The availability of curative hepatitis C therapies has created an opportunity to improve treatment delivery and access. Local providers, government, industry, and community groups in Prince Edward Island developed an innovative province-wide care model. Our goal was to describe the first year of program implementation.
Material and methodsUsing a community-based prospective observational study design, all chronic hepatitis C referrals received from April 2015 to April 2016 were recorded in a database. Primary analysis assessed the time from referral to assessment/treatment, as well as the number of referrals, assessments, and treatment initiations. Secondary objectives included: 1) treatment effectiveness using intention-to-treat analysis; and 2) patient treatment experience assessed using demographics, adverse events, and medication adherence.
ResultsDuring the study period 242 referrals were received, 123 patients were seen for intake assessments, and 93 initiated direct-acting antiviral therapy based on medical need. This is compared to 4 treatment initiations in the previous 2 years. The median time from assessment to treatment initiation was 3 weeks. Eighty-two of 84 (97.6%, 95% CI 91.7 - 99.7%) patients for whom outcome data were available achieved sustained virologic response at 12 weeks post-treatment; 1 was lost to follow-up and 1 died from an unrelated event. In the voluntary registry, 39.7% of patients reported missed treatment doses.
ConclusionIn conclusion, results from the first 12 months of this multi-phase hepatitis C elimination strategy demonstrate improved access to treatment, and high rates of safe engagement and cure for patients living with chronic hepatitis C genotype 1 infections.
Chronic hepatitis C virus (HCV) infection is an epidemic that increases all-cause mortality and is a leading cause of liver failure, liver transplantation, and hepatocel-lular carcinoma.1-3 Treatment and subsequent cure of HCV infection is associated with reduced risk of liver failure, hepatocellular carcinoma, and a reduction in all-cause mortality.4-7 Recently approved direct-acting antiviral (DAA) therapies have the potential to change HCV care from epidemic management to disease elimination. However, to realize the potential of these therapies, realignment of clinical resources and care models must occur to facilitate greater patient access to care.8 Criteria for access to HCV therapy are currently widely heterogeneous, and often restrict treatment access for those with minimal fibrosis or persons with a recent history of alcohol or substance abuse.9,10 Modelling studies have demonstrated that improved treatment access, especially in high risk groups, may prevent incident HCV infections and reduce the prevalence of HCV infection.11,12 HCV care is ideally provided in a comprehensive multi-disciplinary model including experienced physicians, nurses, clinical psychologists, and those with expertise in addiction management.13 Currently in Canada, a limited number of specialist physicians are treating the majority of HCV infections, limiting patient access to curative therapy. Models to expand, expedite, and facilitate HCV care, especially in marginalized populations and unders-erved areas, are greatly needed.
Prince Edward Island (PEI) has a population of approximately 148,000 persons14 with an estimated HCV prevalence of 0.43%.15 The small and geographically defined island population was identified as a key strength in creating a proof-of-principle, comprehensive HCV treatment program. Our goal was to describe program implementation in the first 12 months by assessing access to care and wait times, as well as determine if real world rates of HCV cure in this nurse-led model were comparable to DAA registration trials.
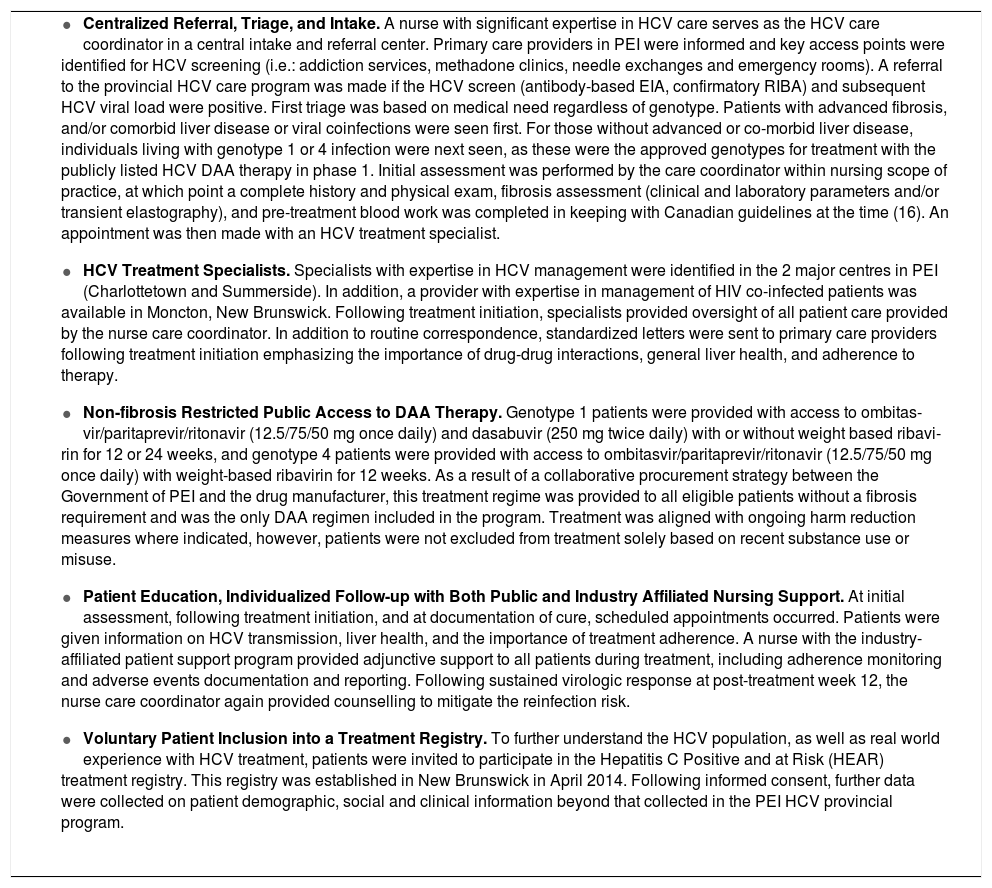
Material and MethodsProgram implementationBetween September 2014 and October 2015 local care providers, government, industry, and HCV community groups in PEI created a province-wide model of care. A program based on this model was then launched in April of 2015 across the province. Core components of the program included: centralized referral, triage, and intake by a nurse care coordinator; HCV treatment specialists; non-fi-brosis restricted access to DAA therapy; patient education and individualized follow-up; and voluntary treatment registry enrollment (Table 1).
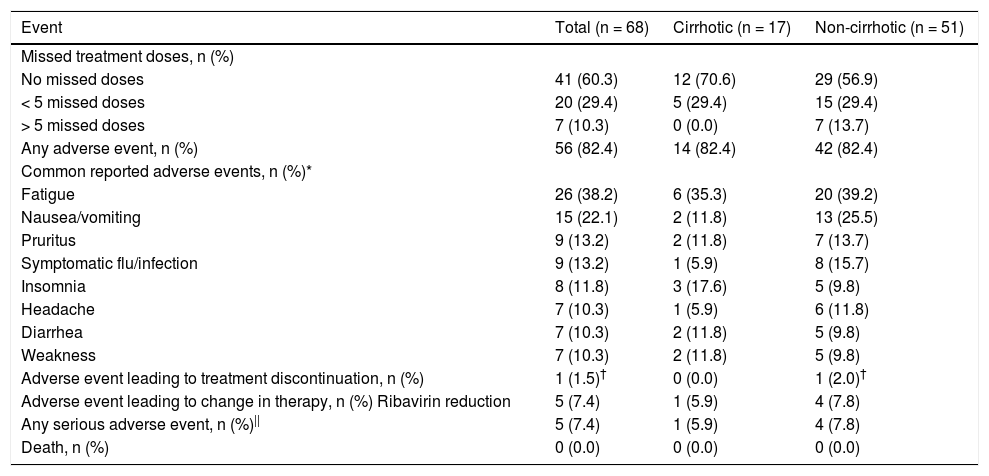
Core components of the PEI HCV care model.
|
HCV: hepatitis c. PEI: Prince Edward Island. EIA: enzyme immuno-assay. RIBA: recombinant immunoblot assay. DAA: direct-acting antiviral. HIV: human immunodeficiency virus.
A nurse with significant expertise in HCV care was designated to serve as the HCV care coordinator in a central intake and referral center. Primary care providers in PEI were informed of the program and key access points were identified for HCV screening (i.e.: addiction services, methadone clinics, needle exchanges and emergency rooms). A referral to the provincial HCV care program was made if the HCV screen (antibody-based EIA, confirmatory RIBA) and subsequent HCV viral load were positive. Patient stratification for intake assessment was performed according to principles previously described.13 First triage was based on medical need regardless of genotype. Patients with advanced fibrosis, and/or comorbid liver disease or viral coinfections were seen first. For those without advanced or comorbid liver disease, individuals living with genotype 1 or 4 infection were next seen, as these were the approved genotypes for treatment with the publicly listed HCV DAA therapy in phase 1 of the program.
Initial assessment was performed by the care coordinator within a nursing scope of practice, at which point a complete history and physical exam, fibrosis assessment, and pre-treatment blood work was completed in keeping with Canadian guidelines at the time.16 An appointment was then made to an HCV treatment specialist. As a result of a collaborative procurement strategy between the Government of PEI and the drug manufacturer, treatment was provided free of charge to all eligible patients without a fibrosis requirement. Genotype 1 patients were provided with access to ombitasvir/paritaprevir/ritonavir (12.5/75/50 mg once daily) and dasabuvir (250 mg twice daily) with or without weight based ribavirin for 12 or 24 weeks, and genotype 4 patients were provided with access to ombitas-vir/paritaprevir/ritonavir (12.5/75/50 mg once daily) with weight-based ribavirin for 12 weeks. Therapy was aligned with ongoing harm reduction measures where indicated, however, patients were not excluded from treatment solely based on recent substance use or misuse.
Individualized patient education and follow-up care was delivered by the nurse care coordinator at scheduled visits with oversight provided by HCV treatment specialists. Patients were given information on HCV transmission, liver health, and the importance of treatment adherence. Following sustained virologic response (SVR) at post-treatment week 12, the nurse care coordinator again provided counselling to mitigate the reinfection risk.
Data collection and analysisAll referrals to the program were prospectively recorded in the provincial HCV treatment database. For the community-based prospective observational study, chronic HCV referrals received from April 2015 to April 2016 were identified by the nurse care coordinator for outcome assessment. De-identified information was extracted from the provincial database and included referral and visit dates, patient demographics, disease characteristics and treatment outcomes. Missing information was abstracted from HCV provider charts by the care-coordinator prior to de-identification. The date of referral was defined as the date the referral was received, or as April 1, 2015 if received prior to the program start date. Wait times were estimated by time from referral to assessment, and from assessment to treatment initiation. The number of referrals, assessments, and treatment initiations during the initial 12-month period were reported using basic proportions.
The secondary analysis measured treatment effectiveness among all treated patients in the provincial HCV program using intent-to-treat analysis. All patients who received at least 1 dose of therapy were included in the analysis of effectiveness. SVR12 is considered an HCV cure, and was defined as HCV RNA negativity by PCR at 12 weeks following treatment completion. SVR12 rates were analyzed overall, by genotype and cirrhotic status. Transient elastography (FibroScan, Echosens, Paris) was used to estimate METAVIR fibrosis stage.17 Ninety-five percent confidence intervals of the binomial proportions were calculated using the Clopper-Pearson method.
The community treatment experience, including detailed demographics and patient-reported adherence/ tolerability, was assessed in treated patients consenting to participate in the hepatitis C positive and at Risk (HEAR) voluntary treatment registry. All patients seen for intake assessment by the nurse care coordinator were invited to participate, and informed consent in writing was obtained. Consented patients initiated on treatment between April 2015 and April 2016 were defined for analysis. Standardized patient and provider-completed study forms were filled out at the initial assessment and subsequent visits. Baseline demographic data, disease characteristics, treatment-emergent adverse events and HCV therapy adherence were extracted from the HEAR database and reported using proportions and means, as appropriate. Missing data was obtained, with permission, from electronic medical records. Patient-reported treatment-emergent adverse events and medication adherence was documented by the nurse care coordinator during a treatment-completion visit. All cases were reconciled with source charts and the industry-sponsored patient support program reporting of adverse events. Adverse events were assessed independently by 2 investigators for severity and relation to DAA therapy, and only adverse events associated with new or worse symptoms during the treatment period were recorded.
The study protocol was approved by the research ethics committees of both Health PEI (PEI, Canada) and Horizon Health Network (NB, Canada), and all research procedures were followed in accordance with the Canadian Government Tri-Council Policy Statement on Human Research (TCPS2) and the Helsinki Declaration of 1975, as revised in 2000.
ResultsPEI HCV treatment program- •
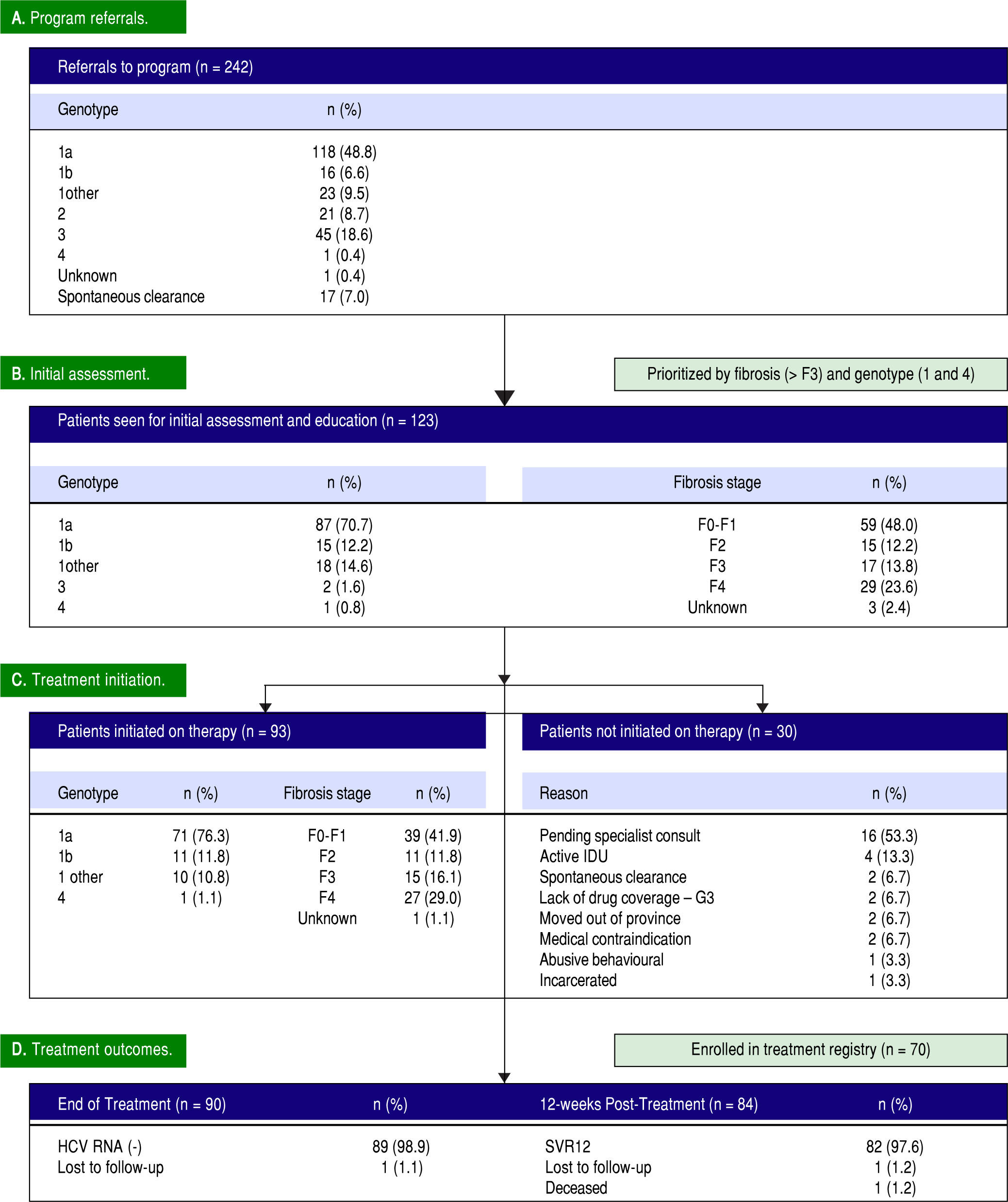
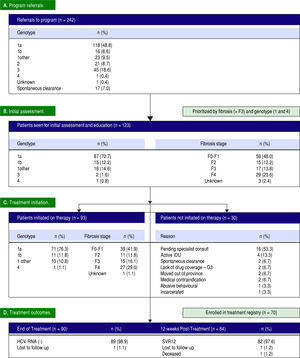
Efficient triage and access to care. During the first year of the PEI HCV Treatment Program, 242 referrals were received. The referred cohort consisted of 118/ 242 (48.8%) genotype 1a infections, followed by genotype 3 with 45 (18.6%) referrals (Figure 1). In total, 123/242 (50.8%) patients were seen for an initial assessment and education session by the nurse care coordinator. Of the assessed patients, 120 (97.6%) patients had genotype 1 infections, 29 (23.6%) had cirrhosis and 24 (19.5%) were treatment experienced with an interfer-on-based regimen. The median time from program referral to intake assessment was 7 weeks. In the first 12 months, 49/123 (39.8%) assessed patients had referrals received on or before April 1, 2015 (i.e.: the program start date).
Thirty of the 123 patients seen for an initial assessment were not started on therapy (Figure 1). The majority, 16/30 (53.3%), were anticipated to start therapy pending an HCV specialist consult. Other reasons for not initiating therapy included: active injection drug use with perceived or reported medication non-adherence (4/30, 13.3%); spontaneous virus clearance (2/30, 6.7%); moved out of province (2/30, 6.7%); incarcerated (1/30, 3.3%); abusive behaviour (1/30, 3.3%); pregnancy (1/30, 3.3%); and Crohn's disease exacerbation (1/30, 3.3%). The median time from assessment to treatment initiation was 3 weeks. In total, 93 patients were initiated on therapy in the first 12 months including 42 of the 46 patients (91.3%) assessed as F3 or F4/cirrhotic (Figure 1). Eighty-six of the 93 (92.4%) treated patients received 12 weeks of ombitasvir/paritaprevir/ritonavir and dasabu-vir with ribavirin 79/93 (84.9%) or without ribavirin 7/ 93 (7.5%). Six patients (6.5%) were cirrhotic and previous treatment null-responders with genotype 1a or non-subtypable genotype 1 infections, and received 24 weeks of ombitasvir/paritaprevir/ritonavir and dasabu-vir with ribavirin as per the Canadian product monograph. The single genotype 4 infection referred to the program (1.0%) was treated with 12 weeks of ombitas-vir/paritaprevir/ritonavir with ribavirin. Only 1 individual was lost to follow-up with the HCV nurse care coordinator. The remainder of individuals were engaged in care for the duration of treatment and 12 weeks of follow-up beyond treatment.
- •
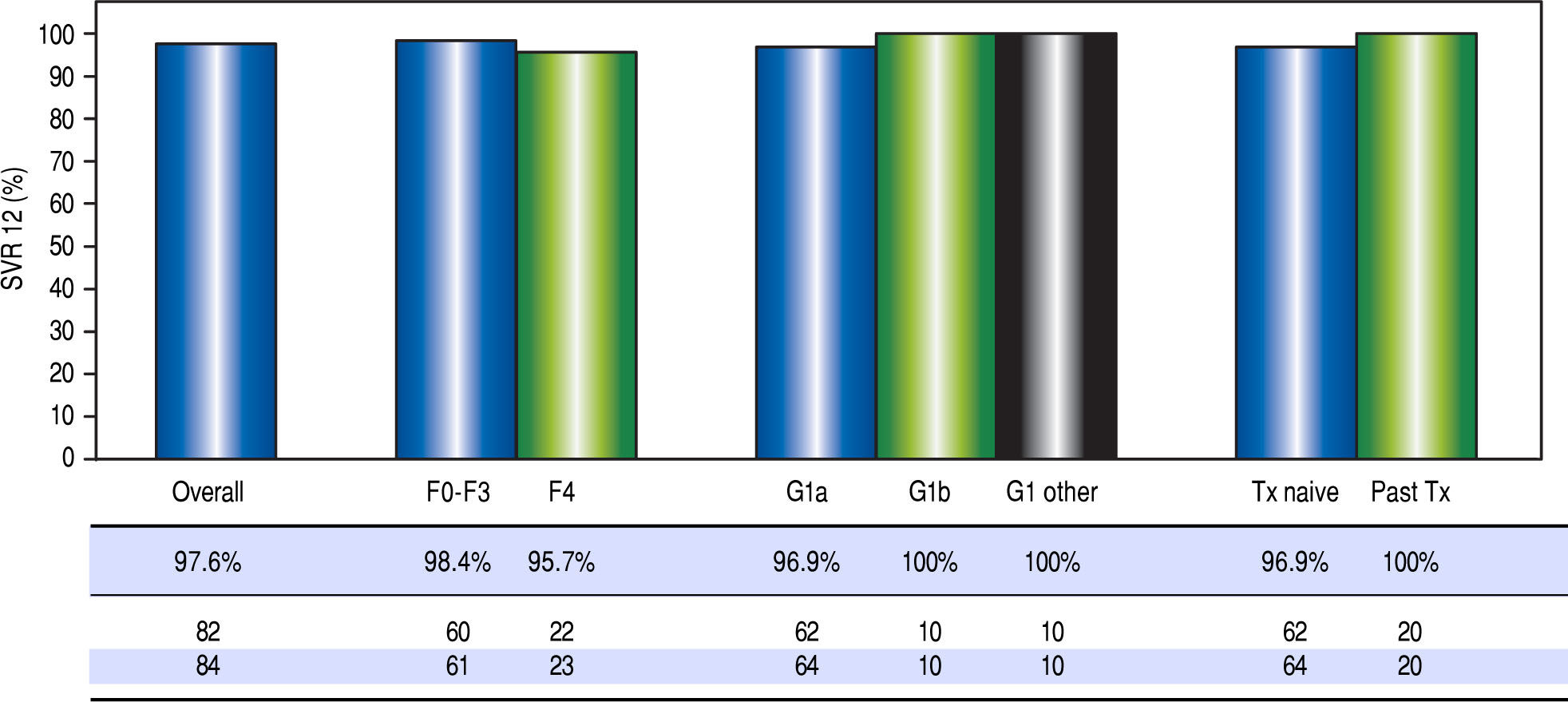
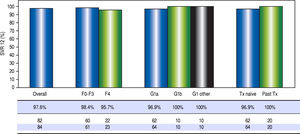
High rates of HCV cure, even in a population enriched for cirrhotic patients. A total of 84 patients had reached 12 weeks post-treatment with SVR12 results at the time of analysis, with the remaining 9 individuals still on active treatment or awaiting SVR12 results (Figure 1). In the intention-to-treat analysis, 82 (97.6%, 95% CI 91.7 - 99.7%) achieved SVR12. No vi-rologic failures were observed; however, 1 patient was lost to follow-up and another died following the end of treatment of an unrelated event. Sub-analysis by genotype demonstrated SVR12 rates of 96.9% (95% CI 89.2-99.6%) in genotype 1a, 100% (95% CI 69.2 - 100.0%) in genotype 1b and 100% (95% CI 69.2-100.0%) in non-subtypable genotype 1 infections (Figure 2). By fibro-sis stage, 95.7% (95% CI 78.1 - 99.9%) of cirrhotic patients and 98.4% (95% CI 91.2 - 100.0%) of non-cir-rhotic patients achieved SVR12. Among treatment naïve patients 96.9% (95% CI 89.2 - 99.6%) and 100.0% (95% CI 83.2 - 100%) of treatment experienced achieved SVR12 (Figure 2). All patients with SVR12 bloodwork were cured following treatment.
Figure 2.Virologic response. Sustained virologic response at post-treatment week 12 among patients treated in the first 12 months of the PEI HCV treatment program (n = 84). Individual proportions are presented. Patients received: 12 weeks paritaprevir/ritonavir/ombitasvir/dasabuvir with ribavirin (n = 73) or without ribavirin (n = 7); 24 weeks paritaprevir / ritonavir / ombitasvir / dasabuvir with ribavirin (n = 4). ^ One loss to follow-up; 1 unrelated death after therapy. SVR12: sustained virologic response at post-treatment week 12. Tx: treatment.
(0.14MB).
From April 2015 to April 2016, a total of 70 treated patients were enrolled in the HEAR voluntary treatment registry.
- •
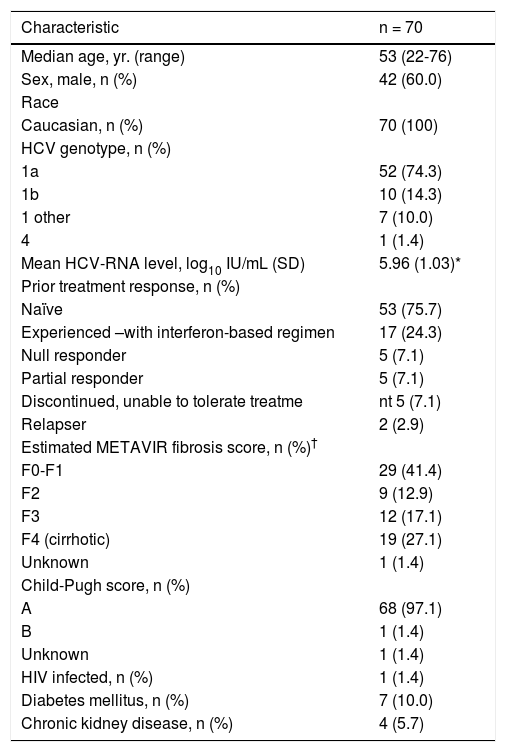
Patient characteristics. Baseline patient characteristics are shown in Table 2. A majority of the patients were male (60.0%) and infected with HCV genotype 1a (74.3%). A total of 19 patients (27.1%) were cirrhotic and 31 (44.3%) had stage F3 or greater fibrosis. Of the cirrhotic patients, only 1/19 (5.3%) patients had Child-Pugh B liver disease; the remaining were Child-Pugh A. Seventeen patients (24.3%) had received previous HCV treatment, all with interferon-based regimens without the use of DAAs. The majority (10/17, 58.8%) of previously treated patients had no response or a partial virologic response to previous therapy, 5 (29.4%) discontinued therapy due to side effects and 2 (11.8%) had relapsed after treatment. The registry-enrolled cohort included 1 (1.4%) person with HIV co-infection, 7 patients (10.0%) with diabetes mellitus and 4 patients (5.7%) with renal comorbidities (Table 2).
Table 2.Demographics and baseline disease characteristics of HEAR registry enrolled patients.
Characteristic n = 70 Median age, yr. (range) 53 (22-76) Sex, male, n (%) 42 (60.0) Race Caucasian, n (%) 70 (100) HCV genotype, n (%) 1a 52 (74.3) 1b 10 (14.3) 1 other 7 (10.0) 4 1 (1.4) Mean HCV-RNA level, log10 IU/mL (SD) 5.96 (1.03)* Prior treatment response, n (%) Naïve 53 (75.7) Experienced –with interferon-based regimen 17 (24.3) Null responder 5 (7.1) Partial responder 5 (7.1) Discontinued, unable to tolerate treatme nt 5 (7.1) Relapser 2 (2.9) Estimated METAVIR fibrosis score, n (%)† F0-F1 29 (41.4) F2 9 (12.9) F3 12 (17.1) F4 (cirrhotic) 19 (27.1) Unknown 1 (1.4) Child-Pugh score, n (%) A 68 (97.1) B 1 (1.4) Unknown 1 (1.4) HIV infected, n (%) 1 (1.4) Diabetes mellitus, n (%) 7 (10.0) Chronic kidney disease, n (%) 4 (5.7) Viral loads recent within 6 months of assessment not available for 7 patients, therefore excluded from calculation. † The fibrosis score was determined by means of Fibroscan (Echosens). HCV: hepatitis C virus. HIV: human immunodeficiency virus. SD: standard deviation.
- •
High rates of cure despite less than optimal adherence. Although treatment was generally well-tolerated, only 41 out of 68 (60.3%) patients reported no missed doses (Table 3). The majority (20/27; 74.1%) of patients who did report missing a dose, missed less than 5. The 7 patients missing greater than 5 treatment doses were all non-cirrhotic. However, despite the less than optimal adherence, all patients who had SVR12 bloodwork were cured.
Table 3.Adverse events and adherence during the treatment period of HEAR registry enrolled patients.
Event Total (n = 68) Cirrhotic (n = 17) Non-cirrhotic (n = 51) Missed treatment doses, n (%) No missed doses 41 (60.3) 12 (70.6) 29 (56.9) < 5 missed doses 20 (29.4) 5 (29.4) 15 (29.4) > 5 missed doses 7 (10.3) 0 (0.0) 7 (13.7) Any adverse event, n (%) 56 (82.4) 14 (82.4) 42 (82.4) Common reported adverse events, n (%)* Fatigue 26 (38.2) 6 (35.3) 20 (39.2) Nausea/vomiting 15 (22.1) 2 (11.8) 13 (25.5) Pruritus 9 (13.2) 2 (11.8) 7 (13.7) Symptomatic flu/infection 9 (13.2) 1 (5.9) 8 (15.7) Insomnia 8 (11.8) 3 (17.6) 5 (9.8) Headache 7 (10.3) 1 (5.9) 6 (11.8) Diarrhea 7 (10.3) 2 (11.8) 5 (9.8) Weakness 7 (10.3) 2 (11.8) 5 (9.8) Adverse event leading to treatment discontinuation, n (%) 1 (1.5)† 0 (0.0) 1 (2.0)† Adverse event leading to change in therapy, n (%) Ribavirin reduction 5 (7.4) 1 (5.9) 4 (7.8) Any serious adverse event, n (%)|| 5 (7.4) 1 (5.9) 4 (7.8) Death, n (%) 0 (0.0) 0 (0.0) 0 (0.0) - •
No serious medication-associated adverse events. Only 1 patient discontinued treatment from an adverse event and it was deemed to be unrelated therapy; a cir-rhotic genotype 1a infected patient stopped all HCV treatment during week 7 due to nausea that was later attributed to a mesenteric arterial stent occlusion. In this case, SVR12 was still achieved without additional treatment. The majority of patients (56/68; 82.4%) reported experiencing at least 1 adverse event while on treatment (Table 3). The most common adverse events reported were fatigue (26/68; 38.2%), nausea/vomiting (15/68; 22.1%) and pruritus (9/68; 13.2%). Five patients (7.1%) required a reduction in ribavirin dose due to anemia or associated symptoms but all of these patients did subsequently achieve SVR12. No other dose modifications or changes in HCV therapy were reported. There were no statistically significant differences (p < 0.05) in adverse events between cirrhotic and non-cirrhotic patients. Serious adverse events during the treatment period included: psychiatric admission, pneumonia, allergic reaction to an unrelated medication, mesenteric arterial stent occlusion and rise in blood glucose of a diabetic patient requiring intravenous fluids in hospital. No serious adverse event was assessed as being related to HCV therapy by investigators. No deaths occurred in this cohort while on treatment (Table 3).
PEI was the first province in Canada to implement a multi-phase open access HCV elimination strategy. Two hundred forty patient referrals, 123 initial assessments, and 93 treatment initiations occurred in the first 12 months, compared to only 4 treatment initiations in the province in the 2 years prior to program implementation (Health PEI, unpublished data). Treatment was well-tolerated with a high rate of virologic cure and no treatment failures have been documented to date.
Timely access to intake assessment was identified as a challenge in the first year of the program. The median time from referral to an intake visit was 7 weeks, and only half of referrals received were seen for assessment. However, almost 40% of intake visits stemmed from referrals to HCV providers that predated the program start date. This high volume of referrals at the onset of the program suggests that a substantial number of known HCV positive individuals on PEI were previously unwilling or unable to access treatment. Expanded access to treatment in-province with this model and improved tolerance/efficacy of novel DAAs are both likely contributing factors. No data currently exists on the total number of PEI residents seeking treatment in previous years or HCV referral practices of PEI providers. The significant burden of clinical, research and administrative responsibilities on a single nurse care coordinator may have also limited the number of referred patients that could be seen for intake assessment and subsequently treated. An internal review of infrastructure and staffing needs is underway to optimize capacity in future iterations of the program.
The model of care established in PEI recognized the limited availability of subspecialty HCV providers, as is often the case throughout Canada.16 Centralized intake, triage, and assessment of patients by a nurse coordinator in this program significantly reduced the demands on consultant HCV physicians. While program initiation was concurrent with novel access to all oral curative regimens in PEI, the new model of care likely contributed significantly to the large increase in treated patients in PEI compared to previous years. However, even in this enhanced model, access to subspecialist care for the patient with non-advanced liver disease living with HCV remains a rate-limiting step to HCV care and public elimination.
In the first year of the program, referrals were triaged for assessment based on:
- •
Medical necessity and then;
- •
Genotype, with priority given to genotypes that were approved for treatment with the single publicly listed HCV DAA regime.
Although treatment was available in a non-fibrosis restricted fashion, close to half of patients treated in the first year had F3 or F4 level disease, in keeping with principles of medical necessity as the first stratifying factor. The close follow-up of this patient population by a nurse-coordinator, with specialist availability when needed, demonstrates how care can be safely provided in the community setting, and how similar models may be used to expand care access across Canada, especially in areas with a limited number of specialist prescribers.
In phase 1, the program provided access to 1 HCV DAA regimen used for treatment of HCV genotypes 1 and 4. However, the development of a treatment registry revealed a moderate size cohort of genotype 2 and genotype 3 patients in need of treatment. Since the program inception, further advances in HCV care have occurred and better treatment options now exist for other HCV genotypes.18-20 Program expansion to include treatment options for these patients is planned.
The cohort was inclusive of a significant proportion of cirrhotic patients as well as those with various medical co-morbidities including diabetes, HIV co-infection, and chronic kidney disease. An overall SVR12 of 97.6% is highly reassuring, with no virologic failures noted to date. The high rates of virologic cure are in keeping with those seen in registration trials,21,22 and while the cohort is currently of moderate size, it is reassuring to see the high HCV cure rates in a real world Canadian setting. Ribavirin dose modification was required in only 7% of cases, with no patient discontinuing therapy owing to adverse events associated with ribavirin including anemia. This is also consistent with controlled trials,21,22 and supports the safe use of ribavirin-containing DAA regimes with minimal negative consequences in the community setting. The loss of only a single patient to follow-up is also notable and reflects the keen patient engagement into this nurse-directed care program.
Elimination of a chronic HCV infection requires treatment of those with medical need due to advanced liver disease at the same time as treatment for those who have the potential to transmit the infection.13 For this reason, the lack of fibrosis restriction is very important in the context of the PEI model. In Canada, the majority of HCV incident infections now occur in persons who use drugs through the sharing of injecting equipment,15 and targeted treatment of this cohort is both safe and essential to achieve meaningful impact on community HCV transmis-sion.11,23,24 A concurrent study of treatment of incarcerated persons in PEI (NCT02460133) aims to further establish the feasibility of treating another core transmitter group and will aid in providing DAA access to higher risk persons in the province. In addition to treatment scale-up, the comprehensive care model in PEI also facilitates expanded HCV testing at key access points (i.e.: addiction services, methadone clinics, needle exchanges and emergency departments) through patient/provider education and resources. Ongoing enhancement of the HCV cascade of care, with structured program evaluation, will expedite elimination goals in PEI.
Early phase 1 results from the first 12 months of this multi-phase HCV elimination strategy demonstrate improved access to HCV care, and high rates of safe engagement and cure for patients living with chronic HCV genotype 1 infection. It is hoped that similar models of care throughout Canada will facilitate broader access to curative HCV therapies, especially in under-resourced settings.
Abbreviations- •
DAA: direct-acting antiviral.
- •
HCV: hepatitis C virus.
- •
HEAR: hepatitis C positive and at risk.
- •
PEI: Prince Edward Island.
- •
SVR: sustained virologic response.
Health Research Foundation of Innovative Medicines Canada.
Dalhousie Medical Research Foundation (DMRF) La-lia B. Chase Studentship Grant.
Dalhousie Medicine New Brunswick Student Research Program Studentship Grant.
Conflict of InterestDisclosure of Potential Conflicts of Interest. Annex 1.