Background/Aims: Thymalfasin has shown efficacy in the treatment of chronic HCV infection. The aim of this study was to evaluate the efficacy and tolerability of triple therapy with thymalfasin, peginterferon α-2a (PEG-IFN α-2a), and ribavirin in Hispanic patients with chronic viral hepatitis C who were nonresponders to prior treatment with interferon alfa (IFN-α)/ribavirin. Methods: In this open-label study, 40 subjects received thymalfasin (1.6 mg twice a week), PEG-IFN α-2a (180 μg once a week), and ribavirin (800-1,000 mg/ day) for 48 weeks. All patients had positive HCV RNA by PCR analysis, abnormal levels of ALT, compensated hepatic disease, and liver biopsy with chronic damage. Results: Viral response was observed in 52.5% patients at week 12 and 50% at week 24. Of the per protocol group, 52.6% showed an end-of-treatment response at week 48 and 21.1% achieved an SVR at week 72. Among genotype 1 patients, 23.5% achieved an SVR at week 72. A reduction of the dose of PEG IFN α-2a and ribavirin was required. Thymalfasin was well tolerated without dose reduction. Conclusion: Triple therapy with thymalfasin, PEG IFN α-2a, and ribavirin is an effective treatment option for difficult-to-treat HCV patients who are refractory to prior conventional treatment, with adequate tolerability.
Hepatitis C virus (HCV) is a leading cause of liver cirrhosis and hepatocellular carcinoma1,14,24 and the most common indication for liver transplantation. The World Health Organization (WHO) estimates that 170 million individuals are infected with HCV worldwide.2 Nearly 4 million people in the United States alone are infected with HCV(3) and 864,000 Mexicans according to a recent open population based National Survey involving 20,288 participants.4
According to the American Association for the Study of Liver Disease (AASLD), the goal of treatment is to prevent complications of HCV by eradicating the infection and achieving a sustained virologic response (SVR), which is defined as normal alanine aminotransferase (ALT) levels and the absence of HCV RNA in serum at 6 months after cessation of therapy. Patients who achieve an SVR almost always have a dramatic early virologic response (EVR), which is generally referred to as a 2-log drop or loss of HCV RNA at 12 weeks of therapy. Continued absence of detectable virus at termination of treatment is referred to as end of treatment response (ETR). A patient is considered to have relapsed when HCV RNA becomes undetectable with treatment but is detected again after discontinuation of treatment. Nonresponders are patients who are not HCV RNA negative at the end of therapy. Nonresponders to standard therapies currently have limited therapeutic options for re-treatment.
The majority of patients who are infected with genotype 1 HCV and have high viral loads do not achieve a sustained response.5 Of genotype 1 patients who did not respond to an initial 12-week course of IFN α-2a and ribavirin, only 12% achieved a sustained response when retreated with PEG IFN α-2a plus ribavirin.6 In addition, ethnicity tends to impact treatment outcomes, with African American and Hispanic HCV patients among the least responsive to treatment.7 The need to discontinue therapy due to adverse events further reduces the likelihood of achieving SVR.
Because current standard therapy does not adequately clear HCV RNA,8 especially in these difficult-to-treat populations,6 triple therapy regimens have been investigated.9,10 In treatment-naïve patients with chronic hepatitis C, 65.3% of patients treated with peginterferon alpha-2a (PEG IFN α-2a, Pegasys®, Roche Pharmaceuticals, NJ, USA), ribavirin (Rebetol™, Schering Corp, NJ, USA), and amantadine (Symmetrel®, Endo Pharmaceuticals Inc, PA, USA) achieved SVR vs 33.3% of patients treated with interferon alfa (IFN-α, Roferon® A, Roche Pharmaceuticals, NJ, USA), ribavirin, and amantadine.10 The efficacy of a triple therapy regimen including amantadine, IFN-α, and ribavirin for the treatment of nonresponders to previous IFN-α/ribavirin therapy is of particular interest and has shown encouraging results.11 In this study, 25% of patients achieved SVR at 1 year. However, among the subgroup of genotype 1 patients, only 12% achieved SVR.11
More recently, thymalfasin (thymosin alpha 1, Tα1, ZADAXIN™, SciClone Pharmaceuticals, Inc., CA, USA) has shown efficacy in the treatment of chronic HCV infection. Thymalfasin is a 28 amino acid peptide that increases the proliferation of lymphocytes CD3, CD4 and CD8, increases the activity of NK cells, and stimulates interferon alpha, interferon gamma, and IL2. In addition, it has a direct antiviral effect by increasing the expression of class I major histocompatibility complex (MHC) molecules and reducing viral replication.
Investigative studies in treatment-naïve patients have shown that thymalfasin plus IFN-α nearly doubles HCV RNA viral clearance compared to IFN-α therapy alone.1,12Among HCV genotype 1 nonresponders with high viral loads, thymalfasin in combination with PEG-IFN α-2a demonstrated a 30% early virological response at 12 weeks.13 These results suggest that many patients who were nonresponders to previous therapies may now be considered candidates for retreatment with more effective combination regimens.
Therefore, the objective of this pilot study was to determine whether triple therapy with thymalfasin in combination with a standard regimen of PEG IFN α-2a and ribavirin for the treatment of chronic hepatitis C nonresponders results in an increase in sustained virologic response (SVR). Nonresponders in this study were those patients who did not achieve HCV RNA clearance after at least 6 months of IFN-α/ribavirin combination therapy.
Patients and methodsThis was a multicenter, open-label trial to determine the safety and efficacy of 48 months of treatment with thymalfasin in combination PEG IFN α-2a plus ribavirin in adult patients with chronic hepatitis C who did not respond to previous therapy. The definition of «nonresponder » for this study applies to all the patients who did not show a virological (ie, HCV RNA clearance) response at the end of at least 6 months of IFN-α/ribavirin therapy.
PatientsForty adult patients from 4 centers in Mexico were enrolled in the study. All patients were infected with chronic HCV (positive HCV RNA) and were nonresponders to a previous 6-month course of IFN-α/ribavirin therapy. All patients provided written informed consent forms, which were sent to the institutional review board (IRB) of each center for approval.
Patients were 23 to 65 years of age (mean 48) and were positive for the anti-HCV antibody. The presence of HCV RNA was measured by quantitative PCR (Roche AmplicorTM) which was the method available at our institution at the time the study was started. Liver biopsy was consistent with chronic hepatitis C within 18 months before treatment began. Only Child-Pugh A cirrhosis was permitted. Patients had elevated ALT on at least 2 occasions 3 to 6 months apart prior to study entry. All patients were nonresponders to at least 6 months of previous therapy with IFN plus ribavirin. Patients were included if they had alpha-fetoprotein < 100 ng/L prior to study entry. In addition, patients with compensated liver disease with prothrombin activity > 50%, total bilirubin < 2 mg/dL, and no history of hepatic encephalopathy, esophageal varices or ascites were included. Patients were negative for hepatocellular carcinoma (HCC) by ultrasound and had the following lab values: hematocrit > 30%, hemoglobin > 10g/dL, platelet count > 100 x 109/L, white blood count > 3 x 109/L, polymorphonuclear white cell count > 1.5 x 109/ L, and serum creatinine level < 1.5 mg/dL. Normal TSH or hypothyroidism under hormone replacement therapy was also required for inclusion.
Patients were excluded from the study if they used systemic corticosteroids, any drug known to be hepatotoxic, any drug (other than the study drugs) known or suspected to have therapeutic activity in hepatitis C (such as aspirin or other non-steroidal anti-inflammatory drugs), or any immunosuppressive drug (azatioprine, or cyclosporine among others). Patients were also excluded if they had any other liver disease including hepatitis B, hepatitis delta, alcoholic liver disease, drug-induced liver injury, primary biliary cirrhosis, sclerosing cholangitis, autoimmune hepatitis, hemochromatosis, alpha 1-antitrypsin deficiency, or Wilson’s disease. Exclusion criteria also included decompensated liver disease based on a history of hepatic encephalopathy, esophageal varices or ascites, Child-Pugh B or C, HIV infection, concomitant or prior history of malignancy other than curatively treated skin cancer or surgically cured in situ carcinoma of the cervix, active infectious process other than HCV that is not of a self-limited nature (eg, tuberculosis and AIDS), rheumatoid arthritis or other autoimmune disease (serum ANA > 1:160, hemoglobinopathy or any disease associated with hemolysis, history of significant cardiac or pulmonary diseases that could be exacerbated by anemia, alpha-fetoprotein > 100 ng/L, pregnancy as documented by a urine pregnancy test, and alcohol or intravenous drug abuse within the previous 1 year.
All participants had a baseline liver biopsy. The degree of fibrosis was graded according to METAVIR scoring system.
Study design and treatment regimensThe study protocol was conducted in compliance with the International Standard GCP procedures and the principles of the Declaration of Helsinki. The protocol was approved by the local IRBs and the ministry of health.
Treatment consisted of PEG IFN α-2a (180 μg once weekly at bedtime) for 48 weeks plus thymalfasin (1.6 mg/twice a week in the morning) for 48 weeks plus ribavirin (800-1,000 mg/day in two doses with lunch and dinner) for 48 weeks. Patients were followed for an additional 24 weeks for a total follow-up of 72 weeks. In this study, the dose of ribavirin administered was lower than the standard recommended dose because of the body mass index (BMI) and tolerability profile of this geographic patient population.
Clinical and laboratory evaluationDuring therapy, blood counts and serum transaminases were measured at 2 to 12-week intervals. Serum HCV RNA was determined at weeks 12, 24, 36, 48, and 72. The presence of HCV RNA was measured by quantitative PCR (Roche AmplicorTM) with a rather poor sensibility threshold (600 IU/mL) and a 500,000 IU/mL upper limit. The final (72-w) serum HCV RNA sample was evaluated by qualitative PCR (Roche AmplicorTM) with a 50 IU/mL lower limit of detection.
Patients were evaluated for vital signs, ECG, hematological measures and measures of liver and kidney function as well as the frequency of adverse events during the treatment period and for 6 months following the last administration of thymalfasin. Monitoring for safety and toxicity was performed throughout the study. When necessary, appropriate medical intervention was provided and investigational agents were discontinued.
Any treated patient who experienced severe or clinical laboratory toxicity (grade 3, modified ACTG graded toxicity scale) was temporarily discontinued from the study medications until the toxicity returned to baseline. Study medications were permanently discontinued if the toxicity persisted despite the interruption of study medications or if a patient experienced life-threatening (WHO grade modified ACTG graded toxicity scale) toxicities.
Patient results were collected from the 4 participating medical centers in Mexico and analyzed at a central lab.
Study end pointsThe primary efficacy endpoint was SVR defined as clearance of HCV RNA (< 600 IU/mL) at the end of the 72-week follow-up. Secondary efficacy endpoints included normal ALT biochemical levels at weeks 48 and 72 and a reduction of > 2 log10 in HCV RNA load (Amplicor Monitor™) at weeks 12.
Early virologic response was defined as >2 log10 drop or negative HCV RNA viral load at week 12. End of treatment response was defined as negative HCV RNA at week 48. Sustained virologic response was negative HCV RNA at the end of the follow-up period, which was 72 weeks from the start of the study.
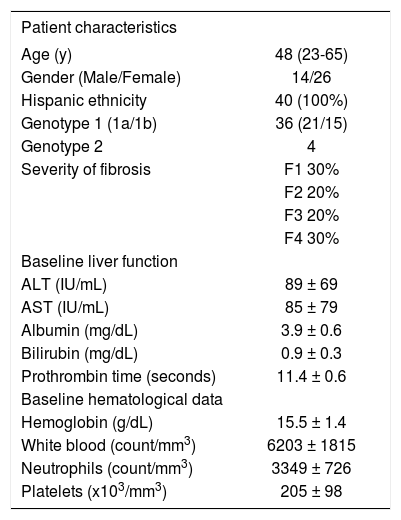
ResultsA total of 40 Hispanic patients from 4 medical centers in Mexico were enrolled in the study in 2003 and followed for a total of 72 weeks (Table I). The average age was 48 (range 23–64). All patients had a positive viral load and 23 patients had HCV RNA > 500,000 IU/mL. Twenty-one patients were infected with genotype 1a, 15 with genotype 1b, and 4 with genotype 2. Thirty-eight patients completed 48 weeks of therapy and 24 weeks of follow-up. Two patients interrupted treatment at week 24 with positive HCV RNA and withdrew from the study due to side effects.
Baseline patient characteristics.
| Patient characteristics | |
|---|---|
| Age (y) | 48 (23-65) |
| Gender (Male/Female) | 14/26 |
| Hispanic ethnicity | 40 (100%) |
| Genotype 1 (1a/1b) | 36 (21/15) |
| Genotype 2 | 4 |
| Severity of fibrosis | F1 30% |
| F2 20% | |
| F3 20% | |
| F4 30% | |
| Baseline liver function | |
| ALT (IU/mL) | 89 ± 69 |
| AST (IU/mL) | 85 ± 79 |
| Albumin (mg/dL) | 3.9 ± 0.6 |
| Bilirubin (mg/dL) | 0.9 ± 0.3 |
| Prothrombin time (seconds) | 11.4 ± 0.6 |
| Baseline hematological data | |
| Hemoglobin (g/dL) | 15.5 ± 1.4 |
| White blood (count/mm3) | 6203 ± 1815 |
| Neutrophils (count/mm3) | 3349 ± 726 |
| Platelets (x103/mm3) | 205 ± 98 |
At 12 weeks of therapy, 57.5% of patients experienced a > 2 log10 drop in HCV RNA and 52.5% of patients were HCV RNA negative (< 600 IU/mL). At week 24, 55% of patients experienced a > 2 log10 drop in HCV RNA and 50% of patients were HCV RNA negative (Figure 1).
Two patients failed to complete the treatment. In the per protocol analysis at 48 weeks (end of treatment), 52.6% of patients were HCV RNA negative. Patients were followed for an additional 24 weeks for a total follow-up period of 72 weeks from the start of the study. At 72 weeks, 21.1% of the total patient population was HCV RNA negative (Figure 2).
Of the patients with genotype 1 HCV, 52.9% were HCV RNA negative at week 48 and 23.5% were HCV RNA negative at week 72. Interestingly, the genotype 1 SVR was higher than the SVR of the total patient population, which may be due to the fact that 4 genotype 2 patients did not achieve SVR at 72 weeks.
In the intent-to-treat (ITT) analysis, 50% of patients were HCV RNA negative at the end of treatment (48 weeks) and 20% were HCV RNA negative at 72 weeks. Of the HCV genotype 1 patient population, 50% were HCV RNA negative at the end of treatment (48 weeks) and 22.2% were HCV RNA negative at 72 weeks.
Normalization of ALT was observed in 30% of patients at week 12 and 37% of patients achieved normal AST levels at week 72. Reported adverse events were those typical of PEG IFN α-2a and ribavirin therapies (Table II). Headache and fatigue were the most frequent adverse events followed by alopecia, nausea, anorexia, and insomnia. No adverse events were associated with thymalfasin during the treatment period. No life-threatening adverse events were observed throughout the study.
Reported adverse events.
| Adverse Event | Patients, % |
|---|---|
| Flu-like symptoms | |
| Fatigue | 33 |
| Headache | 53 |
| Gastrointestinal symptoms | |
| Nausea | 23 |
| Anorexia | 17 |
| Diarrhea | 3 |
| Psychiatric symptoms | |
| Depression | 13 |
| Insomnia | 17 |
| Anxiety | 10 |
| Respiratory tract symptoms | |
| Cough | 10 |
| Rhinitis | 3 |
| Epistaxis | 3 |
| Dermatologic symptoms | |
| Alopecia | 20 |
| Pruritus | 3 |
| Rash | 3 |
| Itching | 10 |
Dose reductions were required for both PEG IFN α-2a and ribavirin at weeks 12, 24, and 48. The most frequent causes of dose reduction included anemia, neutropenia, and thrombocytopenia. However, no dose reduction of thymalfasin was required at any time point (Table III).
Dose reductions.
| Therapy | Week 12 | Week 24 | Week 48 |
|---|---|---|---|
| Thymosin alpha 1 | (0/40) 0% | (0/40) 0% | (0/38) 0% |
| PEG IFN α-2a* | (6/40) 15% | (10/40) 25% | (9/38) 23.7% |
| Ribavirin | (3/40) 7.5% | (7/40) 17.5% | (7/38) 18.4% |
As the population of HCV treatment nonresponders continues to grow, the need for new therapeutic options and more efficacious treatment strategies escalates. Among US HCV patients, 70% to 75% are chronically infected with genotype 1, which is the least responsive to available therapies.14 Fewer than 50% of treatmentnaïve patients with genotype 1 HCV achieve an SVR with initial PEG IFN α-2a plus ribavirin therapy.15 Of HCV patients who do not respond to an initial 12-week course of IFN α-2a and ribavirin, only 12% achieve a sustained response when re-treated with PEG IFN α-2a plus ribavirin.6
In addition, ethnicity has been shown to impact SVR. In a study of 661 treatment-naïve HCV patients who were treated with IFN-α and ribavirin combination therapy, SVR was lowest among African Americans (14%) and Hispanics (23%) and highest among Asians (61%) and Caucasians (39%).7
Among difficult-to-treat patients who are nonresponders to current standard treatment regimens, there are few options available for re-treatment. We must consider the approach of Sherman M,22 and Krawing EL,23 among others, to just re-treat patients with current standard of care peg-interferon and ribavirin dosages which may give a sustained viral response in some patients. However, it is important to mention that a mixed population of relapsers and non-responders were included in both studies raising the possibility of a selection bias in terms of previous complete or incomplete treatment. The vast majority of the patients were Caucasians (85-90%). According to authors a final SVR in non-responders with genotype 1 was obtained in 20 % and 17% respectively. Therefore, the potential efficacy of new approaches to difficult to treat population requires to be investigated.
A double-blind, randomized study of triple therapy with amantadine, IFN α-2b, and ribavirin did not show a significant increase in HCV RNA clearance among treatment- naïve HCV patients.9 Another study by Adinolfi et al11 evaluated the same triple therapy regimen for the treatment of HCV patients who failed previous monotherapy with IFN-α administered for at least 4 months. While 25% of the overall patient population of IFN-α nonresponders achieved SVR at 1 year, only 12% of genotype 1 patients achieved SVR. A study by Teuber et al16also evaluated the efficacy of amantadine/IFN-α/ribavirin therapy among patients who were nonresponders to a combination treatment regimen of IFN-α/ribavirin. The overall SVR for patients treated with the triple therapy was 25%. Among genotype 1 patients with high viral loads (≥1 x 106 copies/mL), 14% achieved a sustained virologic response with triple therapy.
More recently, amantadine has been studied in combination with PEG-IFN and ribavirin for treatment-naïve patients with chronic hepatitis C.10 In a subgroup of genotype 1 patients who were treated for 48 weeks with triple therapy, 55% achieved SVR at 72 weeks. However, previous nonresponders were not evaluated in this study.
Thymalfasin is a chemically synthesized 28 amino acid polypeptide with a unique, multi-modal mechanism of action. Thymalfasin is able to fight disease by stimulating the immune system via a number of immunomodulating activities. Its effects include stimulation of natural killer (NK) cells and cytotoxic lymphocytes (CD8),17which directly kill virally infected cells. Thymalfasin also increases the production of cytokines such as interleukin 2 (IL-2) and decreases the production of Th2 cytokines (IL-4 and IL-10), which are counterproductive in fighting viral infection.18 Evidence has shown that thymalfasin also has direct effects on virally infected cells by decreasing viral replication and increasing the expression of surface-marker proteins (MHC-1), thus providing the immune system with a target for the detection and destruction of diseased cells.19
Thymalfasin nearly doubled response rates when combined with IFN α-2b in a randomized, controlled study by Sherman et al.12 The study compared the biological activity of thymalfasin and IFN α-2b combination therapy vs IFN α-2b therapy alone vs placebo. A total of 109 patients were included in the study and the majority had genotype 1 hepatitis C. At the end of treatment, patients treated with combination thymalfasin/IFN α-2b had higher rates of biochemical, virological, and histological responses.
Similar results were demonstrated in an earlier study by Rasi et al.20 This study included 15 patients with serum HCV RNA positive chronic hepatitis C, most of whom (87%) had genotype 1. All patients were treated with thymalfasin (1 mg twice weekly) and lymphoblastoid interferon (L-IFN) (3MU 3 times weekly) for 1 year. At the end of treatment, 73% of patients were HCV RNA negative. Six months after cessation of therapy, 40% achieved SVR characterized by a negative serum HCV RNA. Of the patients who had genotype 1 hepatitis C, 69% responded to treatment and 39% achieved SVR 6 months after cessation of therapy. In addition, no significant adverse events were observed other than mild influenza- like symptoms associated with L-IFN therapy.
In addition, while other antiviral therapies often produce adverse events that require dose reduction or discontinuation of therapy, thymalfasin has demonstrated a consistent pattern of tolerability and safety in clinical trials. More than 3,000 patients treated with thymalfasin have been evaluated in more than 70 clinical trials since 1979. These patients have been treated with doses ranging from 0.5 to 9.6 mg/m2 for treatment periods from 1 day to 18 months. No serious adverse events have been reported in any of these studies and thymalfasin has been consistently well tolerated. To date, < 1% of patients have experienced any thymalfasin-related adverse events.
Our pilot study21 evaluated the efficacy and safety of triple therapy with thymalfasin, PEG IFN α-2a, and ribavirin for the treatment of a particularly difficult-to-treat population. However, among the main limitations of our study we have to recognize the relatively small sample size and the uncontrolled design. The study group included genotype 1 HCV patients who were Hispanic, had high viral loads, and did not respond to a previous 6-month course of IFN-α/ribavirin combination therapy.
At week 12 of this study, 57.5% of patients experienced a > 2 log10 drop in HCV RNA and 52.5% of patients were HCV RNA negative (< 600 IU/mL). This may be clinically relevant because, in general, patients who achieve a SVR almost always have a dramatic EVR, which is referred to as a 2 log10 drop or loss of HCV RNA at 12 weeks of therapy.
At week 24, 55% of patients experienced a >2 log10 drop in HCV RNA and 50% of patients were HCV RNA negative. In addition, the drop in viral load was significant at weeks 8, 12, 24, 36, and 48, demonstrating an effective inhibition of viral replication.
Of the patients with genotype 1 HCV, per protocol analysis showed that 52.9% were HCV RNA negative at week 48 and 23.5% at week 72. Interestingly, the genotype 1 SVR was higher than the SVR of the total patient population at 72 weeks.
Dose reductions of both PEG IFN α-2a and ribavirin were required at weeks 12, 24, and 48. The most frequent causes of dose reduction included anemia, neutropenia, and thrombocytopenia. However, no dose reduction of thymalfasin was required at any time point.
From this observation, it is not clear whether dose reductions of PEG IFN α-2a and ribavirin decreased the efficacy of this triple therapy regimen. However, an analysis by Shiffman et al6 showed that reducing the dose of PEG IFN α-2a during the first 20 weeks of treatment from > 80% to < 60% of the target dose did not appear to significantly impact either virologic response at week 20 or SVR at week 72. In contrast, reducing the dose of ribavirin during the first 20 weeks of treatment from > 80% to < 60% of the target dose significantly reduced virologic response and SVR. However, dose reductions of either therapy after week 20 had no significant impact on SVR. This is encouraging because dose reductions of PEG IFN α-2a and ribavirin without compromising efficacy could minimize adverse events and potentially improve adherence. Further study is needed to make that determination.
In conclusion, our data indicate that the triple therapy regimen including thymalfasin/ PEG IFN α-2a/ribavirin offers advantages for the re-treatment of genotype 1 nonresponders. Both initial response and sustained response were observed in this 72-week follow-up study. However, careful extrapolation to overt clinical practice utilization must me paid because a larger population and a controlled design will be needed to evaluate the final efficacy of this new and promising therapeutic hypothesis. Indeed, a large, randomized, controlled triple-therapy trial is currently being conducted in Europe, which may confirm these promising results.