Background and rationale for the study. FGF19/15 is a gut-derived hormone presumably governing bile acid (BA) synthesis and gallbladder (GB) refilling. FGF19 mRNA is present in human GB cholangiocytes (hGBECs); however, the physiological significance of GB-derived FGF19 remains unknown. We investigated whether hGBECs secrete FGF19 and the effects of cholecystectomy on serum FGF19 ([FGF19]s) and BA synthesis.
Material and methods. FGF19 expression was assessed by qRT-PCRs and immunostaining in hGBECs and terminal ileum, and quantified in bile and serum by ELISA. Basal and BA (chenodexycholic acid, CDCA) induced FGF19 expression and secretion was analyzed in primary cultured hGBECs and GB-d1 cell line. Pre and postprandial serum changes in [FGF19]s, 7α-hydroxy-4-cholestene-3-one (C4, a marker of BA synthesis) and BA were evaluated in plasma of gallstone disease patients at baseline and after cholecystectomy.
Results. FGF19 mRNA levels were -250-fold higher in hGBECs compared to distal ileum. GB bile contained -23-fold higher FGF19 levels compared to serum (p < 0.0001). CDCA induced dose-dependent expression and secretion of FGF19 in hGBECs and GB-d1 cells. Cholecystectomy increased plasma BA synthesis ≥ 2-fold (p < 0.0001), and altered the diurnal rhythm and significantly reduced [FGF19]s noon peak. BA serum levels, serum cholesterol and triglyceride content remained unchanged.
Conclusions. In conclusion human GB cholangiocytes constitutively express and secrete high levels of FGF19 in a process regulated by BA. Resection of this organ doubles BA synthesis concomitantly with changes in [FGF19]s. These findings suggest a potential connection between GB cholangiocytes-derived FGF19 and BA metabolism that could lead to metabolic dysregulation following cholecystectomy.
- •
FGF19 is highly expressed and secreted in human gallbladder and its secretion is stimulated by chenodeoxyxholic acid, a natural farnesoid X receptor ligand.
- •
In subjects with cholesterol gallstone disease, gallbladder cholangiocytes FGF19 expression is significantly reduced.
- •
Cholecystectomy modifies serum FGF19 diurnal rhythm by inducing a significant reduction on its noon peak. These changes are associated with a two fold increase in C4, a bile acid synthesis biomarker.
- •
Taken together these findings suggest gallbladder has an important function in BA homeostasis regulation, probably mediated by FGF19 secretion.
The human fibroblast growth factor 19 (FGF19) is considered an atypical member of the fibroblast growth factor protein family. Similar to FGF21 and FGF23, FGF19 shows reduced affinity to bind heparin, which allows it to diffuse and act as an endocrine hormone.1 FGF19 functions have been mainly explored on its mouse ortholog Fgf15.2–4
In gut this protein is produced mainly by ileum enterocytes, where its expression is induced by bile acids (BA) signaling through the farnesoid X receptor (FXR). Ileum derived FGF19/Fgf15 is presumably transported to the liver through the portal system where it ultimately suppresses BA synthesis.2,5 Administration of high doses of recombinant FGF19 to human hepatocytes, or to mice, inhibits BA synthesis through repression of cholesterol 7-alpha-hydroxylase (CYP7A1), the first and rate-limiting enzyme in the classical BA biosynthetic pathway.6 In addition, FGF19/Fgf15 stimulates gallbladder (GB) refilling in mice by a cAMP mediated smooth muscle cells relaxation.3 In humans, correlative evidence arising from a few recent studies supports a fundamental role of FGF19 as a gut derived hormone controlling BA synthesis.7–10 While the above data strongly support that FGF19/Fgf15 is a complementary pathway participating in the postprandial negative feedback loop for BA homeostasis and GB motility, more data is needed to obtain more insight about the role of FGF19 as metabolic regulator in humans.11
Recent data suggest that FGF19 may also be involved in the modulation of metabolic syndrome.12,13 Animal models exposed to supra physiologic levels of recombinant FGF19 show reduced body weight and adiposity, and are less prone to develop dyslipidemia, hepatic steatosis, hyperinsulinemia, hyperleptinemia and insulin resistance.14,15 Mechanisms known to participate include increased energy expenditure and fatty acid oxidation. Thus, FGF19 mediated pathways could play key roles in human metabolism beyond BA homeostasis.16
FGF19 was first cloned and described by Xie, et al., who demonstrated by in situ hybridization its mRNA transcripts in a variety of human tissues including adult GB epithelium.17 Since cholangiocytes constitute an essential part of the entero-hepato-biliary axis with relevant implications in gastrointestinal physiology we aimed to:
- •
Explore if FGF19 is synthesized and secreted by human GB cholangiocytes.
- •
Investigate whether FGF19 synthesis and secretion in human cholangiocytes is regulated by the FXR natural ligand chenodeoxycholic acid (CDCA), and
- •
Explore the effect of cholecystectomy (CCT) on FGF19 serum levels and BA metabolism.
In addition, we evaluated whether the expression level of FGF19 in normal GBs differs from GBs of patients with cholesterol gallstones, the most prevalent biliary disease in western countries.
Material and MethodsCollection of human samplesInformed consent was obtained from all subjects included in this study. Experiments using human samples were conducted according to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board of the Faculty of Medicine at the Pontificia Universidad Católica de Chile. We collected biopsies from macroscopically normal duodenal (2nd to 3rd segment; n = 14) and terminal ileum mucosa (5 to 10 cm proximal to the ileocecal valve, n = 15) donated by subjects in outpatient clinic undergoing endoscopy or colonoscopy for symptoms of common non-malignant and non-inflammatory diseases. Four biopsies per patient were taken; one was fixed in formalin and examined histologically and 3 were snap frozen in liquid nitrogen and maintained at -80 °C until further analysis. Cases with histopathological alterations were excluded from the study.
Human GB tissue and bile specimens were obtained from non-obese adult subjects undergoing elective CCT essentially as described.18 We recruited prospectively recruited 8 patients with cholesterol gallstone disease (GSD) and 8 control GSD-free subjects who underwent incidental CCT during liver (focal lesions), pancreatic (tumors) or gastric (gastric cancer) surgery. Subjects with clinical or laboratory evidence of hepatic, kidney or intestinal disease, or taking medications known to alter lipid metabolism were excluded. Additional exclusion criteria for GSD patients were presence of large gallstones (> 20 mm), multiple gallstones filling more than 30% of GB lumen or recent event of biliary-related pain (< 1 week from recruitment). Fragments of the freshly excised GBs were frozen in liquid nitrogen and stored at -80 oC until use. Additional fragments were fixed in formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin-eosin. Most of GB specimens were used to isolate primary hGBECs through a combined enzymatic/mechanical procedure as previously described.19 Microscopic examination of an aliquot of freshly isolated hGBECs revealed that more than 95% of cells had epithelial features. HGBECs were frozen in liquid nitrogen and kept at -80 °C for subsequent protein and RNA extraction as described below. Some fresh harvested hGBECs samples were immediately processed for primary culture experiments.
Cell isolation, culture and treatmentFresh hGBECs were isolated from gallstone free subjects and cultured as detailed elsewhere.20 GB-d1 cells, a human gallbladder carcinoma derived cell line21 (generously provided by Dr. Juan Carlos Roa, Universidad de la Frontera, Temuco, Chile), were cultured in the same conditions as described for hG-BEC. For details see Supplementary Materials. To study the role of FXR in the regulation of FGF19, hGBEC and GB-d1 cells were treated with CDCA, a potent natural ligand of FXR.22 All media were supplemented with 10% FBS unless otherwise specified. Cells were first cultured with BA-depleted medium (DMEM with 10% FBS charcol-dextran) for 16 hrs. Subsequently, the culture medium was changed and cells were incubated for 24 h with 50 or 100 CDCA in 0.1% DMSO. Control cells were incubated with vehicle only. Then, cells were harvested for immunostaining and reporter gene assays. The supernatant was kept at -80°C for FGF19 quantification. For details see Supplementary Material and Methods section.
Prospective follow up study in patients with GSD undergoing elective cholecystectomyTo evaluate the effect of GB resection on FGF19 serum levels and BA metabolism, a cohort of 10 consecutive GSD patients undergoing elective CCT and meeting the inclusion criteria (see Collection of human samples) were included. Patients underwent a functional ultrasound GB study, as described elsewhere.23 Only patients with normal GB emptying capacity (ejection fraction > 50%), were included. Resected GB presented only minor-to-moderate chronic cholecystitis on biopsy and cholesterol gallstones as defined by cholesterol content of > 60% of dry weight. All subjects were studied at baseline (visit 1), 14 days (visit 2) and 90 days after CCT (visit 3). Each time, subjects were evaluated after overnight fasting and received a standardized breakfast (Ensure plusMR, 355 Kcal, 50.5 g carbohydrates, 11 g fat and 13.5 g protein) (AbbottMR, IL, USA) and lunch (501 Kcal, 85 g carbohydrates, 9 g fat and, 22 g protein) at 8:05 AM and 12:05 PM, respectively. Serum samples were taken at 8:00 AM (before breakfast), 10:30 AM, 12:00 PM (before lunch), 1:30 PM and 3:00 PM. Serum FGF19, C4, total BA content, triglycerides and total cholesterol were measured. All subjects were taking a similar diet and performing similar physical activity before and after CCT, according to a 7-day diet history evaluation and a standardized physical activity questionnaire (data not shown) by a registered dietitian. The patients had normal results for fasting liver-function tests, glucose, serum lipids and insulin during the follow-up period.
Additional experimental proceduresDetailed descriptions are provided in the supplementary Materials and Methods.
StatisticsUnless otherwise noted, all data are presented as mean ± SEM. The significance of differences was tested by two-tailed unpaired or paired (as applicable) t-test using GraphPad Prism 4.03 (GraphPad Software Inc., San Diego, CA, USA) for Windows. The level of significance for all statistical analysis was set at P-value < 0.05. Log-transformation was performed to normalize FGF19, C4 and total BA data distribution. Comparison of temporal changes in the follow-up study was performed by one-way ANOVA with Dunnett’s test, using 08:00 h data as baseline reference. For comparisons of serum diurnal variations of the variables of interest at visits-1, -2 and -3, and to determine potential interactions of serum diurnal curves and different study visits, linear mixed effects models24 and generalized estimating equations (GEE) were used.25 R software was used for analyses (R Development Core Team 2010).26
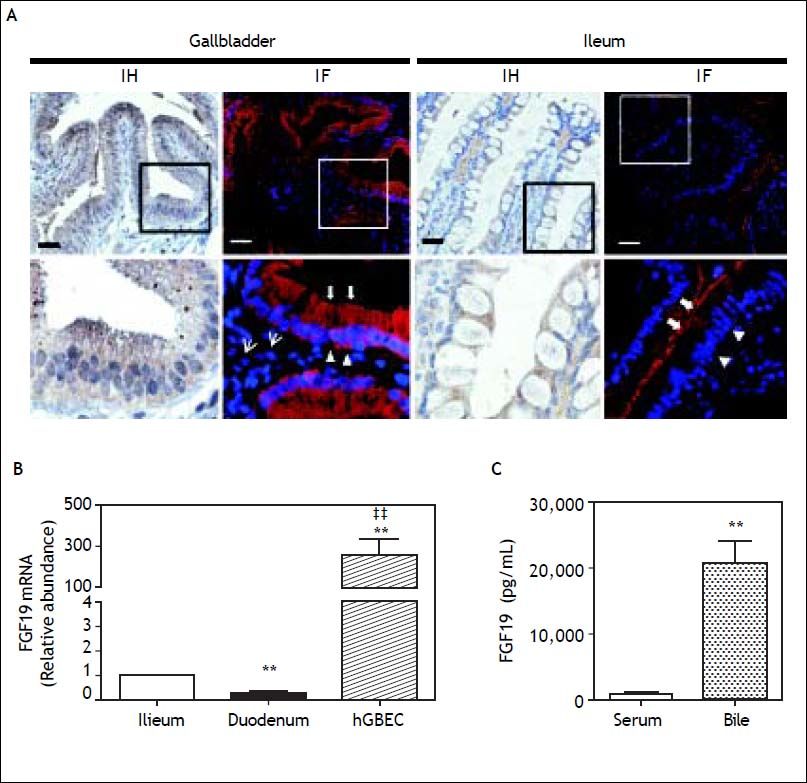
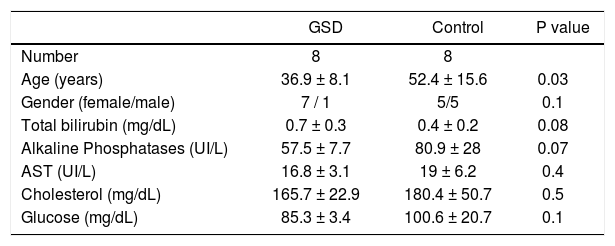
ResultsFGF19 is abundantly expressed and secreted by human gallbladder epithelium. The FGF19 mRNA level was measured in the GB epithelium (n = 15) and mucosa of terminal ileum (n = 15) and duodenum (n = 14). Compared with GSD subjects, non-GSD patients were older but otherwise similar considering gender, liver-function, serum glucose and lipids (Table 1). Of note, the FGF19 mRNA level was ~250-fold higher in hGBECs than distal ileum mucosa (P = 0.001) (Figure 1B). As expected, FGF19 expression was 70% lower in duodenal mucosa than in the terminal ileum. FGF19 protein expression in the GB epithelium was first demonstrated through immunohistochemistry (IH) and immunofluorescence (IF) (Figure 1A, negative control in figure 2). A similar homogeneous and predominant cytoplasmic signal was observed in both cholangiocytes and enterocytes, with a fine granular dot-like signal pattern, compatible with vesicular transport and/or storage. However, under identical experimental conditions, GB cholangiocytes exhibited a clearly stronger signal concordant with the higher mRNA levels. Interestingly, the IF signal for FGF19 in enterocytes was stronger towards the apical membrane in contrast to a more basal/lateral type membranous signal pattern in cholangiocytes (Figure 1A).
Main clinical characteristics of gallstone disease and control subjects.
| GSD | Control | P value | |
|---|---|---|---|
| Number | 8 | 8 | |
| Age (years) | 36.9 ± 8.1 | 52.4 ± 15.6 | 0.03 |
| Gender (female/male) | 7 / 1 | 5/5 | 0.1 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 | 0.4 ± 0.2 | 0.08 |
| Alkaline Phosphatases (UI/L) | 57.5 ± 7.7 | 80.9 ± 28 | 0.07 |
| AST (UI/L) | 16.8 ± 3.1 | 19 ± 6.2 | 0.4 |
| Cholesterol (mg/dL) | 165.7 ± 22.9 | 180.4 ± 50.7 | 0.5 |
| Glucose (mg/dL) | 85.3 ± 3.4 | 100.6 ± 20.7 | 0.1 |
Data are expressed as mean ± SD; mean values were compared using Student’s t test and χ2 test. GSD: gallstone disease. AST: aspartate aminotransferase.
Expression of FGF19 in human gallbladder and gut. A. A positive immunoperoxidase reaction (IH) and immunofluorescense signal (IF) for FGF19 protein is evident predominantly in the cytoplasmatic compartment of gallbladder and distal ileum epithelia. GB cholangiocytes presented a fine granular dot-like signal pattern for FGF19 protein extending from apical (arrows) to basal domains (open arrowhead). Ileum enterocytes showed increased FGF19 signal towards the apical cell compartment. Some FGF19 positive cells were observed in GB lamina propria, most probably of myofibroblastic type (thin arrows). B. FGF19 expression by qRT-PCR in hGBECs (n = 15) is 250-fold higher than ileum (n = 15) and duodenum (n = 14). ** P<0.01 and P < 0.01 compared to ileum and duodenum respectively. C. FGF19 content in GB bile (n = 8) is 23-fold higher than serum levels (n = 45) measured by ELISA. ** P < 0.01.
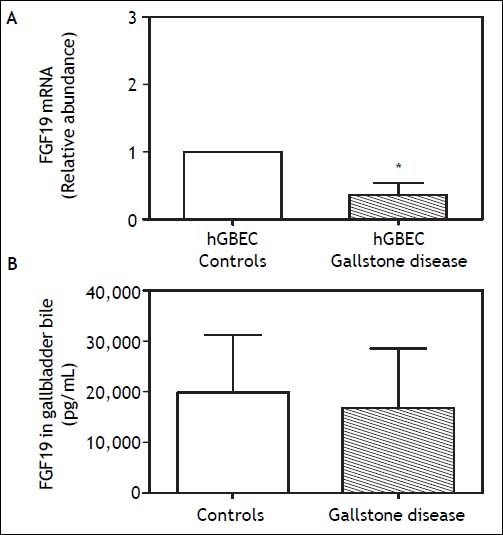
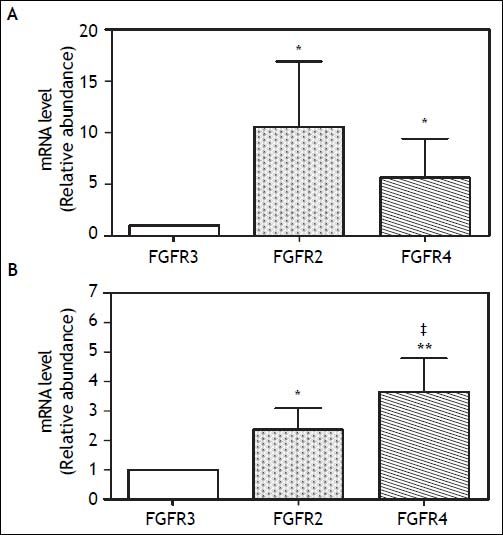
Noteworthy, the FGF19 concentration in GB bile was ~20 fold higher than in serum (20,000 ± 3,400 vs. 880 ± 63 pg/mL, respectively; P < 0.001) (Figure 1C). No differences in FGF19 concentration was observed between observed fresh GB bile of GSD patients (n = 8) and control subjects (n = 8) (Figure 3B). The abundant expression in GB mucosa and its elevated levels in the bile, suggested a potential function of FGF19 in the biliary tree and/or gut mucosa. Hence, the expression level of FGF receptors, known to be activated by FGF19, was also explored in hGBECs and duodenal mucosa.27 HGBECs and duodenal mucosa expressed significant mRNA levels of FGFR2 and FGFR4, with negligible levels of FGFR3 (Figures 4A and 4B, respectively).
A. FGF19 mRNA expression levels in human gallbladder cholangiocytes (hGBECs) of cholesterol gallstones disease patients (n = 8) is significantly lower compared to non-gallstone control patients (n = 8) measured by qRT-PCR and normalized with 18S mRNA gene expression. B. No significant differences were observed in FGF19 content in gallbladder bile of gallstone and control patients in the same subjects. *P < 0.05 compared to control subjects. Results are express as mean ± SEM.
FGFR2, FGFR3 and FGFR4 mRNA expression levels in (A) human gallbladder cholangiocytes (hGBECs) and (B) duodenum biopsies measured by qRT-PCR and normalized with 18S mRNA gene expression. *P < 0.05 compared to FGFR3 expression. Results are express as mean ± SEM. ** P < 0.01 compared to FGFR3 expression. * P < 0.05 compared to FGFR2.
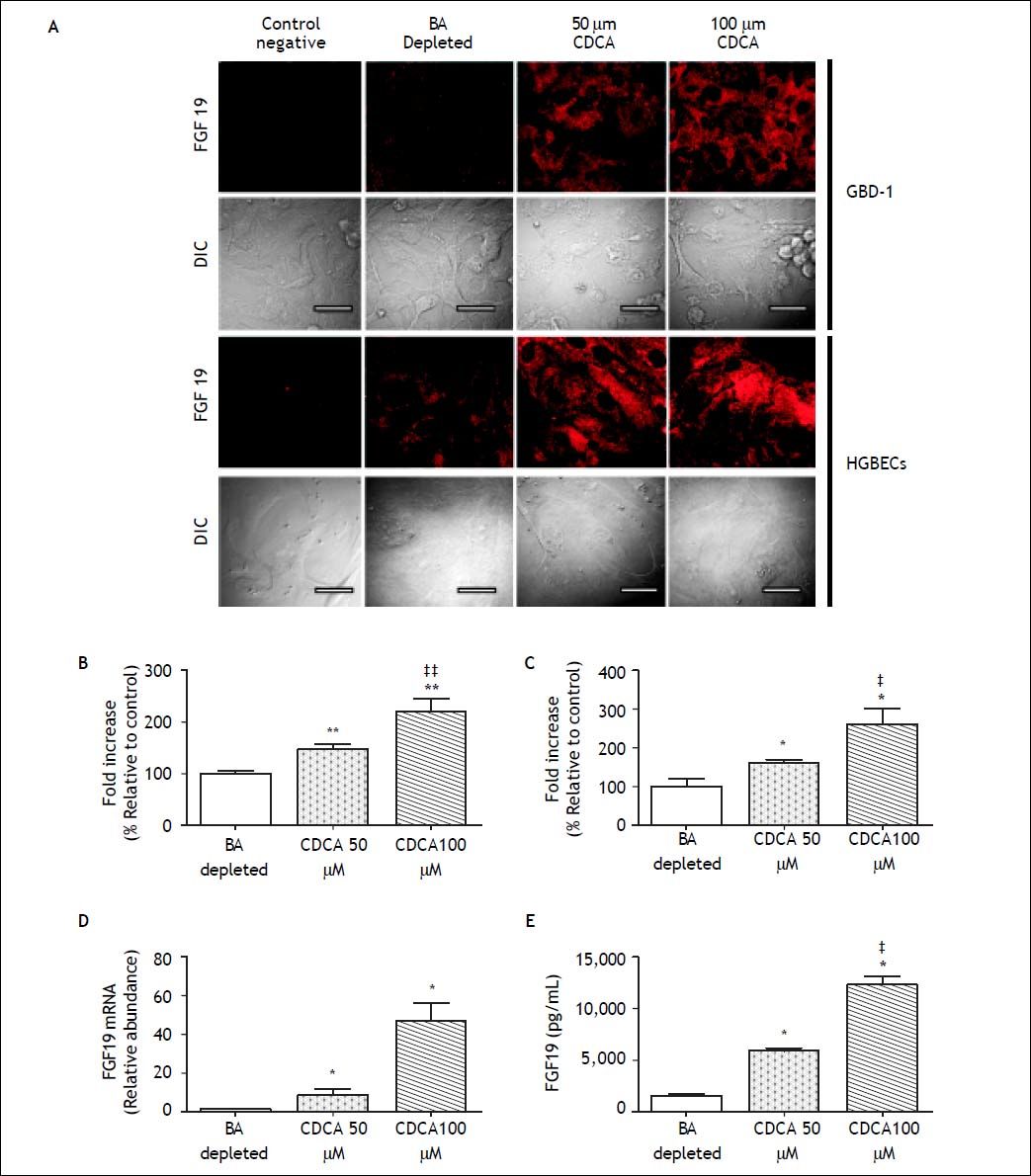
To investigate the role of BAs in the regulation of FGF19 expression and secretion on human cholangiocytes, we treated primary cultured hGBECs and GB-d1 cells with CDCA, a potent natural ligand of FXR, and assessed the mRNA and protein FGF19 expression levels and secretion. Because of the elevated basal FGF19 expression in the primary hG-BECs (Figure 1A), cells were first cultured in BA-depleted medium to reduce the FGF19 expression. The BA depletion resulted in a marked reduction of FGF19 expression in hGBECs (Figure 5A), whereas CDCA (50 and 100 μΜ) prominently increased FGF19 mRNA and protein levels in a dosedependent manner in both, hGBEC and GB-d1 cells. As shown in figures 5A-5C, treatment with CDCA caused a robust dose-dependent increase in intracellular fluorescent signal for FGF19 in both GB epithelial cells. There was also a prominent dose-dependent induction of FGF19 mRNA expression by about 45-fold (Figure 5D). Moreover, FGF19 protein was actively secreted into the culture medium and CDCA treatment induced a three- to eightfold dose-dependent increase in FGF19 secretion in GBD-1 cell cultures (control 1,593 ± 165 pg/mL vs. CDCA 50 µΜ 5900 ± 351 pg/mL, and CDCA 100 µΜ 12,545 ± 767 pg/mL, P < 0.01) (Figure 5E). Taken together, these results confirm that FGF19 is constitutively expressed and secreted by hGBECs under a BA-mediated signaling mechanism, probably through FXR as has been shown for other epithelial cells of the gastrointestinal tract such as enterocytes and hepatocytes.6,22,29
Analysis for FGF19 in human cholangiocyte cultures. Cells were incubated with FBS charcoal-dextran at baseline, followed by 50 or 100 µΜ CDCA added in medium. Negative controls performed omitting the primary antibody. A. Confocal microscopy revealed a dose-dependent increase in FGF19 signal after CDCA exposure (Scale bars, 20 μm). The pixel density of the fluorescent signal was quantified as described in method section in (B) GB-d1 (n = 5) and (C) primary hGBECS cultures (n = 5). D. FGF19 expression (qRT-PCR) and (E) protein quantification in culture media (ELISA) confirm a dose-dependent induction of transcription and secretion in GBD-1 cell cultures. * P < 0.05 and ** P < 0.01 compared to baseline ‡ P < 0.05 and ‡‡ P < 0.01 compared to 50 µΜ CDCA.
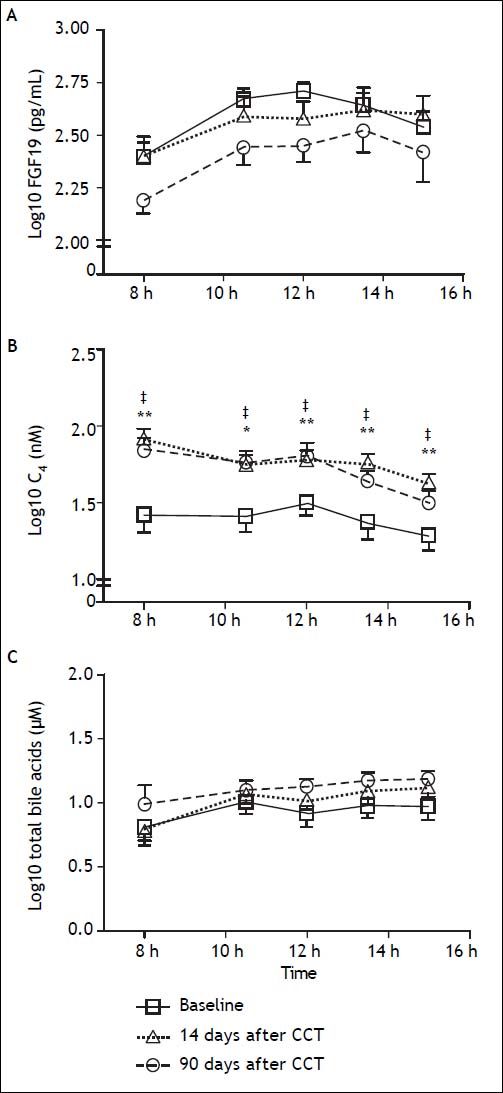
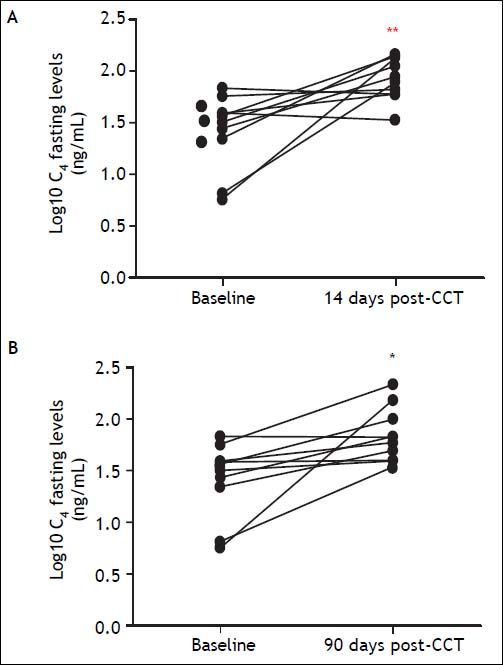
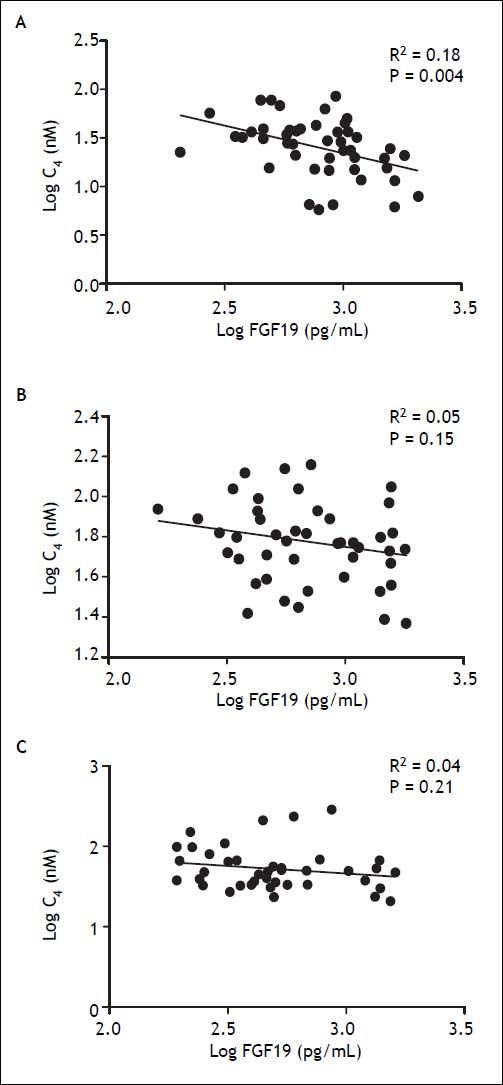
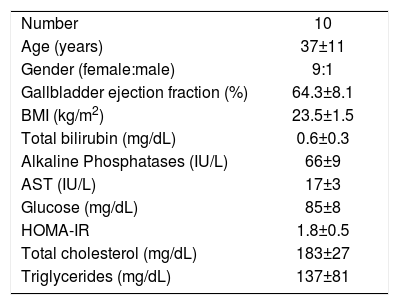
To assess the physiological relevance of FGF19 expression in the GB mucosa, we designed a prospective follow-up study in GSD patients undergoing elective CCT. GSD patients showed a 70% lower expression of FGF19 in hGBECs compared to control subjects (Figure 3A), although this expression level is still ~70-fold higher than in terminal ileum (data not shown) permitting GSD patients to be a good clinical model for studying the effect of CCT on circulating levels of FGF19 and its potential metabolic consequences. The baseline characteristics of the 10 patients included in this prospective cohort are listed in table 2. We designed a protocol aimed to evaluate the effect of GB resection on basal and postprandial serum levels of FGF19, C47,30,31 and total BA. Serum levels of FGF19 and C4 are shown in figures 6A and 6B, respectively. Prior to surgery FGF19 and C4 levels showed significant daily changes with a major peak at noon. Interestingly, GEE ANOVA analysis demonstrated significant differences between baseline, 14 days and 90 days after CCT (P < 0.01). For further analysis we conducted a GEE regression analysis that demonstrated no significant slope at baseline visit (P = 0.08), compared to a significant slope at 14 and 90 days after CCT (P = 0.002 and P < 0.001, respectively). These analysis showed that CCT changed the diurnal rhythm of circulating FGF19 as early as two weeks after surgery. Furthermore, FGF19 serum levels tend to decline three months after CCT, reaching statistical significance at noon (1,086 ± 114 pg/mL vs. 651 ± 115 pg/mL P = 0.02). Same results were obtained after conducting a pairwise ANOVA analysis with Hochberg multiple comparison post-hoc test (P = 0.04). Moreover, fasting C4 levels were consistently increased by more than two-fold as early as 2 weeks after surgery (33.3 ± 6.2 vs. 91.2 ± 12.2 nM, P ≤ 0.01) with a declining curve during the day, which differs significantly compared to baseline (Figures 6B and 7). This change in the C4 daytime curve remained similar three months after CCT. Interestingly, FGF19 was related inversely to BA synthesis (C4) before CCT (R2 = 0.18, P = 0.004), but this correlation was lost after CCT (Figure 8). Similar results were observed after adjusting C4 levels for total serum cholesterol. Total serum BA did not significantly change after CCT as compared with preoperative values (Figure 6C).
Main clinical characteristics of gallstone patients undergoing elective cholecystectomy and enrolled in the cohort study.
| Number | 10 |
| Age (years) | 37±11 |
| Gender (female:male) | 9:1 |
| Gallbladder ejection fraction (%) | 64.3±8.1 |
| BMI (kg/m2) | 23.5±1.5 |
| Total bilirubin (mg/dL) | 0.6±0.3 |
| Alkaline Phosphatases (IU/L) | 66±9 |
| AST (IU/L) | 17±3 |
| Glucose (mg/dL) | 85±8 |
| HOMA-IR | 1.8±0.5 |
| Total cholesterol (mg/dL) | 183±27 |
| Triglycerides (mg/dL) | 137±81 |
Values are expressed as mean ± S.D.BMI: Body mass index. AST: aspartate aminotransferase. HOMA-IR: homeostasis model assessment of insulin resistance.
Changes in FGF19 serum levels and bile acid synthesis after cholecystectomy (CCT). A. Levels of FGF19. B. BA synthesis (C4) and (C) total serum BA at baseline, 14 and 90 days after CCT in ten patients with GSD. Blood samples were collected after 8 hrs fasting at 8:00 h, and after standardized breakfast (10:30, 12:00 h) and lunch (13:30 and 15:00 h). Standardized meals were provided after 8:05 and 12:05 h. Data are expressed as mean ± SEM. * P < 0.05 and ** P < 0.01 at 14 days after CCT compared to baseline; * P < 0.05 90 days after CCT compared to baseline.
The results of the present study identify human GB cholangiocytes as a major source of FGF19. High FGF19 mRNA levels, together with positive immunostaining and a similar cytoplasmic pattern to distal ileum enterocytes were detected in hGBECs. This was accompanied with an elevated concentration of FGF19 protein in GB bile. Furthermore, we demonstrated that this expression was induced in a dose-dependent manner by CDCA in human GB derived cholangiocytes, indicating a BA induced synthesis and secretion, most probably mediated by activation of the nuclear receptor FXR.6,22,29 Zweers, et al.28 recently reported similar findings using cultured tissue explants from GBs and common bile ducts. These results and the present observations indicate an active secretion of FGF19 by cholangiocytes into bile and consequently to intestine, representing a potential intraluminal signaling pathway between these systems. However, the functional role of biliary FGF19 remains to be elucidated.28
Since human cholangiocytes and enterocytes express FGFRs, biliary FGF19 may participate in diverse processes, such as tropism and defense of epithelial cells against bile acid mediated apoptosis, and regulation of GB and intestinal motility.3 FGF19 has also been involved in regulation of apical sodium bile transfer protein (ASBT) expression in human gallbladder, ileum and human cholangiocytes cell lines.29
Recent studies have described an oncogenic role for FGF19 in colon cancer, thus theoretically after its transit through small bowel, bile-derived FGF19 might promote malignant transformation in colonocytes.32 However, before its arrival to colon, bile is mixed with chymus and undergoes enzymatic digestion. As consequence luminal FGF19 concentration is significantly reduced. In addition, colon cancer cells have been described to synthesize and secrete FGF19 in an autocrine manner.32 In contrast to colonocytes, cholangiocytes are directly exposed to elevated FGF19 concentrations from bile, hence an oncogenic role of bile-derived FGF19 is more plausible for cholangiocarcinoma.33
Of note, we demonstrated that hGBECs derived from GBs of GSD patients expressed lower FGF19 transcripts than non-GSD controls, however, biliary FGF19 content remained comparable in both groups (Figure 3). GB bile FGF19 levels may not correlate with GB cholangiocytes FGF19 mRNA levels because FGF19 derived from cholangiocytes of the intrahepatic biliary tree,28 is mixed and concentrated with GB-derived FGF19 in the GB lumen. The finding of a lower FGF19 mRNA level in GB cholangiocytes of GSD patients is intriguing. Theoretically, the secretion of biliary FGF19 could have a role on gallstone pathogenesis by affecting GB motor function3 and/or BA metabolism. Alternatively, the reduced hGBECs FGF19 mRNA levels might occur merely as a consequence of the local inflammatory process frequently accompanying GB disease.
Besides biliary secretion of FGF19 by cholangiocytes, the question remains whether hGBECs also contribute to regional (hepatic) and/or systemic FGF19 circulating levels and metabolic functions. Currently, the best human model to test this hypothesis is measuring the effect of GB resection on FGF19 levels and some of its best-known metabolic effects, such as regulation of BA homeostasis.7–10 The second finding of our study was that CCT induced a pronounced increase in BA synthesis, changes in the physiologic daily oscillations of BA synthesis, and significantly altered the diurnal FGF19 rhythm and reduced [FGF19]s levels at noon. Thus, these findings suggest that the GB actively participates in BA homeostasis and FGF19 circulating levels.
Our clinical study was carried out in a selected cohort of GSD patients with small stones and functioning GB’s, who underwent elective laparoscopic CCT. Since FGF19 levels and BA synthesis show important inter-individual and circadian fluctuations,34 we included serum samples of all subject obtained at standardized time points (fasting and post prandial), before and after CCT. All analysis were performed on a strict pairwise basis. Accordingly, the observed modifications of BA metabolism and circulating FGF19 seem to be mediated either directly or indirectly by CCT.
The fact that hGBECs secrete FGF19 into bile (apical membrane sorting) does not exclude the possibility of simultaneous intracellular traffic of FGF19 to the basolateral plasma membrane and secretion into the systemic circulation. There are several examples of hormones and proteins that present a bidirectional secretion or destination including: leptin in fundic stomach cells;35 angiotensin in proximal tubular renal cells;36 and the HDL-cholesterol receptor SR-BI, which is expressed in the basal membrane of hepatocytes, but in the apical membrane of hGBECs and enterocytes.18 Interestingly, a clear apical signal for FGF19 was evident in the distal ileum enterocytes (Figure 1A), suggesting that besides its sorting to the basolateral membrane, FGF19 could also be transported to the apical membrane and secreted into the intestinal lumen. Further studies aimed to precisely define the intracellular trafficking of FGF19 in cholangiocytes and enterocytes are needed to clarify these issues.
The effect of CCT on BA synthesis was extensively studied by the gold standard isotope dilution technique during the 70’s and 80’s, before the discovery of FGF19. All these studies were performed in small numbers of GSD patients that differed in gender, age, and time points after surgery. The most recent study designed to investigate the effect of CCT on BA kinetics at time points similar to our study (6 weeks and 3 month) was published in 1989.37 While the effect of CCT on BA synthesis, pool size and loss from the enterohepatic circulation have been controversial,34,38 the effect on BA fractional turnover rates has been more consistent, showing an enhanced postoperative enterohepatic cycling of BA.34 This means that the absence of the GB decreases the sequestration of BA pool in the biliary tree between meals, so that hepatic bile secretion into the intestine is more continuous and ileal BA concentration and exposure may therefore increase.39 In this scenario, we postulate that an enhanced enterohepatic cycling of BA after CCT may maintain a higher active FXR signaling in the gut and a reduction of ileal FGF19 synthesis and secretion is therefore unlikely. Based on our data, we postulate that the extraction of the GB is the main factor determining the observed changes in diurnal rhythm and reduction in [FGF19]s, presumably by hampering the contribution of cholangocytes-derived FGF19 to the circulation levels.
The markedly enhanced fasting BA synthesis measured as C4 and observed as early as 2 weeks and maintained 3 month after CCT is consistent with previous reports using the same surrogate marker of BA synthesis before and after CCT.10,34,40 These results are in apparent contradiction with most studies performed using the isotope dilution technique, in which no change of BA synthesis rates after CCT was found, either at short34,37 or long term41 after surgery. So, how can we explain this apparent discrepancy? BA synthesis measured by the isotope dilution technique reflects total daily BA synthesis, whereas BA synthesis assessed by the C4 method reflects BA synthesis at a fixed point. CYP7A1, the rate-limiting and major regulatory enzyme in the synthesis has a short life of 2-3 h. C4 in peripheral blood mirrors the hepatic CYP7A1 enzymatic activity, and its half-life is also less than 5 h.34 Indeed, we observed that the diurnal level of C4 changes drastically after CCT, starting with 2-fold higher fasting levels and a sustained decrease during the day (Figure 6B). Unfortunately, because of logistic reasons we were unable to assess C4 serum levels during a 24 h period. The area under the curve of C4 serum levels during our 8h protocol was significantly greater 2 weeks and 3 month after CCT compared to pre-CCT values (data not shown), but a 24h monitoring of C4 serum levels is needed to demonstrate if BA synthesis assessed by this surrogate marker may fit with data generated by the isotope dilution assay.
In conclusion, GB expresses and secretes high levels of FGF19. The expression level of FGF19 is lower in GB cholangiocytes of GSD patients, but FGF19 content in GB bile remains similar. CCT changes [FGF19]s diurnal rhythm, reduces its noon levels, and this intervention is coupled to a drastic increase in fasting BA synthesis. Given that available knowledge suggests that enterohepatic cycling of BA increases several times after CCT, we postulate that the absence of GB - and not ileal-derived FGF19 - may be responsible for this effect on circulating FGF19 and BA synthesis. Since we have recently shown that CCT mice evolve with significant metabolic changes expressed by increase serum and hepatic triglyceride concentrations and hepatic VLDL production,42 and supra physiologic levels FGF19 prevent diet induced hepatic steatosis in mice,15 our results suggests that CCT may also have major systemic metabolic effects in humans. Interestingly in a recent epidemiologic study Ruhl, et al. demonstrated that cholecystectomy was independently associated to a significant increase in non-alcoholic fatty liver disease even after adjusting for all the common risk factors associated with these diseases.43 Thus the generally accepted medical concept that CCT, one of the most frequent surgeries performed worldwide, is largely innocuous requires further systematic evaluation.44
Abbreviations- •
BA: bile acids.
- •
C4: 7a-hydroxy-4-cholesten-3-one.
- •
CA: cholic acid.
- •
CCT: cholecystectomy.
- •
CDCA: chenodeoxycolic acid.
- •
CI: confidence interval.
- •
CYP7A1: cytochrome p450 7A1.
- •
DCA: deoxycholate acid.
- •
DMEM: Dulbecco’s Modified Eagle Medium.
- •
DMSO: Dimethyl sulfoxide.
- •
FBS: fetal bovine serum.
- •
FGF15: fibroblast growth factor 15.
- •
FGF19: fibroblast growth factor 19.
- •
[FGF19]s: fibroblast growth factor 19 serum concentration.
- •
FGFR: fibroblast growth factor receptor.
- •
FXR: farnesoid X receptor.
- •
GB: gallbladder.
- •
GSD: gallstone disease.
- •
hGBECs: human gallbladder epithelial cells.
- •
HPLC: high performance liquid chromatography.
- •
IF: immunofluorescence assay.
- •
IH: immunohistochemistry assay.
- •
LCA: lithocholic acid.
- •
OR: odds ratio.
- •
SEM: standard error of mean.
- •
UDCA: ursodeoxycholate acid.
The authors thank J.C. Roa for the generous gift of the GB-d1 cells, M.P. Marzolo for helping in standardizing primary hGBECs culture, and M. Arrese for critical review of the manuscript and helpful discussions. The authors acknowledge the Humboldt Foundation (Germany) for donating the ABI 7500 sequence detection system used for gene expression analysis and Nanodrop1000 for RNA and DNA quantification. Part of this work was presented at the Annual Meeting of The American Association of the Study of Liver Diseases, San Francisco, USA, 3-7 November 2011 and published as an abstract form in Hepatology 2011; 54: 728A.
Conflicts of InterestThe authors disclose no conflicts.
FundingSupported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grant N° 1080325 and 1130303 to Juan Francisco Miquel, grant N° 1130146 to Flavio Nervi and grant N° 1150311 to Francisco Barrera.