Pediatric acute liver failure (PALF) is a progressive, potentially fatal clinical syndrome occurring in previously healthy children. Our study aimed to determine the current leading causes of PALF in a single center in Germany, identifying possible prognostic markers. Thirty-seven pediatric patients with PALF were included. Medical records were reviewed for demographic, laboratory and clinical data. Laboratory results on admission and at peak value, PELD and MELD score on admission, and intensive care support were assessed. Fifteen patients recovered spontaneously, 14 died without transplantation, and 8 received a liver transplant. Patients who survived were significantly older than patients who died. Specific causes of PALF could be identified as infectious diseases (16%), metabolic diseases (14%), toxic liver injury (11%), immunologic diseases (8%), or vascular diseases (8%). Causes of PALF remained indeterminate in 43%. High ammonia, low albumin, and low ALT levels on admission were associated with worse outcome. Absence of need of ventilation, hemodialysis, and circulatory support predicted spontaneous recovery. In conclusion, infections are the most common known cause of PALF. However, in a large proportion of patients the cause for PALF remains cryptic. Ammonia and albumin levels may be of prognostic value to predict outcomes.
Pediatric acute liver failure (PALF) is a rapidly progressive, potentially fatal clinical syndrome occurring in previously healthy children. PALF is defined as biochemical evidence of liver injury and coagulopathy not correctable by vitamin K with International Normalized Ratio (INR) ≥ 1.5 in the presence of hepatic encephalopathy (HE) or INR ≥ 2.0 regardless of presence or absence of HE.1
The clinical course of PALF is highly variable, depending on etiology, age, genetic background, and geographical region. The most common occurrence of PALF is in neonates and infants; and the second most common time for occurrence is in the teenage years.1–3 Neonatal liver failure is most frequently caused by immunological (e.g. gestational alloimmune liver disease), viral (e.g. herpes simplex virus), or hematological (e.g. hemophagocytic lymphohistiocytosis) diseases, whereas in teenagers, drug induced liver injury is the most common etiology.1,2 In populations with a high percentage of consanguinity, metabolic disorders such as mitochondrial diseases are more prevalent and associated with poor outcomes.4,5 In developing countries, viral hepatitis A, B, and E are the main causes of PALF. In contrast, in Western countries viral-induced PALF is less prevalent.1,6 In approximately 47% of PALF cases, a definite cause cannot be established.7 The overall mortality in PALF is about 39-54%.4,8 Indeed, neonatal herpes simplex infection is associated with a poor prognosis and only 10% of patients recover with antiviral treatment.9 However, acetaminophen toxicity is associated with an excellent prognosis and 94% of patients recover spontaneously.1 Specific treatments are available in selected cases with known etiology such as autoimmune hepatitis or hepatitis B. However, liver transplantation remains the only curative treatment option in advanced cases of PALF.
Approximately 13% of all pediatric liver transplants go to PALF recipients with a current overall survival rate of 68-74%.8,10 Previous survival rates of liver transplantation for PALF in our center have reached 94%.11 Despite this, reliable prognostic scores to identify and select which patients with PALF are likely to die without transplantation remain elusive. Many efforts have been made to develop prognostic clinical and biochemical scores, but to date these have had inconsistent results.8,12 Moreover, the clinical scores predominantly used in adult acute liver failure [i.e. Kings College Criteria (KCC), Clichy criteria, etc.] do not reliably predict death in PALF.13 The Model of End-stage Liver Disease (MELD) score, which is established in adults, has not been validated in PALF. Previous studies have shown that the predominant etiologies and outcomes in adult acute liver failure (ALF) differ from those in PALF.14
In our study, we evaluated 37 PALF patients over a 4-year period in a single German pediatric transplant center. The aims of our study were to:
- •
Detect the current leading causes of PALF in Germany, and
- •
Identify possible clinical and biochemical markers, which may have prognostic impact in patients who recover spontaneously and those in need of a liver graft.
Forty-four pediatric patients with the admission and/or discharge diagnosis of PALF were identified retrospectively from the hospital patient registry, from admissions during January 2010 to December 2013.
Patients from birth to 18 years of age were eligible for the study. PALF was defined in accordance with the PALF study group criteria: Children with no evidence of chronic liver disease, biochemical evidence of acute liver injury, and hepatic-based coagulopathy (INR ≥ 1.5 not corrected by vitamin K in the presence of HE or INR ≥ 2 independent of HE). After excluding 7 patients (5 patients due to acute-on-chronic liver failure and 2 for death caused by reasons other than liver failure), 37 patients (0-16 years, median age 1 year, males 57%, females 43%) were included in our study. Medical records were reviewed for demographic, biochemical, and clinical information. The study was approved by the local institutional review board (Ethikkommission am Universitätsklinikum Essen).
Clinical parameters, etiology, and outcomePALF was verified by significant alterations of characteristic laboratory parameters. Laboratory results on admission as well as peak values (ALT, AST, creatinine, bilirubin, INR, ammonia) were assessed in each patient. PELD (pediatric end stage liver disease) and MELD score were calculated on admission. Growth failure was not listed for the PELD score due to the acute nature of the disease.
Specific causes of PALF were determined using established diagnostic guidelines.2,3 Diagnostic workup included patients’ history (e.g. drug history, consanguinity), and extensive investigation of viral (e.g. HSV-PCR especially in neonates; HAV, HBV, HEV, etc.), immunologic (e.g. ANA, SMA, SLA and LKM-1 antibodies, etc.), hematologic (e.g. hemophagocytic lymphohistiocytosis), and vascular (e.g. Budd-Chiari syndrome) diseases. Acetaminophen intoxication was excluded by determining blood levels and taking clinical history. Additionally, liver ultrasound was performed repeatedly in order to exclude chronic liver injury or any specific (vascular) cause of liver damage. Clinical parameters (ventilation, hemodialysis, and circulatory support) were assessed on admission and in the clinical course. The patients were divided into two groups according to outcome at 12 weeks post admission. The first group was defined as natural survival in the absence of indication for transplantation (SR), and the other group as alive or lost, either by death or transplant (NSR).
Data analysis and statisticsData are given as means and standard deviation for numeric variables. Univariate analyses were performed for laboratory or clinical parameters associated with death or transplantation, i.e. age, AST, ALT, INR, creatinine, bilirubin, ammonia, PELD, and MELD. Two-sided unpaired t-tests for metric parameters were calculated (age, AST, ALT, INR, creatinine, bilirubin, and ammonia). Mann-Whitney U test was used for MELD and PELD scores. Fisher’s exact test was used for clinical parameters (ventilation, hemodialysis, circulatory support). Data analysis was performed using EXCEL 2010 (Microsoft, Redmond, WA, USA) and SPSS, version 22 (Chicago, IL, USA). A probability value of less than 0.05 was considered statistically significant.
ResultsPatients’ demographicsThirty-seven patients [21 (57%) males and 16 (43%) females, median age 1 year, range 0-16 years] were included in this study. All patients were from a Caucasian population. Fifteen (41%) patients recovered spontaneously (SR), 14 (38%) patients died without transplantation, and 8 (21%) patients received a liver transplant (grouped as NSR). At the end of the study period, all patients who had been transplanted were alive. One child died one year after transplantation due to secondary graft failure and obstructive cardiomyopathy.
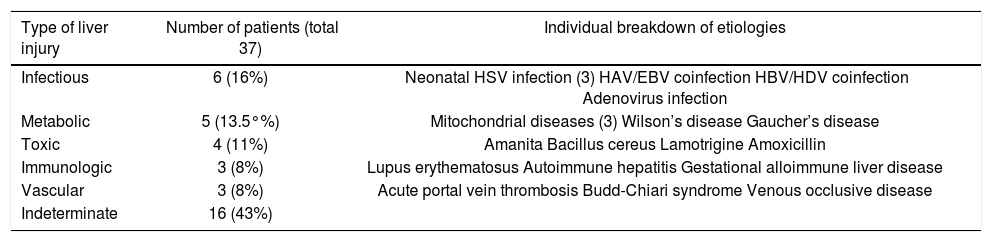
In PALF a high proportion of etiologies remain crypticA specific cause of PALF could only be identified in 21 patients (57%). Specific etiologies include infectious diseases (16%), metabolic diseases (14%), toxic liver injury (11%), immunologic diseases (8%), or vascular diseases (8%). The causes could not be determined in 16 patients (43%). A detailed distribution of individual causes is given in table 1.
Distribution of etiologies in PALF patients.
| Type of liver injury | Number of patients (total 37) | Individual breakdown of etiologies |
|---|---|---|
| Infectious | 6 (16%) | Neonatal HSV infection (3) HAV/EBV coinfection HBV/HDV coinfection Adenovirus infection |
| Metabolic | 5 (13.5°%) | Mitochondrial diseases (3) Wilson’s disease Gaucher’s disease |
| Toxic | 4 (11%) | Amanita Bacillus cereus Lamotrigine Amoxicillin |
| Immunologic | 3 (8%) | Lupus erythematosus Autoimmune hepatitis Gestational alloimmune liver disease |
| Vascular | 3 (8%) | Acute portal vein thrombosis Budd-Chiari syndrome Venous occlusive disease |
| Indeterminate | 16 (43%) |
Infectious diseases represented the largest group of specific causes of PALF (6/21 patients). In three patients, the cause was neonatal herpes simplex infection. A three-month old child died from fulminant adenovirus infection. Two older children with viral coinfection survived without transplantation; one with HAV-EBV and the other with HBV/HDV coinfection, the latter receiving antiviral treatment (Table 1).
Metabolic diseases as the cause of PALF occurred in five patients. Three patients were diagnosed with mitochondrial disorder, and all of these died due to multiple organ failure. One child with Gaucher’s disease and multiple organ involvement died weighing only 2 kg from abdominal compartment syndrome. One teenager with Wilson’s disease received a liver transplant and survived (Table 1).
Toxin-induced PALF was diagnosed in four cases. One teenager with amanita poisoning survived under specific therapy with silibinin. Two children received a liver transplant: one toddler for drug-induced hypereosinophilic syndrome (DRESS) due to amoxicillin and the other for Bacillus cereus toxin poisoning after ingestion of a reheated rice dish. Both children survived. One child died due to lamotrigine-induced liver failure, presenting with multiple organ failure (Table 1).
Immunologically-induced PALF was seen in three children: one with lupus erythematosus and one with autoimmune hepatitis, who survived after initiating steroid therapy. One infant died from gestational alloimmune liver disease (Table 1).
Vascular-associated PALF was seen in three children. One neonate with acute portal vein thrombosis survived after spontaneous resolution of the thrombosis. One child with Budd-Chiari syndrome received a liver transplant and survived. One child with venous occlusive disease after stem cell transplantation died without transplantation (Table 1).
Survival rate increases with higher age at onset of PALFA great proportion (17/37 = 46%) of our pediatric patients consisted of neonates and infants, the median age of all patients was 1 year (range 0-16 years). NSR was associated with younger age [median age 0 year (range 0-15 years) vs. 3 years (range 0-16 years)], however the difference relative to the SR group did not reach statistical significance (p = 0.096). Moreover, overall survival (with or without transplantation) was significantly lower in younger children [median age 0 year (range 0-15 years) vs. 3 years (range 0-16 years), p = 0.039].
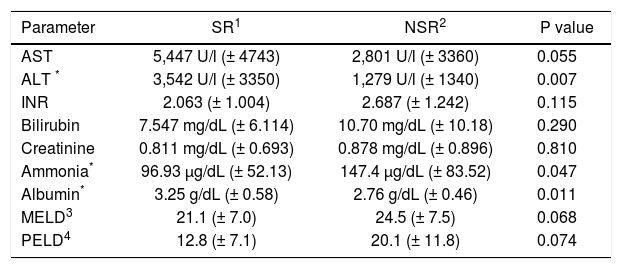
Higher liver enzymes are associated with better outcome in PALFIn order to evaluate the relevance of liver enzyme concentrations to clinical outcomes in PALF, AST and ALT were analyzed on admission as well as peak values (Tables 2 and 3). On admission, AST and ALT were higher in the SR group compared to NSR patients, although statistically significant only for ALT (p = 0.007; AST: p = 0.055). Peak values of AST and ALT did not differ between the two groups (p = 0.966 and p = 0.403).
Clinical parameters of PALF patients grouped by outcome.
| Parameter | SR1 | NSR2 | P value |
|---|---|---|---|
| AST | 5,447 U/l (± 4743) | 2,801 U/l (± 3360) | 0.055 |
| ALT * | 3,542 U/l (± 3350) | 1,279 U/l (± 1340) | 0.007 |
| INR | 2.063 (± 1.004) | 2.687 (± 1.242) | 0.115 |
| Bilirubin | 7.547 mg/dL (± 6.114) | 10.70 mg/dL (± 10.18) | 0.290 |
| Creatinine | 0.811 mg/dL (± 0.693) | 0.878 mg/dL (± 0.896) | 0.810 |
| Ammonia* | 96.93 μg/dL (± 52.13) | 147.4 μg/dL (± 83.52) | 0.047 |
| Albumin* | 3.25 g/dL (± 0.58) | 2.76 g/dL (± 0.46) | 0.011 |
| MELD3 | 21.1 (± 7.0) | 24.5 (± 7.5) | 0.068 |
| PELD4 | 12.8 (± 7.1) | 20.1 (± 11.8) | 0.074 |
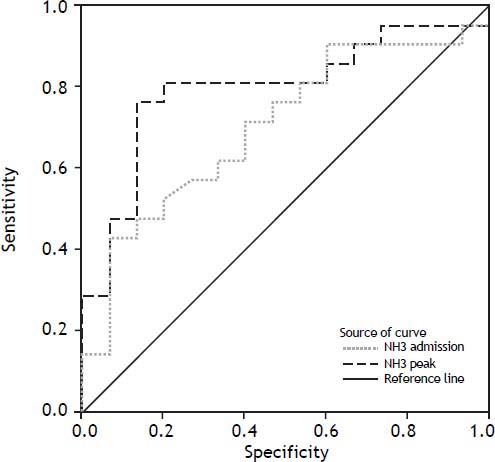
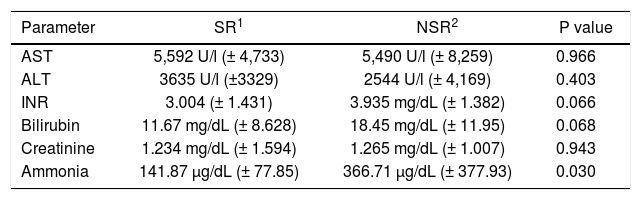
Blood ammonia levels were determined on admission and during the clinical course. In patients with SR, blood ammonia levels were markedly lower than in NSR. This could be observed on admission (p = 0.047) as well as in peak values (p = 0.030; tables 2 and 3). To assess the predictive accuracy of blood ammonia levels for outcome, ROC curves were calculated for ammonia levels at admission and at peak value (Figure 1). AUCs for these curves were 0.7032 at admission (CI: 0.5305 to 0.8759) and 0.7968 (CI: 0.6449 to 0.9488) at peak value, respectively.
Creatinine levels do not predict clinical outcome in PALFPELD and MELD score were calculated on admission for all patients (Table 2). Higher PELD scores on admission were observed in patients with poor outcome, but without statistical significance (p = 0.074). Comparing the single PELD parameters, albumin had the best prognostic value (p = 0.011) followed by INR (p = 0.115) and bilirubin (p = 0.290). An age below one year contributed significantly to the PELD score (+4.36) which was consistent with the worse outcomes in young children observed in our study. There were higher MELD scores in the SR group (p = 0.068). The three single MELD parameters also did not differ between SR patients and NSR (serum creatinine: p = 0.810; bilirubin: p = 0.290; INR: p = 0.115). There was only a modest overall increase of serum creatinine in most children (Table 3).
Clinical parameters at peak value.
| Parameter | SR1 | NSR2 | P value |
|---|---|---|---|
| AST | 5,592 U/l (± 4,733) | 5,490 U/l (± 8,259) | 0.966 |
| ALT | 3635 U/l (±3329) | 2544 U/l (± 4,169) | 0.403 |
| INR | 3.004 (± 1.431) | 3.935 mg/dL (± 1.382) | 0.066 |
| Bilirubin | 11.67 mg/dL (± 8.628) | 18.45 mg/dL (± 11.95) | 0.068 |
| Creatinine | 1.234 mg/dL (± 1.594) | 1.265 mg/dL (± 1.007) | 0.943 |
| Ammonia | 141.87 μg/dL (± 77.85) | 366.71 μg/dL (± 377.93) | 0.030 |
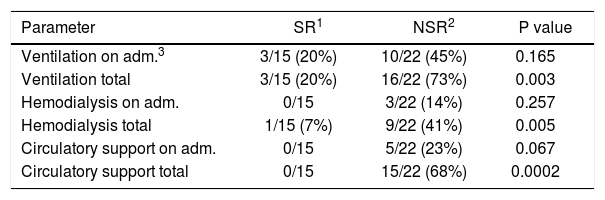
Clinical parameters (ventilation, hemodialysis, circulatory support) were assessed on admission and during the clinical course. On admission, patients with poor outcome more often required ventilation, hemodialysis, and circulatory support but without statistical significance (p = 0.165, p = 0.257, p = 0.067). However, patients not requiring ventilation, hemodialysis, and circulatory support in the clinical course had a significantly better chance to survive without transplantation (p = 0.003, p = 0.005, p = 0.0002) (Table 4).
Clinical parameters of PALF patients grouped by outcome.
| Parameter | SR1 | NSR2 | P value |
|---|---|---|---|
| Ventilation on adm.3 | 3/15 (20%) | 10/22 (45%) | 0.165 |
| Ventilation total | 3/15 (20%) | 16/22 (73%) | 0.003 |
| Hemodialysis on adm. | 0/15 | 3/22 (14%) | 0.257 |
| Hemodialysis total | 1/15 (7%) | 9/22 (41%) | 0.005 |
| Circulatory support on adm. | 0/15 | 5/22 (23%) | 0.067 |
| Circulatory support total | 0/15 | 15/22 (68%) | 0.0002 |
Pediatric ALF is a rare disease with a high mortality. Our study confirms the known high mortality, whereby there was a mortality rate of 38% for patients without transplantation. This highlights the need for reliable prognostic markers to distinguish between patients with spontaneous recovery and organ loss. Our data indicates that:
- •
Infectious diseases are the most common causes of PALF,
- •
High AST and ALT levels are not associated with a worse outcome,
- •
Blood ammonia and albumin levels could predict outcome in PALF,
- •
Creatinine has no predictive value for PALF, and
- •
Clinical parameters are very useful for predicting outcomes in PALF.
Infectious diseases are the most common causes of PALF in our cohort. Amongst those six patients, three were diagnosed of neonatal herpes simplex infection with a high mortality rate of 2/3 which was in accordance with the data of Verma, et al.9 The second most common causes of PALF in our cohort are metabolic diseases. Among those five patients, three were diagnosed with mitochon-drial disorder with a 100% mortality rate. This adverse overall outcome of mitochondrial disorders is in line with previous studies.4,5 Both diseases are rarely seen as a cause of adult ALF. Indeterminate PALF was seen in 43% which is in line with previous findings from the PALF data-base.7 Nevertheless, the number of pediatric patients with indeterminate cause of PALF is much higher than in adult ALF.15
High AST and ALT levels are not associated with a worse outcome. Highly elevated liver enzymes are usually associated with liver damage and could therefore be interpreted as worse outcome in PALF. We found that patients with spontaneous recovery had higher AST and ALT levels on admission, which was statistically significant. This data supports the findings of Rajanayagam, et al.16 They found that AST and ALT levels were higher in PALF patients who survived spontaneously. One explanation could be lower liver enzymes in pre-damaged livers such as gestational alloimmune liver disease and Wilson’s disease, which are commonly associated with worse outcome. Indeed, this is paralleled by results observed in adults.15
Blood ammonia levels could predict outcome in PALF. A significant difference in biochemical markers on admission and as peak value with notably higher values in the group with organ loss was only noted for ammonia. This was also shown by Liu, et al.17 who could show in their cohort of 81 children with PALF that high ammonia levels were significantly associated with death or need for liver transplant. In comparison with adult acute liver failure, ammonia seems to be a far better prognostic parameter for children than for adults. Zhao, et al. showed in their study of 32 children and 177 adults with acute liver failure that ammonia levels were significantly higher in children, and higher levels were significantly associated with worse outcome.14 Taken together, the evidence suggests that ammonia levels may predict outcome in PALF.
Creatinine has no predictive value for PALF. The MELD score can, to some extent, predict ALF outcome in adults. Therefore, we checked its prognostic relevance in PALF. Among the classical values of the MELD score there was a tendency for higher peak INR and bilirubin levels in children with worse outcome, however creatinine did not differ between the two groups. This may be because kidney function is rarely impaired in children - even in liver failure. Zhao, et al. reported a similar finding with creatinine being significantly lower in children with PALF, irrespective of the disease outcome.14 In children with chronic liver disease, PELD score is used to predict outcome. We showed that higher PELD scores were present in PALF with poor outcomes, which did not reach statistical significance. This is in accordance with previous studies.16
Clinical parameters can predict outcomes in PALF. We found that extrahepatic organ support (ventilation, hemodialysis, circulatory support) was associated with poor prognosis. This is in accordance with previous studies such as Poddar, et al.18 who showed in children and adults with acute liver failure that extrahepatic organ involvement was associated with worse outcomes. Additionally, Bajaj, et al. described the impact of extrahepatic organ failure on survival and outcome in infection-related acute on-chronic liver failure, showing only 23% survivals in patients with multiple organ failure and hepatic encephalopathy.19
We conclude that the overall outcome of PALF is still in need of improvement. Prompt referral to a transplant center is needed. Biochemical markers and clinical parameters may help to identify patients in need of a liver graft. A crucial step towards improving clinical management of pediatric ALF patients would be to identify the underlying cause in a greater proportion of affected children.
Abbreviations- •
ALF: (adult) acute liver failure.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
AUC: area under the curve.
- •
CI: confidence interval.
- •
DRESS: drug-induced hypereosinophile syndrome.
- •
EBV: Eppstein-Barr virus.
- •
HAV/HBV/HDV: hepatitis A/B/D virus.
- •
HE: hepatic encephalopathy.
- •
HSV: herpes simplex virus.
- •
INR: international normalized ratio.
- •
KCC: King’s College Criteria.
- •
MELD: model of end-stage liver disease.
- •
NSR: non-spontaneous remission.
- •
PALF: pediatric acute liver failure.
- •
PELD: pediatric end-stage liver disease.
- •
SR: spontaneous remission.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, grant 267/8-1 and 267/131 to A.C. and BE 3967/3-1 to L.P.B.).
Conflict of InterestThe authors declare nothing to disclose.
Author ContributionsSK conceptualized and designed the study, collected data, evaluated data, drafted the initial manuscript, and approved the final manuscript as submitted.
LPB, PM, JPS evaluated data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
PG, EL, AD collected data, and approved the final manuscript as submitted.
PFH, AC conceptualized and designed the study, reviewed and revised the manuscript, and approved the final manuscript as submitted.
AEF reviewed and revised the manuscript, and approved the final manuscript as submitted.
AcknowledgementsThe authors thank Jason D Coombes, PhD (Regeneration and Repair Group, Foundation for Liver Research, The Institute of Hepatology, London, United Kingdom), for editorial assistance.