA systematic review of Brazilian studies on drug and herb-induced liver injury was carried out. Searches were performed in the PubMed, SciELO, Science Direct, and CAPES databases.
Portuguese and English DILI descriptors were used in the search, according to the following syntax: (“Hepatotoxicity” or “drug induced liver injury” or “liver injury” or “hepatotoxic adverse drug”) and (“Brasil” or “Brazil” or “Brazilian”). All study designs were initially included, but for a better assessment of results, in this study only case reports were analyzed. The systematic review was recorded in the PROSPERO platform, under code CRD42017067817.
The selection was performed by two independent reviewers, in three sequential stages comprised of reading the title, the abstract, and then the full text. Disagreements were resolved by a third reviewer. Eligibility criteria were the following: being a Brazilian study, being a case report, and suspicion of DILI due to drug or herb use.
The following variables were analyzed: age; sex; patient's underlying disease; report location; drug; symptom onset time; length of hospital stay and normalization of liver enzymes; associated treatment; outcome; type of liver injury; use of algorithm to support diagnosis. Whenever possible, the liver injury was classified as hepatocellular, cholestatic, or mixed.
The collected data were compared to those of international studies [8,16–22]. A descriptive statistical analysis was performed using Excel 2013.
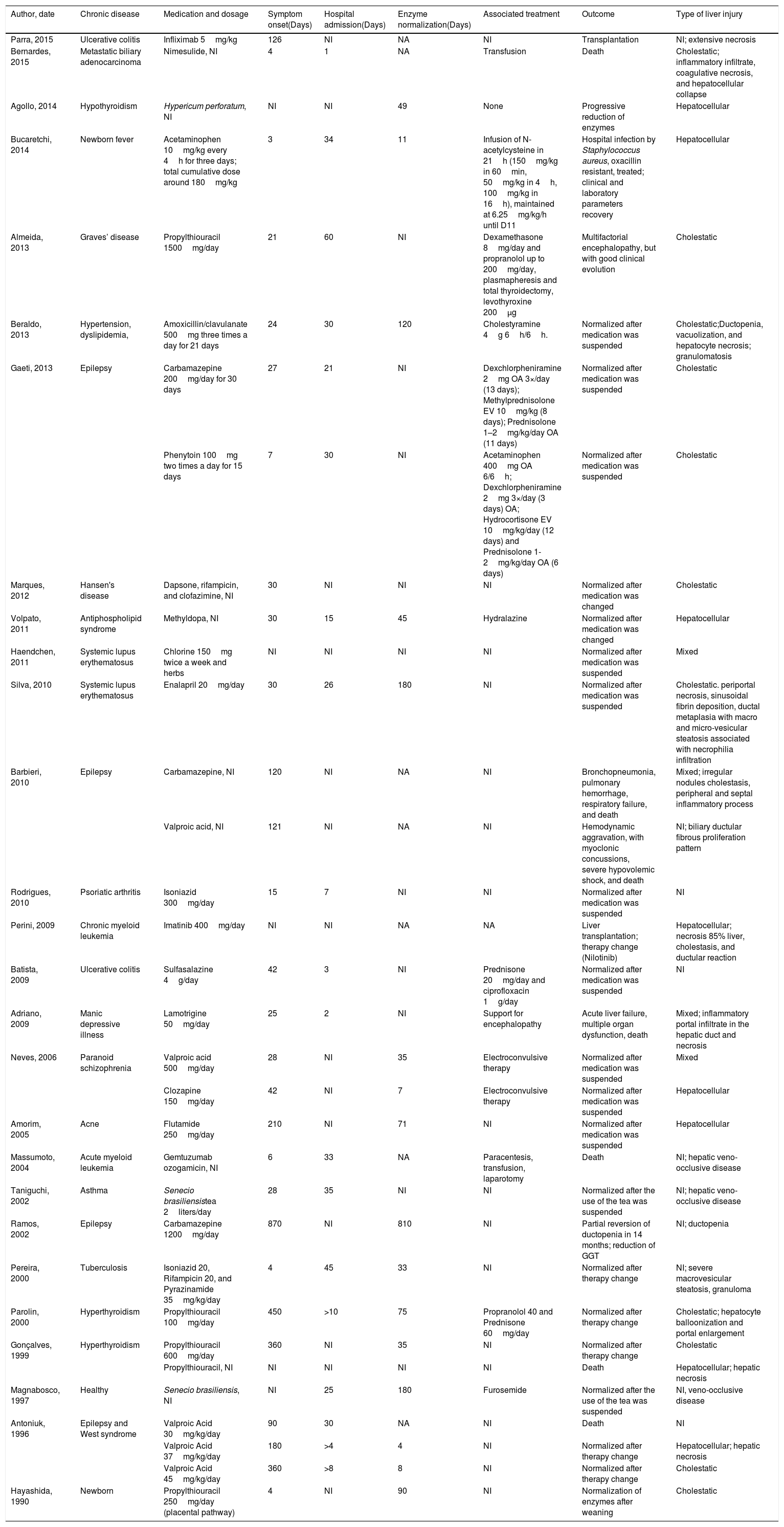
3ResultsA total of 337 records was initially identified. Among these, 264 were excluded during title and abstract screening, and the 73 remaining articles underwent a full-text review. Twenty-seven publications were case reports, describing a total of 32 DILI cases in Brazil [23–49]. Studies from 9 Brazilian states were found, with the state of São Paulo accounting for 41% of all studies. DILI identification occurred predominantly in hospitals (n=28). Average patient age was 27 years. Patients were older people (above 60 years old; n=4), adults (between 18 and 59 years old; n=15), and children (under 18 years old; n=13). Males accounted for 56% of the cases. Data on patients, drugs, clinical manifestation onset time, hospital length of stay, outcomes, and type of hepatic injury were summarized in Table 1.
Characteristics of Brazilian case reports of drug-induced liver injury.
| Author, date | Chronic disease | Medication and dosage | Symptom onset(Days) | Hospital admission(Days) | Enzyme normalization(Days) | Associated treatment | Outcome | Type of liver injury |
|---|---|---|---|---|---|---|---|---|
| Parra, 2015 | Ulcerative colitis | Infliximab 5mg/kg | 126 | NI | NA | NI | Transplantation | NI; extensive necrosis |
| Bernardes, 2015 | Metastatic biliary adenocarcinoma | Nimesulide, NI | 4 | 1 | NA | Transfusion | Death | Cholestatic; inflammatory infiltrate, coagulative necrosis, and hepatocellular collapse |
| Agollo, 2014 | Hypothyroidism | Hypericum perforatum, NI | NI | NI | 49 | None | Progressive reduction of enzymes | Hepatocellular |
| Bucaretchi, 2014 | Newborn fever | Acetaminophen 10mg/kg every 4h for three days; total cumulative dose around 180mg/kg | 3 | 34 | 11 | Infusion of N-acetylcysteine in 21h (150mg/kg in 60min, 50mg/kg in 4h, 100mg/kg in 16h), maintained at 6.25mg/kg/h until D11 | Hospital infection by Staphylococcus aureus, oxacillin resistant, treated; clinical and laboratory parameters recovery | Hepatocellular |
| Almeida, 2013 | Graves’ disease | Propylthiouracil 1500mg/day | 21 | 60 | NI | Dexamethasone 8mg/day and propranolol up to 200mg/day, plasmapheresis and total thyroidectomy, levothyroxine 200μg | Multifactorial encephalopathy, but with good clinical evolution | Cholestatic |
| Beraldo, 2013 | Hypertension, dyslipidemia, | Amoxicillin/clavulanate 500mg three times a day for 21 days | 24 | 30 | 120 | Cholestyramine 4g 6h/6h. | Normalized after medication was suspended | Cholestatic;Ductopenia, vacuolization, and hepatocyte necrosis; granulomatosis |
| Gaeti, 2013 | Epilepsy | Carbamazepine 200mg/day for 30 days | 27 | 21 | NI | Dexchlorpheniramine 2mg OA 3×/day (13 days); Methylprednisolone EV 10mg/kg (8 days); Prednisolone 1–2mg/kg/day OA (11 days) | Normalized after medication was suspended | Cholestatic |
| Phenytoin 100mg two times a day for 15 days | 7 | 30 | NI | Acetaminophen 400mg OA 6/6h; Dexchlorpheniramine 2mg 3×/day (3 days) OA; Hydrocortisone EV 10mg/kg/day (12 days) and Prednisolone 1-2mg/kg/day OA (6 days) | Normalized after medication was suspended | Cholestatic | ||
| Marques, 2012 | Hansen's disease | Dapsone, rifampicin, and clofazimine, NI | 30 | NI | NI | NI | Normalized after medication was changed | Cholestatic |
| Volpato, 2011 | Antiphospholipid syndrome | Methyldopa, NI | 30 | 15 | 45 | Hydralazine | Normalized after medication was changed | Hepatocellular |
| Haendchen, 2011 | Systemic lupus erythematosus | Chlorine 150mg twice a week and herbs | NI | NI | NI | NI | Normalized after medication was suspended | Mixed |
| Silva, 2010 | Systemic lupus erythematosus | Enalapril 20mg/day | 30 | 26 | 180 | NI | Normalized after medication was suspended | Cholestatic. periportal necrosis, sinusoidal fibrin deposition, ductal metaplasia with macro and micro-vesicular steatosis associated with necrophilia infiltration |
| Barbieri, 2010 | Epilepsy | Carbamazepine, NI | 120 | NI | NA | NI | Bronchopneumonia, pulmonary hemorrhage, respiratory failure, and death | Mixed; irregular nodules cholestasis, peripheral and septal inflammatory process |
| Valproic acid, NI | 121 | NI | NA | NI | Hemodynamic aggravation, with myoclonic concussions, severe hypovolemic shock, and death | NI; biliary ductular fibrous proliferation pattern | ||
| Rodrigues, 2010 | Psoriatic arthritis | Isoniazid 300mg/day | 15 | 7 | NI | NI | Normalized after medication was suspended | NI |
| Perini, 2009 | Chronic myeloid leukemia | Imatinib 400mg/day | NI | NI | NA | NA | Liver transplantation; therapy change (Nilotinib) | Hepatocellular; necrosis 85% liver, cholestasis, and ductular reaction |
| Batista, 2009 | Ulcerative colitis | Sulfasalazine 4g/day | 42 | 3 | NI | Prednisone 20mg/day and ciprofloxacin 1g/day | Normalized after medication was suspended | NI |
| Adriano, 2009 | Manic depressive illness | Lamotrigine 50mg/day | 25 | 2 | NI | Support for encephalopathy | Acute liver failure, multiple organ dysfunction, death | Mixed; inflammatory portal infiltrate in the hepatic duct and necrosis |
| Neves, 2006 | Paranoid schizophrenia | Valproic acid 500mg/day | 28 | NI | 35 | Electroconvulsive therapy | Normalized after medication was suspended | Mixed |
| Clozapine 150mg/day | 42 | NI | 7 | Electroconvulsive therapy | Normalized after medication was suspended | Hepatocellular | ||
| Amorim, 2005 | Acne | Flutamide 250mg/day | 210 | NI | 71 | NI | Normalized after medication was suspended | Hepatocellular |
| Massumoto, 2004 | Acute myeloid leukemia | Gemtuzumab ozogamicin, NI | 6 | 33 | NA | Paracentesis, transfusion, laparotomy | Death | NI; hepatic veno-occlusive disease |
| Taniguchi, 2002 | Asthma | Senecio brasiliensistea 2liters/day | 28 | 35 | NI | NI | Normalized after the use of the tea was suspended | NI; hepatic veno-occlusive disease |
| Ramos, 2002 | Epilepsy | Carbamazepine 1200mg/day | 870 | NI | 810 | NI | Partial reversion of ductopenia in 14 months; reduction of GGT | NI; ductopenia |
| Pereira, 2000 | Tuberculosis | Isoniazid 20, Rifampicin 20, and Pyrazinamide 35mg/kg/day | 4 | 45 | 33 | NI | Normalized after therapy change | NI; severe macrovesicular steatosis, granuloma |
| Parolin, 2000 | Hyperthyroidism | Propylthiouracil 100mg/day | 450 | >10 | 75 | Propranolol 40 and Prednisone 60mg/day | Normalized after therapy change | Cholestatic; hepatocyte balloonization and portal enlargement |
| Gonçalves, 1999 | Hyperthyroidism | Propylthiouracil 600mg/day | 360 | NI | 35 | NI | Normalized after therapy change | Cholestatic |
| Propylthiouracil, NI | NI | NI | NI | NI | Death | Hepatocellular; hepatic necrosis | ||
| Magnabosco, 1997 | Healthy | Senecio brasiliensis, NI | NI | 25 | 180 | Furosemide | Normalized after the use of the tea was suspended | NI, veno-occlusive disease |
| Antoniuk, 1996 | Epilepsy and West syndrome | Valproic Acid 30mg/kg/day | 90 | 30 | NA | NI | Death | NI |
| Valproic Acid 37mg/kg/day | 180 | >4 | 4 | NI | Normalized after therapy change | Hepatocellular; hepatic necrosis | ||
| Valproic Acid 45mg/kg/day | 360 | >8 | 8 | NI | Normalized after therapy change | Cholestatic | ||
| Hayashida, 1990 | Newborn | Propylthiouracil 250mg/day (placental pathway) | 4 | NI | 90 | NI | Normalization of enzymes after weaning | Cholestatic |
NI: not identified; NA: does not apply; OA: oral administration.
Clinical outcomes were as follows: normalization of liver enzymes after medication interruption or change (n=23), jaundice (n=14), liver transplantation (n=2), and death (n=7). Fifteen liver biopsies were performed.
Patient deaths were associated with valproic acid (n=2), nimesulide (n=1), propylthiouracil (n=1), carbamazepine (n=1), lamotrigine (n=1), and gemtuzumab (n=1). Liver transplantation cases were associated with imatinib (n=1) and infliximab (n=1). Liver injuries were classified as hepatocellular (n=8), cholestatic (n=11), and mixed (n=4), or remained undetermined (n=9).
In 50% of the cases, clinical manifestation started within 30 days of drug ingestion. Regarding the decline of liver enzymes, in 50% of the cases normality was reached after drug withdrawal. This took up to 49 days. Enzyme rate normalization remained unreported in 15 cases, and clinical manifestation onset time remained unreported in 4 cases.
Several cases of DILI in chronic disease patients were reported. The most frequent chronic diseases were epilepsy (n=8) and hypothyroidism (n=4). Three authors reported algorithm support for DILI confirmation, and the identified algorithms were: Naranjo [50] 50 (n=1), Maria & Vitorino [51] (n=2), and RUCAM7 (n=1). Of the 20 drugs associated with DILI ((n=1), Maria & Vitorino [51] (n=2) and RUCAM [7] (n=1), Maria & Vitorino [51] (n=2), and RUCAM. Among 21 medicines associated with DILI (Table 1), the most frequently occurring ones were valproic acid (n=5), propylthiouracil (n=5), carbamazepine (n=3), and Senecio brasiliensis (n=2). Anticonvulsants were associated with 28% of the cases. Fifteen (15) out of 21 liver injury-inducing drugs appear in the Brazilian List of Essential Medicines. Most of the cases were associated with drugs for primary pharmaceutical care (n=18), in comparison to specialized care (n=5) and strategic care (n=3).
Valproic acid was the drug most frequently associated with DILI (n=5, four children and one adult). Valproic acid-induced injuries were classified as cholestatic (n=1), mixed (n=1), or remained undetermined (n=3). Propylthiouracil was reported in 3 adult DILI cases (1 death) and in 2 cases involving children. Injuries caused by this drug were cholestatic (n=4). Carbamazepine-induced DILI occurred in three patients, causing cholestatic (n=1), mixed (n=2), or undetermined injuries (n=1).
4DiscussionThis is the first review of Brazilian DILI case reports. In the international literature, on the other hand, DILI case reports are easily identified [8,16,17,52,53]. Brazilian data, however, remain sparse. Considering the magnitude of the Brazilian population and the widespread use of drugs and medicinal plants known to induce liver injury, the fact that only 32 case reports were found points to alarmingly low rates of DILI suspicion and identification.
In a study on herb-induced liver injuries between 1999 and 2009 carried out by the Brazilian Health Surveillance Agency (ANVISA), only 10 cases of hepatotoxicity were reported [54]. None of these cases were found in our review.
Brazilian pharmacovigilance has been growing in the past two decades. In 2001, the creation of a sentinel network was proposed by ANVISA, in which reference hospitals cooperate to build a reliable information system, subsidizing the development of sanitary surveillance actions. In 2009, the Notivisa system was created, an online platform for adverse events notification [55]. Also, the Brazilian Society of Hepatology has the HEPATOX project, in which it is possible to notify case reports in an online database [56]. These efforts notwithstanding, adverse effects including hepatotoxicity remain underreported throughout Brazil.
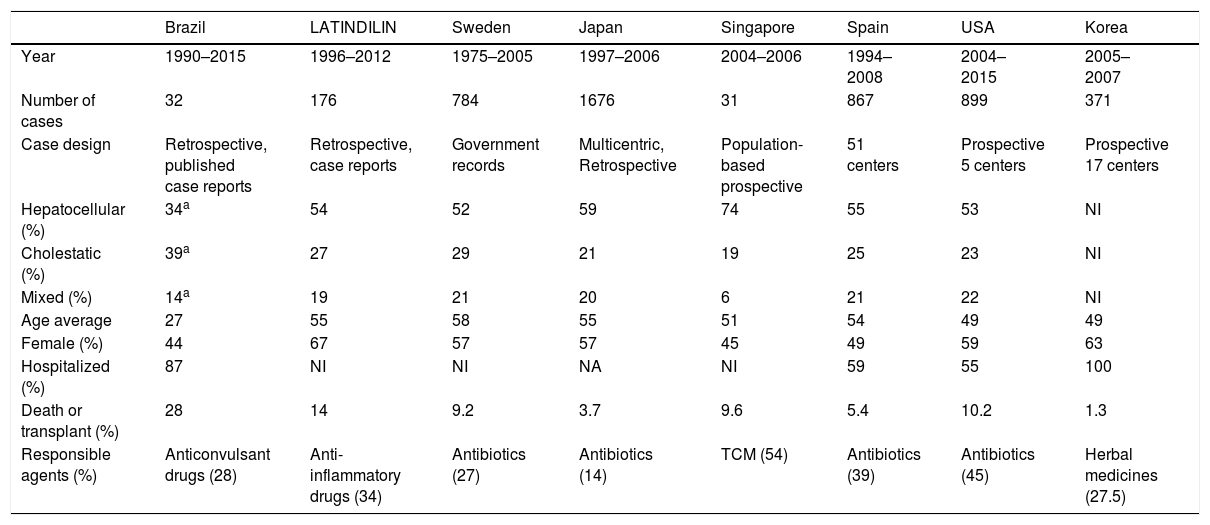
In 2011, the Latin American DILI Network [57] was inaugurated for the prospective follow-up of DILI cases. The network was modeled after similar endeavors in Spain [16] and Iceland [52], both based on RUCAM. A recent study by the LATINDILI group presented new data from Latin America, comprised of 250 DILI case records. Average age was 51 years (15–89 years), and the female sex was predominant (59%). Most DILI cases were from Argentina (47%), followed by Uruguay (32%), Chile (7%), Mexico (5%), Paraguay (3%), Venezuela (3%), Ecuador (2%), Brazil (0.5%), and Peru (0.5%) [58].
Reports found in our research were mostly identified in hospitals. This is to be expected, considering that hospitals have better diagnostic resources, as well as the possibility of multi-professional care, both of which may improve identification rates. Furthermore, health professionals who are involved in academic activities may be more encouraged to identify and publish DILI cases.
As shown in Table 2, few studies contain information on hospital length of stay. In those that do, hospitalization times were significantly longer in comparison to literature results. This comparison must be performed with caution, considering studies’ methodological differences, but it is suspected that several DILI cases originate from outpatient prescriptions, and, due to DILI's acute characteristic, identification is only effectively carried out after hospital admission. The frequency of hospitalized patients with DILI in Iceland was 0.7% [52]. In a study in the United Kingdom, a 0.7% incidence of DILI was found in 1964 consecutive hospital admissions [59]. In Switzerland, a study with 4209 patients found an incidence of 0.7% admissions due to DILI, in addition to 1.4% patients who developed DILI while hospitalized. These studies also suggest high rates of underdiagnosis and underreporting, with no DILI diagnosis being obtained in 50% of cases [60]. While DILI does seem to have an impact on hospital admission, the number of patients hospitalized for other causes who developed DILI during hospitalization was twice as high as the number of patients initially hospitalized due to DILI. These results suggest that it may be important to carefully monitor patients’ liver function, and also gather more data on potentially hepatotoxic drugs used in hospitals. Also, the use of RUCAM whenever DILI is suspected should be stimulated [7].
Comparison between Brazilian and international studies on drug-induced liver injury.
| Brazil | LATINDILIN | Sweden | Japan | Singapore | Spain | USA | Korea | |
|---|---|---|---|---|---|---|---|---|
| Year | 1990–2015 | 1996–2012 | 1975–2005 | 1997–2006 | 2004–2006 | 1994–2008 | 2004–2015 | 2005–2007 |
| Number of cases | 32 | 176 | 784 | 1676 | 31 | 867 | 899 | 371 |
| Case design | Retrospective, published case reports | Retrospective, case reports | Government records | Multicentric, Retrospective | Population-based prospective | 51 centers | Prospective 5 centers | Prospective 17 centers |
| Hepatocellular (%) | 34a | 54 | 52 | 59 | 74 | 55 | 53 | NI |
| Cholestatic (%) | 39a | 27 | 29 | 21 | 19 | 25 | 23 | NI |
| Mixed (%) | 14a | 19 | 21 | 20 | 6 | 21 | 22 | NI |
| Age average | 27 | 55 | 58 | 55 | 51 | 54 | 49 | 49 |
| Female (%) | 44 | 67 | 57 | 57 | 45 | 49 | 59 | 63 |
| Hospitalized (%) | 87 | NI | NI | NA | NI | 59 | 55 | 100 |
| Death or transplant (%) | 28 | 14 | 9.2 | 3.7 | 9.6 | 5.4 | 10.2 | 1.3 |
| Responsible agents (%) | Anticonvulsant drugs (28) | Anti-inflammatory drugs (34) | Antibiotics (27) | Antibiotics (14) | TCM (54) | Antibiotics (39) | Antibiotics (45) | Herbal medicines (27.5) |
TCM: Traditional Chinese Medicine.
The regional coverage of the cases included 9 out of 26 Brazilian states, and almost half of the reported cases came from the state of São Paulo. This distribution may indicate that DILI health concerns have yet to reach several Brazilian regions, with many occurrences possibly neglected. Access to information as well as regional and cultural differences in the reporting of cases may bear on these results, once again reinforcing the central role of the publication and discussion of case reports, in order to assist in the diagnosis and management of similar cases.
Drug-induced liver injury is more common in adults, although the number of cases affecting children is also significant. The average age of DILI patients found in the literature is between 49 and 58 years. In Spain, nearly 50% DILI cases affect older people, with advanced age being regarded as an important predictor of hepatotoxicity. In Brazil, 12.5% of reported cases involved older people. Cholestasis was more strongly associated with advanced age, while hepatocellular injury was more strongly associated with the younger population [61,62], and this considerable age gap may be related to Brazil's socioeconomic profile as a developing country. However, case reports found in this review mostly regarded acute manifestations: chronic manifestations may be less attractive as a subject matter, which may have affected our results.
Hepatocellular injury was present in 35% of cases. A higher frequency was expected due to patients with this type of injury being younger and having a higher mortality rate (hepatocellular injury is more severe). The small rate of hepatocellular damage can be attributed to the unavailability of ALT or AP information in some cases’ descriptions. Consequently, the relative frequency of this type of injury was inverted in comparison to international studies, with more cases of cholestatic injury, as shown in Table 2.
Jaundice, associated with advanced age, was present in 59% of the patients. The most severe cases of cholestatic injury were reported in young patients. When analyzing medications, age groups, and injury types, the data were similar to the literature's.
Anticonvulsant medicines were the most reported, contrasting with other studies, in which antibiotics and anti-inflammatory drugs were the most associated with cases of hepatotoxicity, as shown in Table 2[16–20,63].
Children were more prone to liver injuries caused by valproic acid than adults; hepatocellular injuries were more frequent, but mixed and cholestatic injuries were also reported. A histological pattern of cholestasis with fibrotic lesions of ductile proliferation, with or without ductopenia, was found [64,65]. This suggests caution when administering valproic acid to children. Pediatricians should have a good grasp of the drug's hepatotoxic potential and develop protocols for early liver injury detection.
Cases involving propylthiouracil occurred in adults. One case stands out, in which the drug used by the mother induced liver injury in her newborn. The symptoms appeared at birth, associated with the placental pathway. After a favorable evolution, the newborn was inadvertently breastfed by the mother even after presenting symptoms of liver damage, compounded by the excretion of the drug through breast feeding. Propylthiouracil is considered safe in pregnant women, as long as the antithyroid dose is optimized to avoid fetal complications related to thyroid function. Even so, this case shows that it may be important for doctors accompanying pregnant women to attentively consider the hepatotoxic manifestations of drugs such as propylthiouracil, as well as understand their placental and breast milk excretion. In the abovementioned case, the injury pattern was cholestatic and the outcome was positive; another propylthiouracil case involving a hepatocellular injury, however, resulted in death. Histology for cases associated with this drug was compatible with that found in the literature, except for balloonization, which is characteristic of DILI and unrelated to propylthiouracil [66–68].
Carbamazepine is among the antiepileptic drugs most commonly associated with DILI. There are reports of cross-toxicity with phenytoin and lamotrigine. The injury often varies between the three types, but it is slightly more associated with chronic hepatitis; there are reports of ductopenia, eosinophilia, and granulomas, and, in some cases, steatosis [69,70]. In the Brazilian scenario, drug-induced liver injury in epileptic patients may have become a major issue. The need for combining various anticonvulsant medications can increase the odds of DILI; in fact, this study found that anticonvulsants were responsible for 28% of DILI cases and 57% of DILI deaths. Thus, it is abundantly clear that the more frequent use of hepatotoxic drugs by some specialties means that they must have precise knowledge of DILI.
Medicinal plants were responsible for three intoxication cases, two caused by the consumption of Senecio brasiliensis, and another by the consumption of Hypericum perforatum (also known as St. John's wort). Hepatotoxicity by herbal medicines may be more difficult to detect. Although the RUCAM algorithm can be used for both drugs and herbs, and is able to show the causality of a single chemical when used for drugs, the same is not true for herbs. Medicinal plants usually have many compounds, and properly investigating them is a much more involved process [7]. In the St. John's wort case, the authors suspected an interaction between Hypericum and copaiba oil (a resin oil extracted from the Copaifera sp. trunk and commonly used as an anti-inflammatory). However, they did not fully explain their suspicion and did not discuss other possibilities, such as levothyroxine, glucosamine, and chondroitin.
Our research group has recently published a Brazilian case report of liver transplantation by Kava [71]. The RUCAM resulted in a “probable” causality grading. The chemical profile of the sample used by the patient was investigated, and even though we were unable to identify the specific substance responsible for the toxicity, potential alternatives were successfully excluded, such as fungal contamination and likely hepatotoxic compounds.
In herb-induced liver injury (HILI), causality is generally harder to define than in DILI. Reports of hepatotoxicity by Senecio spp. in humans are rare, and we were unable to find any other Senecio brasiliensis human case, although veterinarian cases have been reported. This herb seems to have no important indication for medicinal use, and is already recognized as toxic [72]. In any case, doctors should always investigate the traditional use of medicinal plants. HILI must be suspected when the patient reports using medicinal teas, since some patients tend to omit or fail to recognize the use of certain herbs as potential health hazards. Brazil is a major consumer of plants for therapeutic purposes and, according to the World Health Organization, up to 80% of the world population uses herbal medicines, mainly in developing countries [73]. In Asian countries, there has been a 19 to 63% increase in HILI cases [74]. Some studies have already pointed to HILI as the main cause of liver injury [20,21].
In this review, medicinal plants accounted for 9% of the case reports. This is similar to Spain's HILI rates, but lower than the United States’, where plants and supplements are the second most frequent cause of liver injury, accounting for 16% of cases [16,17]. In Asian countries, plants and supplements are the leading cause of medicine-induced liver injury [14,20,21]. It is assumed that Brazil's rate may be higher than expected, due to the common consumption of medicinal plants, lack of knowledge about HILI, and underdiagnosis [10–12].
Only three studies reported using causality algorithms for DILI confirmation. This may be due to professionals lacking sufficient knowledge on this type of instrument. Algorithms are an important tool that can be helpful in collecting pertinent clinical information in order to accurately determine causality.
The use of causality algorithms is recommended by specialists, and the Roussel Uclaf Causality Assessment Method (RUCAM) is the most commonly applied variant [8,16,17,19,52]. Methods and strategies for detecting DILI should be widely disseminated, especially among general physicians, considering that, in most case reports, specialist follow-up showed that patients had developed chronic pathologies.
Most patients (84%) were diagnosed by exclusion of other diseases, without any mention of causality algorithms—the use of RUCAM retrospectively is not recommended, so causality cannot be determined post-diagnosis. This was a surprising result: although several suspected cases of hepatotoxicity were identified, the lack of information in these reports makes it impossible to determine causality. We believe this lack of information is due to subpar knowledge of DILI in Brazil, and also with the low quality and quantity of DILI researches in comparison to other countries. In both the medical and pharmaceutical fields, the country has no recognized DILI research groups, and, in this sense, the Latin American DILI research group is a welcome initiative—considering this study's results, we believe that Brazil could offer a much more significant contribution to DILI research. We are confident that the cooperation of Brazilian researchers with the LATINDILI network will favor the adequate use of RUCAM in case identification, so that future Brazilian cases can be integrated into the Latin American database.
Liver biopsy was reported in 47% of cases, and in some reports, it was referred to as an important confirmatory diagnostic method. However, considering DILI's inaccurate histological manifestations, which may lead to it being confused with primary liver and biliary diseases, there is no evidence that biopsy increases diagnostic accuracy. It is more commonly used for the exclusion of other diseases [74], and judiciously requested only for cases of greater severity and diagnostic difficulty. The RUCAM algorithm remains the best method for detecting DILI and determining its causality [75].
Some case reports lacked important information, such as: days elapsed since medication ingestion and symptom onset (n=1), days of hospitalization (n=13), and days until the normalization of hepatic enzymes (n=15). These data should always be collected, since it contains important pieces of information for the determination of causality, epidemiological and economic impacts on the hospital network, and patient prognosis.
Medications associated with hepatotoxicity are included in the Brazilian List of Essential Medicines, which makes them accessible by most of the population. Although widely studied and known, these drugs can still pose risks. On the other hand, as their use increases, more information about these risks can be gathered and reported.
Prescription-free drugs, such as acetaminophen, have also been associated with hepatotoxicity. There are cases of acetaminophen use related to newborn fever and overdoses due to the prescription not being adjusted to the patient's weight. Pharmacist intervention during dispensation could prevent this type of problem, especially in Brazil, where the population has a large number of pharmacies at its disposal, but difficult access to the health system [76–78]. In this context, it is important to stimulate pharmacists to develop clinical and pharmacovigilance skills, mainly in community pharmacies. When pharmacists suspect DILI, they must refer the patient to a medical emergency service.
The promotion of health information to patients during drug dispensation is of great importance, since it can contribute to damage reduction and help include them in systems for pharmacovigilance and monitoring of adverse drug effects. In an Italian study, after a pharmaceutical intervention, the participation of patients in reports of adverse effects increased from 0% to about 7% [79]. In this regard, special attention should be given to prescription-free drugs, anti-inflammatory drugs, and medicinal plants, since these are easily accessible, widely used, and associated with high rates of self-medication [76].
Knowledge on relevant drugs, dosages, patterns of liver damage, and laboratory abnormalities is essential for the early detection of DILI cases, which can limit its impacts. A proactive stance by clinical pharmacists alongside health teams can favor early detection [80].
5ConclusionThe worryingly low number of DILI case reports and the lack of knowledge on the topic stand out as this study's most relevant findings, showing the need to stimulate Brazilian DILI research and further the interactions between Brazilian researchers and the Latin American DILI network.
The populational profile of DILI case reports in Brazil is comprised of young males with chronic diseases. Despite the low number of reports, severe outcomes occurred in almost a third of the cases, pointing to a clear need for the early detection and management of DILI occurrences.
To this end, it is essential to disseminate knowledge on liver injury-inducing medications, DILI signs and symptoms, histology, serology of liver diseases, and the temporal relationship between drug administration and symptom onset. Moreover, RUCAM is an essential instrument in the confirmation of suspected induced liver injury cases. Strengthening the culture around DILI diagnosis and notification can increase professional and public knowledge, promoting drug safety in Brazil and worldwide.AbbreviationsALT alanine aminotransferase alkaline phosphatase Coordination for the Improvement of Higher Education Personnel drug-induced liver injury herb-induced liver injury Roussel Uclaf Causality Assessment Method
The authors have no conflicts of interest to declare.