Background. HCV infection and transfusional iron overload in Thalassemic patients may result in liver disease. HCV treatment in Thalassemia has raised safety concerns.
Aim. Estimate effectiveness and tolerability of interferon-based therapy in HCV-infected Thalassemic patients.
Material and methods. Over a 12-year period, consecutive patients with β Thalassemia major (TM) and chronic hepatitis C received treatment. Liver biopsy, HCV-RNA and genotyping were performed beforehand. Sustained virological response (SVR) was defined as negative HCV-RNA 6 months post-treatment. Forty eight patients (26 M-22 F, mean age 39.8) were enrolled. Twenty nine patients were treated with conventional interferon alpha (IFNa) for 48 weeks (group A). Nineteen patients (10 naïve-9 previously IFNa experienced) received pegylated interferon (PEG-IFN) (group B).
Results. HCV-1 was found in 44%, HCV-2 in 14%, HCV-3 in 23% and HCV-4 in 19%. Group A: ten patients (38.5%) achieved SVR, 2 (7.5%) relapsed and 17 (54%) were non responders. Group B: five (28%) achieved SVR, 8 (44%) relapsed and 6 (28%) never responded. High HCV-RNA levels, genotype 1 and advanced liver fibrosis were independently associated with no response. Four patients (3 treated with IFNα, 1 with PEG-IFN) had to discontinue treatment due to complications.
Conclusions. The response rate of IFN monotherapy in multi-transfused, HCV-infected Thalassemic patients is not inferior to that in non-multi-transfused patients. IFNa administration is well-tolerated and should be recommended as initial treatment schedule in this setting.
Chronic infection with hepatitis C virus affects nearly 170 million people worldwide and is a leading cause of cirrhosis and end-stage liver disease in developed countries.1 Multi-transfused patients with β-Thalassemia are at high risk for hepatitis C infection, particularly if transfused before the introduction of HCV donor screening programs.2 HCV infection is found in almost 2/3 of Thalassemic patients throughout the world. 28–61% anti-HCV positivity among poly-transfused patients with hemoglobinopathies has been reported in Greece.3–5 Hepatitis C virus infection is one of the main causes of chronic liver disease among patients with β-Thalassemia major. In addition, these patients have iron overload, which also causes hepatic damage, as well as disturbances in cardiac and endocrine function. They frequently have increased hepatic iron concentrations and iron-induced liver damage, even given optimal iron chelation treatment.6 It has been shown that iron overload and HCV infection are independent risk factors for progression of liver fibrosis and development of cirrhosis.7 Since sustained virological response after anti-HCV therapy halts the progression of liver disease and decrease liver-related mortality, it is a crucial option in the management of these patients.8,9
Both, conventional and pegylated interferon have been used in the treatment of patients with chronic hepatitis C.10 Current guidelines recommend combination therapy with pegylated interferon (PEG-IFN) and ribavirin in patients with chronic hepatitis C.11 The efficacy and safety of interferon monotherapy, as well as combination treatment with ribavirin in this difficult to treat group of patients have been studied previously.12,13 Interferon alpha was well tolerated but the addition of ribavirin resulted in an increase of blood transfusion requirements. Iron accumulation and risk of iron-related toxicities, during combination therapy remains a major concern in patients with chronic hepatitis C and hemoglobinopathies.14,15 On the other hand the effect of hepatic iron overload on treatment efficacy is controversial.16–19
In the present study, we assessed during a 12-year period the efficacy and safety either conventional or pegylated interferon monotherapy in our cohort of patients with β-thalassemia major and chronic hepatitis C with various genotypes.
Material and MethodsPatients’ selectionWe enrolled consecutive patients with β-Thalassemia major (TM) and chronic hepatitis C (CHC) who were evaluated in the outpatient liver clinic at Laiko General Hospital, Athens University School of Medicine during a period of 12 years (1992–2004) who received antiviral treatment TM had been confirmed in all patients with hemoglobin electrophoresis or DNA testing earlier in their life were enrolled in the study. In addition, all subjects had been receiving regular blood transfusions at 2- to 4- week intervals along with regular therapy with desferrioxamine to maintain hemoglobin levels between 9–11 g/dL. Patients were required to be HCV-RNA positive with persistent elevation of alanine aminotransferase (ALT) for at least six months. Only adults (age over 18 years) were included in the study. Patients with decompensated liver disease, hepatocellular carcinoma, bone marrow or liver transplant, malignant neoplastic disease, severe cardiac or chronic pulmonary disease, renal failure, autoimmune disorders, untreated thyroid disease, active substance abuse, concurrent HBV or HIV co-infection and untreated psychiatric illness were excluded. Patients who did not respond in a previous treatment course with conventional IFN were eligible. All patients were informed about treatment and its possible side effects and written consent was obtained before liver biopsy. IRB approval was obtained before initiation of recombinant or pegylated interferon.
Study designEach patient of the study was referred to the Hepatology Unit of the 1st Department of Propaedeutic Medicine for diagnostic confirmation of chronic hepatitis C, induction of the appropriate therapeutic scheme and regular follow-up, both during treatment and up to 6 months afterwards. At study entry all participants underwent a physical examination and baseline laboratory testing. Liver biopsy was performed before initiation of treatment with a Menghini needle, us-guided. Speciments of at least 1 cm length were evaluated. Hepatic inflammation and fibrosis were graded according to the modified Histology Activity Index, proposed by Ishak, et al.20 The Perl’s staining method with 0–4 score was applied to assess hepatic siderosis.
Serum HCV-RNA was detected by PCR (nested and/or Amplicor, Roche Diagnostics, NJ, USA). Viral load quantification was performed using the Cobas Amplicor HCV Monitor, v2.0 (Roche Diagnostics, NJ, USA) with a lower detection level of 50 IU/ml. HCV was genotyped using the INNO-LIPA II probe assay (Innogenetics, NV, Belgium).
Clinical examination, full blood counts and biochemistry were obtained during treatment in weeks 1, 2, 4, then monthly until the end of therapy and subsequently bi-monthly throughout the follow-up period. The efficacy of treatment was based on the achievement of sustained virological response (SVR) defined as negative HCV-RNA by a sensitive qualitative PCR assay, six months after the end of treatment. Relapse was defined as clearance of viremia at the end of treatment, followed by reappearance of HCV-RNA during follow-up while non-responders were defined by HCV-RNA positivity throughout the study (partial or null responders).
Before the introduction of PEG-IFN as a therapeutic option, patients were treated with 3MU (million units) of interferon alpha (IFN-α) subcutaneously, thrice weekly, for 48 weeks (Intron-A©, Schering, Kenilwoth, NJ, USA or Roferon©, Hoffman La Roche, Basel, Switzerland). Only after 2002 when pegylated interferon-α had been widely implemented as a first-line treatment for chronic hepatitis C, as well as proven safe for Thalassemic patients,15 did we utilize PEG-IFN (Pegintron, Schering, Kenilwoth, NJ, USA and Pegasys, Hoffman La Roche, Basel, Switzerland) in doses of 100, 135 or 180 µg, subcutaneously, once weekly for 24 (genotypes 2, 3) or 48 weeks (genotypes 1, 4).
The interval between transfusions was reduced if it was necessary to maintain the hemoglobin levels between 9 and 11 g/dL. Administration of granulocyte colony stimulating factors (GM-CSF) was permitted in patients with severe neutropenia (absolute neutrophil count < 500/mm3) resulting from interferon treatment.
Statistical analysisValues are expressed as mean (SD) or median (range) as appropriate. Quantitative data were compared with the sample Student’s t-test or with Mann-Whitney U test for non-parametric variables. Categorical data were analyzed using the χ2-test with Yate’s correction or Fisher’s exact test as appropriate. Cox regression analysis (using forward selection) was used to identify independent predictors of SVR. The contribution of each significant variable to the probability of reaching the end-point was estimated by the relative hazard with its 95% confidence intervals (CIs). All statistical analyses were performed using SPSS 16.0 for Windows (SPSS, Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant.
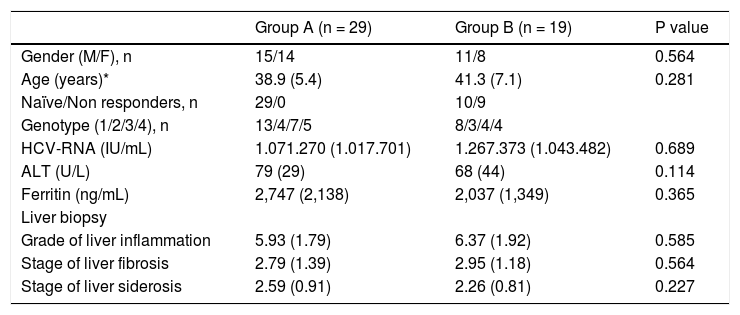
ResultsPatients characteristicsOur cohort consisted of 48 adult patients (26 male-22 female, mean age: 39.8 years) with TM and chronic hepatitis C. Baseline characteristics of the patients who received treatment with conventional interferon (group A) and of those who received Pegylated interferon (group B) are presented in table 1. Of them, 39 (all 29 group A pts and 10 group B pts) had not received treatment previously (naïve) and 9 were previously experienced pts. Genotype 1 predominated (21 pts, 44%) followed by genotypes 3 (11 pts, 23%), 4 (9 pts, 19%) and 2 (7 pts, 14%). Mean (SD) ALT values at the enrollment were 75 (36) IU/mL and mean (SD) ferritin values were 2,707 (1,883) ng/mL. Fifty eight percent of the patients had high HCV-RNA levels of more than 600,000 IU/mL and the mean (SD) HCV-RNA levels before treatment were 1.148.895 (1.491.866) IU/mL. Three patients (6.25%) had cirrhosis and 8 (16.7%) more had severe fibrosis in the liver biopsy. Mean (SD) grade of liver inflammation was 5.63 (1.76), mean (SD) stage of fibrosis 2.7 (1.54) and mean (SD) of liver sidirosis 2.74 (0.94).
Baseline characteristics of the patients receiving either conventional (group A) or pegylated interferon (group B).
| Group A (n = 29) | Group B (n = 19) | P value | |
|---|---|---|---|
| Gender (M/F), n | 15/14 | 11/8 | 0.564 |
| Age (years)* | 38.9 (5.4) | 41.3 (7.1) | 0.281 |
| Naïve/Non responders, n | 29/0 | 10/9 | |
| Genotype (1/2/3/4), n | 13/4/7/5 | 8/3/4/4 | |
| HCV-RNA (IU/mL) | 1.071.270 (1.017.701) | 1.267.373 (1.043.482) | 0.689 |
| ALT (U/L) | 79 (29) | 68 (44) | 0.114 |
| Ferritin (ng/mL) | 2,747 (2,138) | 2,037 (1,349) | 0.365 |
| Liver biopsy | |||
| Grade of liver inflammation | 5.93 (1.79) | 6.37 (1.92) | 0.585 |
| Stage of liver fibrosis | 2.79 (1.39) | 2.95 (1.18) | 0.564 |
| Stage of liver siderosis | 2.59 (0.91) | 2.26 (0.81) | 0.227 |
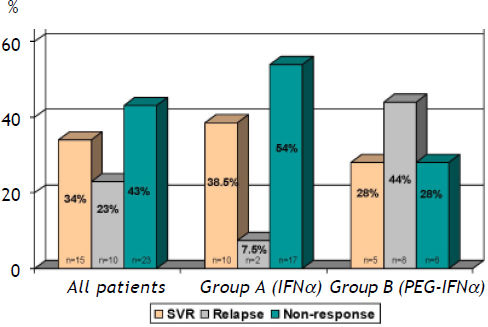
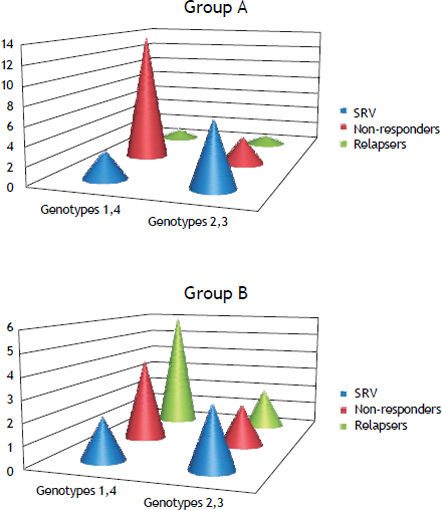
Sustained virological response (SVR) was achieved in 15 (34%) patients, 10 (23%) patients relapsed (negative HCV-RNA at the end of treatment and detectable within six months after the end of treatment) and (43%) were non-responders (nulls and partial responders HCV-RNA) (Figure 1). Twenty nine patients (60%) (15 male-14 female) were treated with conventional IFNa. SVR was achieved in 10 (38.5%) patients, 2 (7.5%) patients relapsed and 17 (54%) patients did not respond to treatment (Figure 1). The remaining 19 patients (40%) (11 male-8 female) received PEG-IFN. Ten patients were naïve and 9 were previous experienced to conventional IFNa. Five patients (28%) achieved SVR, 8 (44%) patients relapsed after the end of treatment and 6 (28%) patients never responded (Figure 1). Response rates were not significantly different in patients who received conventional IFN (group A) as compared to patients who received pegylated IFN (group B) (38.5 vs. 28%, p = 0.531). Moreover, SVR in naïve pts of group B was not statistically different from SVR of those who were previously experienced to conventional IFN (30 vs. 22%, p = 0.998) (Figure 2). Post-treatment aminotransferase levels and ferritin values did not differ significantly in comparison to pre-treatment values.
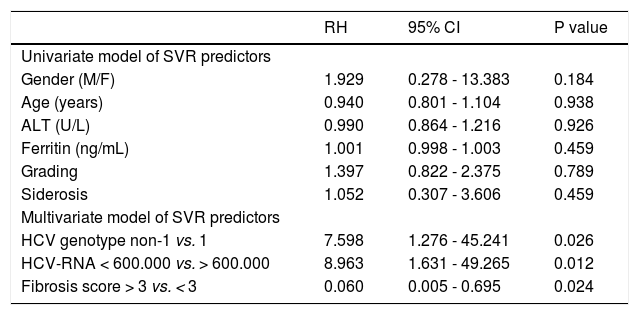
We further investigated baseline variables that might be associated with the achievement of SVR. Variables that were included in the analysis were gender, age, ALT, ferritin, serum HCV-RNA levels (> 600,000 IU/mL vs. < 600,000 IU/mL), genotype (1 vs. non-1), grade of liver inflammation, staging of liver fibrosis (> 3 vs. < 3) and hepatic hemosiderosis. On univariate analysis, gender (p = 0.184), age (p = 0.938), ALT (p = 0.926) ferritin (p = 0.459), grade of liver inflammation (p = 0.789) and hepatic hemosiderosis (p = 0.459) were not correlated with SVR. Multivariate analysis revealed that high HCV-RNA levels, genotype 1 and advanced stage of liver fibrosis were independently associated with no response to treatment (Table 2).
| RH | 95% CI | P value | |
|---|---|---|---|
| Univariate model of SVR predictors | |||
| Gender (M/F) | 1.929 | 0.278 - 13.383 | 0.184 |
| Age (years) | 0.940 | 0.801 - 1.104 | 0.938 |
| ALT (U/L) | 0.990 | 0.864 - 1.216 | 0.926 |
| Ferritin (ng/mL) | 1.001 | 0.998 - 1.003 | 0.459 |
| Grading | 1.397 | 0.822 - 2.375 | 0.789 |
| Siderosis | 1.052 | 0.307 - 3.606 | 0.459 |
| Multivariate model of SVR predictors | |||
| HCV genotype non-1 vs. 1 | 7.598 | 1.276 - 45.241 | 0.026 |
| HCV-RNA < 600.000 vs. > 600.000 | 8.963 | 1.631 - 49.265 | 0.012 |
| Fibrosis score > 3 vs. < 3 | 0.060 | 0.005 - 0.695 | 0.024 |
Both IFN-α and PEG-IFN were generally well-tolerated and the most commonly reported adverse effect was flu-like syndrome (headache, fever, cough, malaise). One patient’s anemia deteriorated and had to temporarily increase the transfusion rate to once weekly, whereas in another patient had to receive granulocyte colony stimulating factor to boost the absolute neutrophil count. In both patients we were able to continue the treatment. Treatment was discontinued in four patients (3 treated with IFN-α, 1 with PEG-IFN). Two patients presented serious adverse events (neutropenic sepsis and severe anemia respectively). They were hospitalized and recovered after therapeutic intervention. The other two withdrew consent to continue therapy due to patient decision because of severe weakness and poor quality of life.
DiscussionThalassemic patients who were transfused before 1990s had a high probability of been infected with HCV, a risk to be proportional to the number of units of blood received and approaching 80% in the adult population.21 Improvements in blood transfusion therapy in combination with iron chelation therapy have achieved a substantial increase in the survival and quality of life of patients with TM. However, liver disease mainly due to HCV infection and hepatic iron overload represents an important cause of morbidity and mortality in these patients.22 Liver transplantation could not be considered in the majority of this group of patients because of other serious causes of comorbitily such as cardiac and infection problems. Therefore, clinical management of chronic hepatitis C in TM patients is mandatory to control liver inflammation, prevent the development of cirrhosis and improve the prognosis.
In this study, we treated with IFNa (conventional or pegylated) our cohort of patients with TM and chronic hepatitis C for a period of 48 weeks. This treatment schedule was effective in 34% of our patients, who achieved SVR. Our results are in agreement with those of other studies where IFNa monotherapy was administered for 6 to 18 months and induced SVR in about 40% of patients.23–25
It is worth to note that our patients have achieved response rates to IFNa monotherapy comparable to those in non-Thalassemics.26 It has been suggested that the young age of Thalassemic patients and the short duration of hepatitis may play important roles in the treatment outcome.5 However, in our study only adults have been included (mean age 39.8 ± 6.2). Alternatively, multiple blood transfusions required by thalassemic patients may have immunomodulatory effects or other biological properties that enhance the therapeutic effect of interferon-α.27
Nineteen of our patients received PEG-IFN after its implementation as a first-line treatment for chronic hepatitis C. Almost half of them (9 out of 19) represented a difficult treatment group as they hadn’t responded in a previous course of therapy with conventional IFNa. This fact has probably contributed to the lower response rate of patients who received PEG-IFN (28%) vs. those received conventional if (38.5%). However this difference was not statistically significant (see results). However, 28% of those patients achieved SVR. This is in agreement with previous studies where PEG-IFN monotherapy has been used in a small number of naïve TM patients with CHC with response rates ranging from 13 to 43%.24,25 These results as well as the results of our study show that a significant number of TM patients infected with HCV benefit from the administration of IFN-α alone. Thus, the addition of ribavirin which is a major factor of SVR, should be considered with cautiousness difficult in this group and may be indicated for the difficulty to treat subgroup of genotype 1 infected patients, with severe fibrosis and increased hepatic iron concentration.
We further investigated the factors that may be associated with SVR in TM patients with CHC. In the multivariate analysis, genotype 1, high viral load and severe fibrosis or cirrhosis were independently associated with less probability of response to treatment. These findings are consistent with the results of previous studies performed in non-Thalassemic patients with CHC.26,28 Moreover, a correlation between increased hepatic iron concentration (HIC) and failure to attain an SVR has been demonstrated in previous studies conducted in non-Thalassemic patients with CHC.17,18 However, this finding is controversial among Thalassemic patients.19,28 Similarly to others, in our cohort, serum ferritin and liver siderosis score did not reach statistical significance for predicting SVR. It is important to clarify that a raised HIC in Thalassemic patients may not be directly comparable to a raised HIC in non-Thalassemic patients as it is primarily due to parenterally acquired iron. Thus, in the latter, a high HIC is probably a better reflection of disease severity than in Thalassemia, where this is not sure to be a reliable fact.29,30
None of our patients received combination therapy with IFN and ribavirin, thus conventional IFN monotherapy has to be extended to 48 weeks independently of HCV genotype while pegylated IFN was given for 24 or 48 weeks according to HCV-genotype. Ribavirin is well tolerated but its major adverse effect is hemolysis related to oxidative damage. The hemolytic effects of ribavirin gain much greater importance in TM patients.21 Limited trials with IFN and ribavirin in patients with TM resulted in a 30–40% increase of blood requirement and prompted an associated increase in chelation therapy.24,32 This has led to specific contraindication to the use of ribavirin in Thalassemia and in other hemolytic anemias. However, recently published studies found a beneficial effect of combination therapy with PEG-IFN and ribavirin in the achievement of SVR in TM patients, providing a greater care is taken in monitoring and, if necessary, increasing chelation therapy.13,21,31 These findings should be considered with cautiousness and subsequent studies may identify certain subgroups of Thalassemic patients who would benefit more from the addition of ribavirin to the treatment regimen.
The treatment was well tolerated in the majority of our patients with minor and common side-effects usually attributed to IFNa. Forty four patients (91%) were able to complete the treatment schedule although dose reductions were required in some of them. We did not observe an increase in transfusion requirements during treatment, probably because of no administration of ribavirin.
Limitations of this study are the small number of group B patients, as well as the fact that 9/19 of them were interferon experienced but their SVR to peg-IFN was not statistically different from that of the naïve patients of this group. These factors may hinder the expected difference between conventional and pegylated IFN response rate. Despite of these limitations, our results indicate that both conventional and pegylated interferon-α treatment is well tolerated and not inferior to that in non-multi-transfused patients receiving IFN monotherapy. This approach should be recommended as initial treatment schedule in this setting, while ribavirin may be reserved for the difficult to treat subgroup of Thalassemic patients.
Abbreviations- •
HCV: hepatitis C virus.
- •
TM: ß Thalassemia major.
- •
SVR: sustained virological response.
- •
IFNa: interferon alpha.
- •
PEG-IFN: pegylated interferon alpha.
None to declare.