Autoimmune liver diseases (AILD) are rare causes hepatocellular carcinoma (HCC), and data on the efficacy and tolerability of anti-tumor therapies are scarce. This pan-European study aimed to assess outcomes in AILD-HCC patients treated with tyrosine kinase inhibitors (TKIs) or transarterial chemoembolization (TACE) compared with patients with more common HCC etiologies, including viral, alcoholic or non-alcoholic fatty liver disease.
Materials and Methods107 patients with HCC-AILD (AIH:55; PBC:52) treated at 13 European centres between 1996 and 2020 were included. 65 received TACE and 28 received TKI therapy. 43 (66 %) were female (median age 73 years) with HCC tumor stage BCLC A (34 %), B (46 %), C (9 %) or D (11 %). For each treatment type, propensity score matching was used to match AILD to non-AILD-HCC on a 1:1 basis, yielding in a final cohort of 130 TACE and 56 TKI patients for comparative analyses of median overall survival (mOS) and treatment tolerability.
ResultsHCC-AILD patients showed comparable mOS to controls for both TACE (19.5 vs. 22.1 months, p = 0.9) and TKI (15.4 vs. 15.1 months, p = 0.5). Adverse events were less frequent in AILD-HCC patients than controls (33 % % vs. 62 %, p = 0.003). For TKIs, there were no significant differences in adverse events (73% vs. 86%, p = 0.2) or interruption rates (44% vs. 36 %, p = 0.7).
ConclusionsIn summary, this study demonstrates comparable mOS for AILD-HCC patients undergoing local and systemic treatments, with better tolerability than HCC of other causes. TKIs remain important therapeutic options for AILD-HCC patients, particularly given their exclusion from recent immunotherapy trials.
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related deaths worldwide [1]. Only early stage tumors are eligible for curative treatment by resection, ablation, or transplantation. However, most patients present with advanced disease and can only be offered palliative treatment, such as transarterial chemoembolization (TACE) or systemic therapy [2]. Until 2017, systemic therapy was limited to the tyrosine kinase inhibitor (TKI) sorafenib. Since then, the treatment landscape has drastically changed, and checkpoint inhibitor-based combination therapies have evolved as a new standard of care in the first-line setting [3,4].
The most common etiologies leading to HCC include alcohol and non-alcoholic steatohepatitis as well as chronic hepatitis B and C. An infrequent cause of HCC is cirrhosis due to autoimmune liver disease (AILD) [5,6]. AILD refers to chronic liver disease with an incidence of approximately 25 per 100 000 individuals and a female predominance (ratio 4:1) [7]. Primary AILD includes autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC). Owing to its low incidence, AILD-associated HCC is underrepresented in studies on systemic therapies. Moreover, patients with AIH have been excluded from recent clinical trials testing new immunotherapeutic therapies because of concerns that exacerbation of the underlying autoimmune disease or potentially severe adverse events may occur [4,8]. Hence, TKI and ramucirumab remain the only established systematic treatment options for patients with active AIH [9]. Overall, data on treatment efficacy and tolerability are scarce, and no specific treatment guidelines exist [10-12].
The objective of this study was to assess both treatment responsiveness and tolerability in patients with AIH- and PBC-associated HCC undergoing systemic therapy with TKI or locoregional therapy with TACE compared to patients with more frequent etiologies including viral, alcohol-associated, or non-alcoholic fatty liver disease.
2Material and methods2.1Study designA retrospective observational study was conducted by the European Reference Network on Hepatological Diseases (ERN RARE-LIVER) in the following 13 European centers: Frankfurt (Germany), Hamburg (Germany), Hannover (Germany), Mainz (Germany), Vienna (Austria), Paris (France), Larissa (Greece), Debrecan (Hungary), Milan (Italy), Leuven (Netherlands), Nijmegen (Netherlands), Warsaw (Poland) and London (United Kingdom), and the study was performed in accordance with the World Medical Association Declaration of Helsinki.
2.2PatientsA total of 107 adults diagnosed with AILD-associated HCC (AIH, n = 55; PBC, n = 52) who were treated between 1996 and 2020 were initially enrolled in this study. Patients who received TACE, TKI therapy, or both were included in the final analysis (n = 72; AIH, n = 38; PBC, n = 34), as indicated in Fig. 1. An additional cohort of 347 HCC patients with underlying viral or non-alcohol-related liver disease from the Medical Center Hamburg-Eppendorf who received TACE, TKI, or both served as the control group.
Patient enrolment and exclusion flowchart.
Propensity Score matching 1:1 was performed for the TACE and systemic treatment groups separately, thereby adjusting for age, Barcelona Clinic for Liver Cancer Classification, Child-Pugh stage, and physical performance status. 21 AILD-HCC patients received subsequently both TACE and systemic treatment and were included in both the groups. AILD, autoimmune liver disease; ASH, alcoholic steatohepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; NASH, non-alcoholic steatohepatitis; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor.
All clinicopathological data were collected using a customized electronic medical record form, including the Barcelona Clinic for Liver Cancer (BCLC) classification, Child-Pugh stage, and Eastern Cooperative Oncology Group (ECOG) classification. Treatment intolerability was defined as the occurrence of at least one adverse event or interruption of treatment due to adverse events. Only treatment-related side effects were considered adverse events. Pre-defined adverse events for TACE included emesis, fatigue, fever, nausea, and right upper quadrant pain as well as diarrhea, emesis, fatigue, fever, hand-foot-skin reaction (HFSR), malaise, nausea, and skin reaction for systemic treatment.
2.4Propensity score matchingFor the comparison of AILD- with non-AILD-associated HCC patients, propensity score matching (PSM) was applied to balance the differences in major clinical confounders. Propensity scores were calculated via logistic regression analysis using the following variables: age, sex, BCLC, Child-Pugh stage, and ECOG physical performance status. Patients with AILD and non-AILD-associated HCC were matched 1:1 based on their propensity scores using the optimal matching method. This was performed separately for the TACE and TKI treatment groups. Due to the extremely imbalanced sex ratio, it was not possible to fully compensate for the gender differences.
2.5Statistical analysisQuantitative non-normally distributed variables were expressed as median ± interquartile range (IQR). Percentages are based on the respective subgroups, as indicated. The associations between the quantitative and qualitative variables were assessed using the Wilcoxon rank test for non-normal distributions. The relationship between categorical variables was tested using the Chi-Square or Fisher's exact test when the sample size was <5. The Kaplan-Meier method was applied to generate median overall survival (mOS) curves, and differences were compared using the log-rank test. The mOS was calculated using associated 95 % confidence intervals (CI). Cox proportional hazard models with hazard ratios (HR) and 95 % CI were used to estimate the group effect, including sex as a covariate. The patients were censored on the day of the last follow-up. For secondary outcome analysis, the Wilcoxon rank test was used to compare the number of patients with at least one adverse event and the overall prevalence of each adverse event between patients with and non-AILD patients. All tests were two-tailed at a significance level of 0.05. Analyses were performed using GraphPad Prism Software (version 9.2.0; GraphPad Software Inc., La Jolla, CA, USA) and R Studio (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
2.6Ethical declarationsThe study was approved by the Ethics Committee of the Ärztekammer Hamburg, Germany (approval No. PV3578, December 2010). All included patients signed a consent form before study inclusion.
3Results3.1Baseline demographics and clinical characteristicsA total of 72 patients with AILD-associated HCC treated with TACE, systemic therapy, or both from 13 centers in 10 European countries were included in the final analysis (Fig. 1). For the evaluation of TACE treatment, 65 AILD-associated HCC patients and an equal number of non-AILD-matched control patients were considered. Therefore, the subtype of AILD was equally distributed in 34 AIH and 31 PBC cases. Disease characteristics were well balanced between AILD-HCC and matched controls, except for a female predominance in the AILD group (66 %), whereas most patients were male in the control group (75 %). The median age was 71 and 70 years in the AILD and matched control groups, respectively. Most patients had an ECOG status of 0 (71 % for AILD vs. 65 % for match) and Child-Pugh A (74 % for AILD vs. 69 % for match). Tumor stage was classified as BCLC A (34 % for AILD vs. 25 % for match), B (46 % for AILD vs. 69 % for match), C (9 %for AILD vs. 12 % for match), or D (11 % for AILD vs. 3 % for match) (Table 1).
Baseline characteristics of propensity score matched patients according to underlying liver disease and treatment received.
| Characteristics | TACE | Systemic treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALD = 65 | AIH = 34 | PBC = 31 | Non-AILD = 65 | p Value (ALD vs. non AILD) | ALD = 28 | AIH = 15 | PBC = 13 | Non-AILD = 28 | p Value (ALD vs. non-AILD) | |
| Male gender, n (%) | 22 (34) | 10 (29) | 12 (39) | 49 (75) | <0.001 | 9 (32) | 4 (27) | 5 (38) | 22 (79) | <0.001 |
| Median age, y [IQR] | 71 [64–77] | 70 [64–75] | 73 [67–78] | 70 [63–75] | 0.3 | 69 [64–74] | 65 [60–72] | 71 [69–74] | 70 [66–73] | 0.8 |
| ECOG, n (%) | 0.7 | 0.4 | ||||||||
| 0 | 46 (71) | 26 (76) | 20 (65) | 42 (65) | 13 (46) | 7 (47) | 6 (46) | 17 (61) | ||
| 1 | 15 (23) | 6 (18) | 9 (29) | 18 (28) | 14 (50) | 7 (47) | 7 (54) | 11 (39) | ||
| 2 | 2 (3) | 1 (3) | 1 (3) | 4 (6) | 1 (4) | 1 (7) | 0 (0) | 0 (0) | ||
| 3 | 2 (3) | 1 (3) | 1 (3) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Child Pugh, n (%) | 0.3 | 0.5 | ||||||||
| A | 48 (74) | 26 (76) | 22 (71) | 45 (69) | 22 (79) | 11 (73) | 11 (85) | 19 (68) | ||
| B | 12 (18) | 7 (21) | 5 (15) | 18 (28) | 6 (21) | 4 (27) | 2 (14) | 9 (32) | ||
| C | 5 (8) | 1 (3) | 4 (12) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| BCLC, n (%) | 0.2 | >0.9 | ||||||||
| A | 22 (34) | 13 (38) | 9 (29) | 16 (25) | 1 (4) | 0 (0) | 1 (8) | 2 (7) | ||
| B | 30 (46) | 17 (50) | 13 (42) | 39 (60) | 9 (32) | 4 (27) | 5 (38) | 9 (32) | ||
| C | 6 (8) | 3 (8) | 3 (9) | 8 (11) | 18 (64) | 11 (73) | 7 (54) | 17 (61) | ||
| D | 7 (10) | 1 (3) | 6 (19) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
Statistical significance was tested using Wilcoxon-rank test for continuous variables and Fisher's exact or Chi-Square test for categorical variables. Bold p-values are significant at p < 0.05. AIH = autoimmune hepatitis, AILD = autoimmune liver disease, BCLC = Barcelona Clinic Liver Cancer Classification, ECOG = Eastern Cooperative Oncology Group Performance Status, IQR = interquartile range, TACE = transarterial chemoembolisation, TKI = tyrosine kinase inhibitor, PBC = primary biliary cholangitis.
In the systematic therapy group, 28 patients with AILD and non-AILD-HCC were included, all of whom were treated with TKI. 15 AIH and 13 with PBC constituted the systemic treatment group. As was the case for TACE, also in the TKI group, AILD patients were mostly female (68 %) and non-AILD patients were mostly male (79 %). The median age was 69 and 70 years in the AILD and matched controls, respectively. Most patients were of ECOG 0 (46 % for AILD vs. 61 % for match) or 1 (50 % for AILD vs. 39 % for match) and Child-Pugh A (79 % for AILD vs. 68 % for match) or B (21 % for AILD vs. 32 % for match). The most frequent tumor stage was BCLC C (64 % for AILD vs. 61 % for match), followed by BCLC B (32 % for both AILD and Match) (Table 1).
3.2Treatment efficacyFor TACE, there was no significant difference in mOS between AILD and non-AILD-associated HCC patients (mOS 19.5 months for AILD vs. 22.1 months for non-AILD, p = 0.9) (Fig. 2A). In patients under TKI treatment, mOS was equally comparable between AILD and matched controls (mOS 15.4 months for AILD vs. 15.1 months for non-AILD, p = 0.4) (Fig. 2B).
Kaplan-Meier estimates comparing median overall survival (mOS) between patients with autoimmune liver disease (AILD) associated and non-AILD associated hepatocellular carcinoma (HCC).
Comparison of median overall survival (mOS) between patients with AILD and non-AILD-associated HCC receiving TACE (A) or TKI (B). Subgroup analysis of mOS in AIH and PBC vs. non-AILD patients treated with TACE (D) or TKI (E). The log-rank test was performed to test for statistical differences between the groups. Sex-adjusted hazard ratios for death are reported along with the p-values. CI, confidence interval; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor.
In the subgroup analysis of AILD patients treated with TACE, a trend of lower mOS in AIH patients compared to PBC and non-AILD-HCC patients emerged, however, the difference was not statistically significant due to the small number of patients (mOS 12.0 months for AIH, 20.4 months for PBC and 22.1 months for Match, p = 0.4) (Fig. 2C). Under systemic treatment with TKI, there was a similar trendency for shorter mOS in AIH (mOS 5.3 months) than in PBC patients (mOS 15.8 months) or matched controls (mOS 15.1 months), but again without reaching statistical significance (p = 0.6) (Fig. 2D).
3.3Treatment safetyFollowing TACE, 17/52 (33 %) patients with AILD in the AILD cohort experienced one or more adverse events of any grade, which was significantly less frequent than in the control group (40/65, 62 %) (p = 0.002) (Table 2). Most notably, post-TACE syndrome-related symptoms occurred significantly less frequently in the AILD-HCC cohort than in matched controls. The symptoms included nausea (3 % for AILD vs. 22 % for controls, p = 0.016), emesis (2 % for AILD vs. 23 % for controls, p < 0.001), fever (0 % for AILD vs. 35 % for controls, p < 0.01), and right upper quadrant pain (2 % for AILD vs. 37 % for controls, p < 0.001). Among the AILD subgroups, patients with PBC had the lowest rate of adverse events 6/24 (25 %).
Adverse events from transarteial chemoembolisation (TACE).
| TACE | ||||||
|---|---|---|---|---|---|---|
| All n = 117 | AILD n = 65 | AIH n = 34 | PBC n = 31 | Non-AILD n = 65 | p Value | |
| Available Data | n = 117 | n = 52 | n = 28 | n = 24 | n = 65 | (AILD vs. non-AILD) |
| RUQ pain | 26 (22) | 2 (4) | 1 (4) | 1 (4) | 24 (37) | <0.001 |
| Fever | 23 (20) | 0 (0) | 0 (0) | 0 (0) | 23 (35) | <0.001 |
| Nausea | 17 (14) | 3 (6) | 1 (4) | 2 (8) | 14 (22) | 0.016 |
| Emesis | 16 (13) | 1 (2) | 0 (0) | 1 (4) | 15 (23) | <0.001 |
| Fatigue | 13 (10) | 2 (4) | 1 (4) | 1 (4) | 11 (16) | 0.025 |
| Other | 23 (20) | 12 (23) | 9 (32) | 3 (11) | 11 (16) | 0.40 |
| ≥1 adverse event | 57 (49) | 17 (33) | 11 (39) | 6 (25) | 40 (62) | 0.002 |
Statistical significance was assessed using Chi-Square test. Bold p-values are significant at p < 0.05. AIH = autoimmune hepatitis, AILD = autoimmune liver disease, RUQ = right upper quadrant, PBC = primary biliary cholangitis.
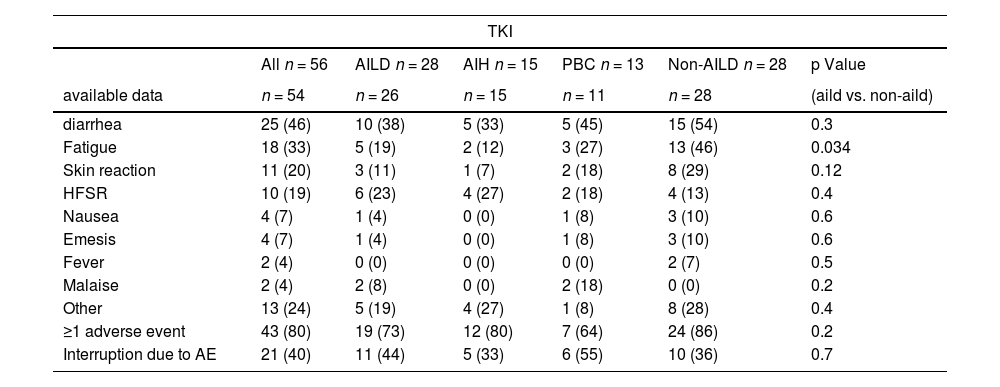
Among patients receiving systemic treatment, there was no significant difference in the occurrence of any grade of adverse events between AILD and non-AILD patients (≥1 adverse event: 19/26, 73 % for AILD vs. 24/28, 86 % for match, p = 0.2) (Table 3). Most importantly, the frequency of treatment interruptions due to severe adverse events was not significantly higher in patients with AILD than in those without non-AILD patients.
Adverse events from systemic tyrosine kinase inhibitor (TKI) treatment.
| TKI | ||||||
|---|---|---|---|---|---|---|
| All n = 56 | AILD n = 28 | AIH n = 15 | PBC n = 13 | Non-AILD n = 28 | p Value | |
| available data | n = 54 | n = 26 | n = 15 | n = 11 | n = 28 | (aild vs. non-aild) |
| diarrhea | 25 (46) | 10 (38) | 5 (33) | 5 (45) | 15 (54) | 0.3 |
| Fatigue | 18 (33) | 5 (19) | 2 (12) | 3 (27) | 13 (46) | 0.034 |
| Skin reaction | 11 (20) | 3 (11) | 1 (7) | 2 (18) | 8 (29) | 0.12 |
| HFSR | 10 (19) | 6 (23) | 4 (27) | 2 (18) | 4 (13) | 0.4 |
| Nausea | 4 (7) | 1 (4) | 0 (0) | 1 (8) | 3 (10) | 0.6 |
| Emesis | 4 (7) | 1 (4) | 0 (0) | 1 (8) | 3 (10) | 0.6 |
| Fever | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (7) | 0.5 |
| Malaise | 2 (4) | 2 (8) | 0 (0) | 2 (18) | 0 (0) | 0.2 |
| Other | 13 (24) | 5 (19) | 4 (27) | 1 (8) | 8 (28) | 0.4 |
| ≥1 adverse event | 43 (80) | 19 (73) | 12 (80) | 7 (64) | 24 (86) | 0.2 |
| Interruption due to AE | 21 (40) | 11 (44) | 5 (33) | 6 (55) | 10 (36) | 0.7 |
Statistical significance was assessed using Chi-Square test. Bold p-values are significant at p < 0.05. AE = adverse event, AIH = autoimmune hepatitis, AILD = autoimmune liver disease, HFSR = hand food skin reaction, PBC = primary biliary cholangitis.
This is the first study to assess the treatment efficacy of both systemic treatment with TKI and locoregional therapy with TACE in an extremely rare patient population with AILD-associated HCC. Overall, AILD patients showed a similar mOS for both treatment modalities compared to a matched cohort of HCC patients with other, more frequent, non-AILD etiologies. In the subgroup analysis, a trend for shorter mOS for AIH patients compared to PBC and non-AILD patients for both TKI and TACE treatment appeared, however, without statistical significance. This potentially inferior response in AIH patients’ needs to be validated and confirmed in future studies with a larger cohort size.
Assessment of TACE treatment tolerability revealed that the incidence of symptoms related to post-TACE syndrome was significantly lower in patients with AILD-HCC than in non-AILD patients. Previous studies have reported that dexamethasone effectively prevents the occurrence of TACE-induced AE [13,14]. Since the standard treatment for AIH patients is based on steroids and azathioprine, the reduced incidence of post-TACE syndrome in this subgroup may be at least partly due to the protective effect of corticosteroids. However, for patients with PBC who do not receive steroid therapy on a regular basis but are mostly treated with ursodeoxycholic acid, the mechanism remains unclear. It is possible that due to the large proportion of patients with toxic liver diseases (NASH and ASH) in the non-AILD-HCC group, who often have multiple comorbidities, AE occurred more frequently in this subgroup. Another possible explanation may be related to the fact that symptoms defining post-TACE embolization syndrome are commonly present in most AILD patients prior to the start of locoregional HCC treatment. Therefore, a mild aggravation of these symptoms caused by TACE therapy may not have been registered as treatment-related AE in these patients but may have been attributed to their AILD.
Regarding the overall incidence of adverse events following TKI treatment, there was no difference between AILD- and non-AILD-HCC patients, with the exception of fatigue, which was significantly less frequent in AILD-HCC patients. This observation was unexpected because fatigue is a common symptom associated with hepatobiliary autoimmune diseases [15,16]. Again, this may be explained by the fact that these patients already suffered from fatigue before the start of HCC treatment; therefore, it was not registered as an AE related to TKI therapy. Most importantly, there was no difference in the rate of treatment interruption owing to severe AE. Subgroup analysis revealed that there was a trend for overall adverse events to be less frequent in PBC; however, a greater proportion led to treatment interruption.
Our study has several limitations. The patients with AILD-associated HCC included in this study were treated at 13 different centers, whereas data on control patients with non-AILD-HCC were retrieved only from a single center. Recording of side effects and scores, such as performance status, depends on the individual judgement of each investigator assessing the patient, may vary between different centers, and there is potential underreporting due to the retrospective design of the study. Specifically, new-onset arterial hypertension as an AE of HCC therapy was not well documented and therefore not included in the final analysis. Moreover, only the overall incidence of AE was registered, and it may be argued that severe AE (grade 3 or higher) are of clinical relevance. However, the fact that a similar number of patients with AILD-HCC and non-AILD-HCC stopped TKI treatment due to AE strongly suggests that severe AE did not occur more frequently in AILD patients. Being a pan-european project involving many tertiary centres, some patients were treated with TACE in advanced BCLC stages (C and D) as individual therapeutic approaches although international guidelines do not routinely recommend TACE as a treatment option in these later stages of HCC. Another limitation of this study was that due to opposing sex ratios in the two groups, it was not possible to adjust sufficiently for sex differences between AILD and matched control patients. To compensate for this imbalance, sex was included as a covariate in the Cox regression model of mOS. Due to the small cohort size in the subgroup analysis, the statistical power was limited and insufficient to determine whether the trend of shorter mOS in AIH patients was due to an inferior treatment response. Further studies with larger sample sizes are needed to evaluate whether there is a significant difference and what this can be attributed to. Owing to the rarity of AILD-associated HCC, prospective trials with a sufficient cohort size are not feasible. Hence, despite the limitations of retrospective studies owing to their design, they remain important tools for evaluating treatment outcomes in underrepresented subgroups of patients with HCC.
5ConclusionsIn this study, we provide compelling evidence that local treatment with TACE and systemic therapy with TKI are efficacious and safe for patients with AILD-associated HCC. Since the majority of these patients have been excluded from recent immunotherapy trials, TKI and TACE remain important treatment options. The potential trend of inferior outcomes in patients with AIH should be further assessed in consecutive studies with larger cohorts.
Author contributionsStudy conception and design: Kornelius Schulze, Johann von Felden, Louisa Stern, Constanting Schmidt. Acquisition of data: Lorenz Kocheise, Vincent Joerg, Christian Casar, Aurélie Walter, Joost P.H. Drenth, Maria Papp, Nikolaos K. Gatselis, Kalliopi Zachou, Matthias Pinter, Bernhard Scheiner, Arndt Vogel, Martha M. Kirstein, Fabian Finkelmeier, Oliver Waidmann, Arndt Weinmann, Piotr Milkiewicz, Douglas Thorburn, Neil Halliday, Ana Lleo, Samuel Huber, George N. Dalekos, Ansgar W. Lohse, Henning Wege, Kornelius Schulze, Johann von Felden, Louisa Stern, Constanting Schmid. Data analysis: Christian Casar, Louisa Stern, Constanting Schmidt. Interpretation of the data: Kornelius Schulze, Johann von Felden, Ansgar W. Lohse, Henning Wege, Louisa Stern, Constanting Schmidt. Manuscript drafting: Louisa Stern,
All the authors participated in revising the article critically and provided their final approval for the publication of this manuscript.