Controlled attenuation parameter (CAP) has been developed as a non-invasive method for detecting liver steatosis. The aim of the study was to determine factors associated with non-obtaining lower IQR-CAP values.
Materials and MethodsRetrospective revision of medical records of CAP studies for steatosis screening. Anthropometrical, biochemical, and quality variables were collected. A logistic regression analysis was performed to determine independent associations with non-obtaining IQR-CAP <30, <20, and <10 in all patients and then adjusted for obesity/overweight and severity of steatosis.
Results5061 studies were analyzed. Median IQR-CAP was 26 [IQR 20–33] dB/m. Steatosis prevalence was 39.4 % (n = 1996). In overweight patients, significant alcohol consumption was an independent factor for non-obtaining IQR-CAP <30; meanwhile, in obese patients glucose impairment, AST, skPa>8 and steatosis severity were independent factors for non-obtaining lower IQR-CAP values. According to steatosis severity, the presence of anthropometric characteristics of obesity and significant alcohol consumption were independent factors for non-obtaining lower IQR-CAP values.
ConclusionsIn steatosis detection by CAP, obesity, significant alcohol consumption, glucose impairments, and minimal liver function test alterations were independent factors associated with non-obtaining lower values of IQR-CAP.
Metabolic-Associated Steatotic Liver Disease (MASLD) (formerly named nonalcoholic fatty liver disease (NAFLD) is a metabolic disorder characterized by evidence of liver steatosis in addition to at least one metabolic abnormality in individuals without significant consumption of alcohol, hepatotoxic medications or other known causes of secondary steatosis [1,2]. Its clinical-pathological spectrum can evolve from simple steatosis to steatohepatitis, progressive fibrosis, cirrhosis, and hepatocellular carcinoma. The estimated global prevalence in the adult population is 24 % [3], which parallels trends for obesity and diabetes, and it must be considered one of the biggest health issues in the next decades as the most prevalent liver disease worldwide. MASLD affects all ethnicities, although Hispanics may be considered the ethnic group with the highest risk, as seen in different epidemiological studies, due to genetic alterations [3–6]; therefore, the importance of timely detection is paramount.
MASLD diagnostic methods have evolved from the gold standard of liver biopsy to less invasive methods, starting with non-invasive biomarkers [7] to imaging options like conventional hepatic ultrasound [8], magnetic resonance spectroscopy [9], and controlled attenuation parameter (CAP) by transient elastography (TE) [10]. However, evidence shows that the sensitivity and specificity of non-invasive biomarkers and hepatic ultrasound are poor; on the other hand, CAP has shown a similar diagnostic accuracy to magnetic resonance, and it is a more accessible method [11].
Developed more than 15 years ago, CAP is based on the ultrasonic properties of the radiofrequency back-propagated signals acquired by the TE; during liver stiffness measurement (LSM) with TE, the CAP algorithm calculates the ultrasound signal attenuation and is expressed in dB/m [12], ranging from 100 to 400 dB/m. TE systems are equipped with two types of probes for adults: an M probe to be used in most patients and an XL probe for obese patients. Criteria for probe size selection could be based on body mass index (BMI) or skin-liver capsule distance (<25 mm for M and ≥25 for XL) [13]. Nowadays, there are established factors that could affect the reliability of LSM, such as obtaining an IQR-kPa >30 or selecting the incorrect probe [14]; however, the liver steatosis diagnosis by CAP scenario is not clear [15,16]. Some factors associated with the reliability of CAP measurement, when manufacturer basic recommendations are accomplished, have been described, such as BMI, fasting conditions, steatosis severity, LSM, waist circumference, extrahepatic cholestasis, and biochemical markers such as triglycerides, glucose, hepatic enzymes levels; however, evidence comes majorly from studies with combined etiologies (NAFLD + hepatitis C virus infection (HCV) or NAFLD + hepatitis B (HBV) virus infection) or only evaluation of NAFLD in HCV patients. When these factors are evaluated in only NAFLD patients, biochemical levels have no significant associations with reliability, and it seems to be that the stronger factors that could affect the reliability of CAP measurements are BMI and steatosis severity [16,17].
No specific recommendations on quality advice for CAP measurement have been made by the manufacturer since the device could indicate if the measurement is valid; nonetheless, recommendations on probe size according to BMI and skin-liver capsule have yielded contradictory results [18,19]. The study performed by Caussy et al. [20] shows that, even with an adequate probe size selection, the liver steatosis grade could be underestimated when it is compared with magnetic resonance, suggesting that different cut-off points should be used for each probe size for liver steatosis diagnosis.
IQR-CAP has been proposed as a quality parameter for liver steatosis measurement; IQR-CAP <40 dB/m and <30 dB/m are the cut-off points that have been related with accuracy in comparison with magnetic resonance imaging or liver biopsy [21]; however, Semmler et al. [22] observed that CAP diagnostic accuracy is not lower than AUROC 0.800, even with IQR-CAP <80 dB/m or <60 dB/m, compared to liver biopsy. On the contrary, in studies that did not meet IQR-CAP <40 dB/m, the diagnostic accuracy is AUROC 0.799. A higher AUROC was observed in IQR-CAP <20 dB/m, but this criterion was only met in 20 % of studies.
Until now, different criteria have been described to obtain a reliable CAP measurement, from the correct size probe to IQR-CAP and IQR-kPa values; however, it is unknown if, while still fulfilling these criteria, when lower IQR-CAP values are desired, there are factors associated with not obtaining them. Therefore, the aim of this study is to determine factors associated with non-obtain lower IQR-CAP in liver steatosis detection by TE.
2Material and Methods2.1Study populationWe retrospectively reviewed the medical records of the TE studies conducted at our institution (Medica Sur Clinic & Foundation, Mexico City, Mexico) from January 2015 to December 2019. We included records of patients 18 years of age and older who underwent a TE to screen liver steatosis. History of other hepatic diseases such as viral infections (HCV and HBV), diabetes mellitus (DM), high blood pressure (HBP), and dyslipidemia were collected for the medical record. Age and gender were recorded, as well as anthropometric measurements of waist circumference (WC) and BMI calculated as weight (kg)/height(m)2. Overweight was determined as BMI ≥ 25 kg/m2; meanwhile obesity was determined as BMI ≥30 kg/m2. Data for fasting metabolic biochemical parameters (glucose, triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and HbA1c) were collected. Alcohol consumption was recorded for medical history in which the physician asked the patient about the number of drinks and consumption frequency; we classified the patients as having significant alcohol consumption (SAC) according to the number of drinks consumed per day (≥2 drinks per day in women and ≥3 drinks per day in men). The presence of liver steatosis was determined by CAP according to cut-offs proposed in the Cao et al. meta-analysis, as follows: S1 ≥268 dB/m, S2 ≥288 dB/m, and S3 ≥313 dB/m [17], Hepatic Steatosis Index (HSI) (HSI = 8 × ALT/AST + BMI(+2 if type 2 DM present, +2 if female)) [23], Triglyceride Glucose Index (TyG) Ln [Tg (mg/dL) × fasting glucose (mg/dL)/2] [24] and TyG-WC (TyG index) × (waist circumference (cm)) [25] were calculated, and liver steatosis was determined with an HSI ≥36, TyG >4.6 and TyGWC >425.6 [26].
2.2Transient elastography studies and transient elastography reliability assessmentLSM and CAP measurements were taken with TE Fibroscan 502 Touch (Echosens, Paris, France) by a single operator who was certified by the manufacturer. The studies were conducted according to the manufacturer's recommendations, which include at least four hours of fasting, the use of an M or XL probe on the right lobe of the liver through the intercostal spaces, and according to the automatic probe selection or under the operator's consideration based on the patient's BMI (≥28 kg/m2, according to Myers et al. [27]). Patients with less than ten valid measurements or without anthropometric measurements were excluded. Reliable studies were defined as those that met at least ten valid measurements, correct probe selection, and IQR-CAP <40 dB/m
2.3Statistical analysisData considered for identifying study reliability-related factors were gender, presence of significant fibrosis (≥8 kPa), overweight and obesity (according to BMI), steatosis severity, history of DM, HBP, SAC, and metabolic abnormalities as follows systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg, fasting glucose ≥100 mg/dL, triglycerides ≥150 mg/dL, HbA1c ≥5.7 %, ALT >36 UI/L, AST >33 UI/L and WC >80 cm in women and >94 cm in men.
We performed an analysis to evaluate the factors associated with lower values of IQR-CAP, classifying the studies according to different cut-offs of IQR-CAP: IQR-CAP<30 dB/m, IQR-CAP<20 dB/m, and IQR-CAP<10 dB/m. Univariate and multivariate analyses were performed to identify the independent associations with non-obtaining lower IQR-CAP values in all patients, and then, the analysis was adjusted according to the presence of overweight (BMI>27 kg/m2), obesity (BMI>30 kg/m2) and each grade of steatosis. Categorical data were compared by Fisher´s exact test; the multivariate analysis by logistic regression included all those variables with a p-value <0.1 in the univariate analysis. Odd ratios and 95 % confidence intervals were calculated for each covariate, and a p-value <0.05 was accepted as significant. Statistical analyses were performed using SPSS v.21 (SPSS, Chicago, IL, USA).
2.4Ethical statementThe study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Médica Sur S.A.B. de C.V (2020-EXT-458 in April 2020 and amendment in March 2024).
3ResultsFrom January 2015 to December 2019, a total of 5061 TE studies that met the inclusion criteria were included. The median age was 46 [IQR 39–53] years, 58 % (n = 2979) of patients were male, the median BMI was 25.8 [IQR 23.5–28.5] kg/m2; the prevalence of overweight and obesity was 43.3 % (n = 2193) and 16.8 % (n = 848), respectively. Prevalence of HCV and HBV were 0.03 % and 0.09 % respectively. General characteristics are shown in Table 1. The median CAP was 251 [IQR 213–294] dB/m, with a median IQR-CAP 26 [IQR 20–33] dB/m. Liver steatosis prevalence was 39.4 % (n = 1996); according to the steatosis severity, the prevalence of S1 was 26.4 % (n = 527), S2 29.3 % (n = 584), and S3 44.3 % (n = 885). Significant liver fibrosis (≥ 8 kPa) was observed in 0.6 % (n = 32) of patients. According to HSI, the prevalence of liver steatosis was 44.4 % (n = 2247); meanwhile, according to TyG indexes, it was 31.7 % (n = 1604) with TyG and 38.7 % (n = 1957) with TyGWC.
General characteristics of transient elastography studies and patients (n = 5061).
| Characteristic | % (n), M [IQR] |
|---|---|
| Male | 58.9 % (2979) |
| Age (years) | 46 [39–53] |
| BMI (kg/m2) | 25.8 [23.5–28.5] |
| Obesity | 16.8 % (848) |
| DM | 4.9 % (248) |
| HBP | 16.8 % (850) |
| Dyslipidemia | 53.8 % (2723) |
| SAC | 12 % (605) |
| WC (cm) | 92 [87–97] |
| FG (mg/dL) | 91 [88–95] |
| HbA1c (%) | 5.4 [5.2–5.5] |
| SBP (mmHg) | 110 [103–119] |
| DBP (mmHg) | 72 [69–78] |
| Triglycerides (mg/dL) | 113 [87–148] |
| ALT (UI/L) | 25 [21–32] |
| AST (UI/L) | 24 [22–28] |
| dB/m | 251 [213–294] |
| Steatosis (>268 dB/m) | 40.2 % (2034) |
| S1 | 11.2 % (565) |
| S2 | 11.5 % (584) |
| S3 | 17.5 % (885) |
| kPa | 4.2 [3.5–4.9] |
| Liver fibrosis (>8 kPa) | 0.6 % (32) |
| Steatosis by HSI | 44.4 % (2247) |
| Steatosis by TyG | 31.7 % (1604) |
| Steatosis by TyGWC | 38.7 % (1957) |
| Studies | |
| IQR dB/m | 26 [20–33] |
| IQR <30 dB/m | 67.2 % (3399) |
| IQR <20 dB/m | 27.9 % (1414) |
| IQR <10 dB/m | 3.6 % (181) |
| kPa IQR (%) | 13 [9.0–18] |
M, median; IQR, interquartile range; BMI, body mass index; DM diabetes mellitus; HBP high blood pressure; SAC significant alcohol consumption; WC waist circumference; FG fasting glucose; SBP systolic blood pressure; DBP diastolic blood pressure; ALT alanine aminotransferase; AST aspartate aminotransferase; S1 ≥268 dB/m; S2≥288 dB/m; S3≥313 dB/m; HSI hepatic steatosis index; TyG triglycerides-glucose index; TyG-WC triglycerides-glucose-waist circumference index.
Respect to IQR-CAP cut-offs, 67.2 % (n = 3399) of the studies accomplished IQR-CAP <30 dB/m, IQR-CAP <20 dB/m was obtained in 27.9 % (n = 1414) of the studies, and IQR-CAP <10 dB/m was obtained in 3.6 % (n = 181) of the studies. (Table 1)
Regarding factors associated with obtaining lower cut-offs of IQR-CAP in all patients, for non-obtain an IQR-CAP <30 dB/m, obesity, SAC, fasting glucose ≥100 mg/dL, and steatosis severity were associated in univariate analysis; in the multivariate analysis obesity (OR 1.59 (CI95 % 1.31–1.93), p ≤ 0.001), SAC (OR 1.20 (CI95 % 1.01–1.44), p = 0.03 glucose ≥100 mg/dL (OR 1.18 (CI95 % 1.0–1.38), p = 0.03) shown independent association with non-obtain an IQR-CAP <30 dB/m. In the analysis for non-obtain an IQR-CAP <20 dB/m, the presence of obesity and ALT >36 UI/L were independently associated (OR 1.74 (CI95 % 1.40–2.17), p ≤ 0.001, and OR 1.19 (CI95 % 1.02–1.38), p = 0.02 respectively). No significant associations were observed in univariate and multivariate analysis for IQR-CAP <10 dB/m (Table 2).
Factors associated with non-obtain IQR <30, IQR<20 and IQR<10 in all patients.
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (CI 95 %) | p | OR (CI 95 %) | p | |
| Correct probe + IQR<30 | ||||
| Overweight | 1.07 (0.98–1.16) | 0.091 | ||
| Obesity | 1.34 (1.22–1.47) | ≤0.001 | 1.54 (1.27–1.88) | ≤0.001 |
| S0 | 0.94 (0.90–0.98) | 0.005 | ||
| S1 | 1.13 (0.99–1.29) | 0.064 | ||
| S3 | 1.20 (1.10–1.32) | ≤0.001 | ||
| SAC | 1.13 (1.01 – 1.27) | 0.003 | 1.20 (1.01–1.44) | 0.03 |
| FG ≥100 mg/dL | 1.11 (1.00 – 1.23) | 0.03 | 1.18 (1.0–1.38) | 0.03 |
| Correct probe + IQR<20 | ||||
| Overweight | 1.05 (1.01–1.08) | 0.007 | ||
| Obesity | 1.14 (1.10–1.18) | ≤0.001 | 1.74 (1.40–2.17) | ≤0.001 |
| S0 | 0.92 (0.84–1.01) | 0.08 | ||
| ALT >36 UI/L | 1.04 (1.00 – 1.09) | 0.02 | 1.19 (1.02–1.38) | 0.02 |
S0 <268 dB/m; S1 ≥268 dB/m; S2≥288 dB/m; S3≥313 dB/m; SAC significant alcohol consumption; FG fasting glucose; ALT alanine aminotransferase.
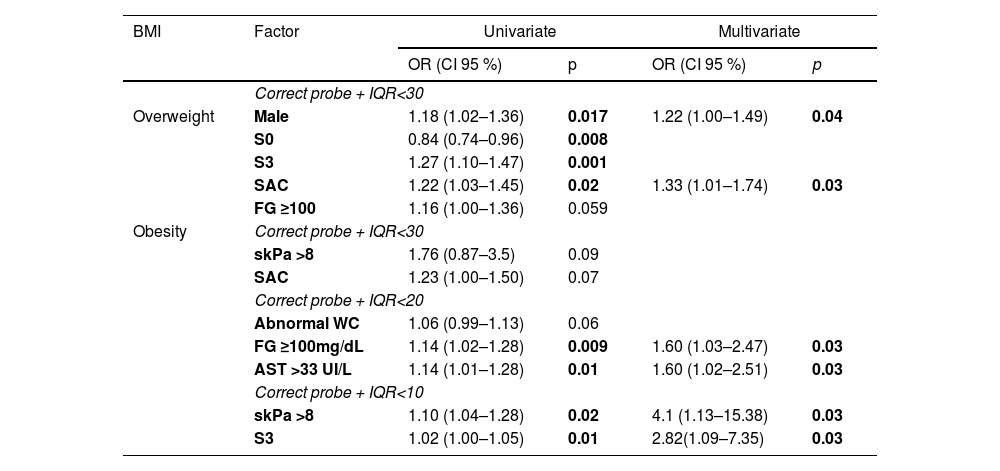
When factors associated with non-obtain lower IQR-CAP values were analyzed, adjusting by the presence of overweight, gender (male) (OR 1.22 (CI95 % 1.00–1.49), p = 0.04) and SAC (OR 1.33 (CI95 % 1.01 – 1.74), p = 0.03) shown an independent association with non-obtain and IQR-CAP <30 dB/m. IQR-CAP <20 dB/m and <10 dB/m have no associated factors in univariate or multivariate analysis. (Table 3)
Factors associated with non-obtain lower IQR values adjusted to overweight (BMI>27 kg/m2) (n = 2193) and obesity (BMI>30 kg/m2) (n = 848).
| BMI | Factor | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| OR (CI 95 %) | p | OR (CI 95 %) | p | ||
| Correct probe + IQR<30 | |||||
| Overweight | Male | 1.18 (1.02–1.36) | 0.017 | 1.22 (1.00–1.49) | 0.04 |
| S0 | 0.84 (0.74–0.96) | 0.008 | |||
| S3 | 1.27 (1.10–1.47) | 0.001 | |||
| SAC | 1.22 (1.03–1.45) | 0.02 | 1.33 (1.01–1.74) | 0.03 | |
| FG ≥100 | 1.16 (1.00–1.36) | 0.059 | |||
| Obesity | Correct probe + IQR<30 | ||||
| skPa >8 | 1.76 (0.87–3.5) | 0.09 | |||
| SAC | 1.23 (1.00–1.50) | 0.07 | |||
| Correct probe + IQR<20 | |||||
| Abnormal WC | 1.06 (0.99–1.13) | 0.06 | |||
| FG ≥100mg/dL | 1.14 (1.02–1.28) | 0.009 | 1.60 (1.03–2.47) | 0.03 | |
| AST >33 UI/L | 1.14 (1.01–1.28) | 0.01 | 1.60 (1.02–2.51) | 0.03 | |
| Correct probe + IQR<10 | |||||
| skPa >8 | 1.10 (1.04–1.28) | 0.02 | 4.1 (1.13–15.38) | 0.03 | |
| S3 | 1.02 (1.00–1.05) | 0.01 | 2.82(1.09–7.35) | 0.03 | |
BMI Body Mass Index; S0 <268 dB/m; S3≥313 dB/m; SAC significant alcohol consumption; FG fasting glucose; WC waist circumference; AST aspartate aminotransferase.
The presence of liver fibrosis (>8 kPa) and SAC showed association with non-obtain IQR-CAP<30 dB/m in obese patients, but only in univariate analysis; however when it was evaluated for non-obtain IQR<20 dB/m fasting glucose ≥100 mg/dL (OR 1.60 (CI95 % 1.03–2.47), p = 0.03) and AST >33 UI/L (OR 1.60 (CI 95 % 1.02 – 2.51), p = 0.03) show independent association with non-obtain an IQR-CAP <20 dB/m. Meanwhile, for non-obtain IQR<10 dB/m, the presence of fibrosis and S3 were independently associated (OR 4.1 (CI95 % 1.13–15.38), p = 0.03 and (OR 2.82 (CI95 % 1.09 – 7.35), p = 0.03), respectively) (Table 3).
When the analysis was adjusted by steatosis severity, in S1, obesity was the only factor that showed an independent association with non-obtain IQR-CAP <30 dB/m (OR 2.38 (CI 95 % 1.53–3.70), p ≤ 0.001). In S2 patients, obesity (OR 1.52 (CI95 % 1.05–2.20), p = 0.02) and SAC (OR 1.82 (CI95 % 1.08–3.07), p = 0.02) were independent factors to non-obtain an IQR-CAP<30 dB/m; for non-obtain an IQR-CAP <20 dB/m, obesity and HbA1c ≥5.7 % show independent association and there were not factors associated with non-obtain an IQR-CAP<10 dB/m in S2 patients (Table 4).
Factors associated with non-obtain lower IQR values adjusted to steatosis severity.
| Steatosis Severity | Factor | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| OR (CI 95 %) | P | OR (CI 95 %) | p | ||
| S1 | Correct probe + IQR<30 | ||||
| Obesity | 1.76 (1.35–2.28) | ≤0.0001 | 2.38 (1.53–3.70) | ≤0.0001 | |
| Dyslipidemia | 1.28 (1.01–1.67) | 0.06 | |||
| FG ≥100mg/dL | 1.33 (1.02–1.82) | 0.09 | |||
| Tg ≥150mg/dL | 1.33 (1.02–1.73) | 0.04 | |||
| Correct probe + IQR<20 | |||||
| Obesity | 1.22 (1.10–1.35) | 0.001 | |||
| Dyslipidemia | 1.10 (1.00–1.22) | 0.059 | |||
| Correct probe + IQR<10 | |||||
| Obesity | 1.03 (1.01–1.05) | 0.052 | |||
| HbA1c ≥5.7 % | 1.03 (1.01–1.05) | 0.08 | |||
| S2 | Correct probe + IQR<30 | ||||
| Obesity | 1.26 (1.00–1.57) | 0.06 | 1.52 (1.05–2.20) | 0.02 | |
| skPa >8 | 1.54 (1.45–1.64) | 0.09 | |||
| SAC | 1.43 (1.07–1.89) | 0.02 | 1.82 (1.08–3.07) | 0.02 | |
| Correct probe + IQR<20 | |||||
| Obesity | 1.20 (1.09–1.32) | ≤0.0001 | 2.17 (1.40–3.38) | 0.001 | |
| HbA1c ≥5.7 | 1.14 (0.98–1.33) | 0.053 | 1.57 (1.00–2.48) | 0.04 | |
| S3 | Correct probe + IQR<30 | ||||
| Obesity | 1.17 (1.00–1.39) | 0.06 | 1.33 (1.01–1.76) | 0.03 | |
| SAC | 1.34 (1.09–1.64) | 0.01 | 1.73 (1.17–2.57) | 0.006 | |
| Abnormal WC | 1.29 (1.09–1.53) | 0.003 | 1.54 (1.16–2.02) | 0.002 | |
| Correct probe + IQR<20 | |||||
| Overweight | 1.07 (0.99–1.62) | 0.090 | |||
| Obesity | 1.10 (1.02–1.19) | 0.01 | |||
| Abnormal WC | 1.16 (1.07–1.26) | ≤0.0001 | 1.72 (1.26–2.35) | 0.001 | |
| FG ≥100mg/dL | 1.11 (0.99–1.25) | 0.051 | |||
| AST >33 UI/L | 1.20 (1.04–1.39) | 0.004 | 1.69 (1.10–2.60) | 0.011 | |
| Correct probe + IQR<10 | |||||
| skPa >8 | 1.11 (0.94–1.31) | 0.052 | |||
S1 ≥268 dB/m; S2≥288 dB/m; S3≥313 dB/m, FG fasting glucose; Tg triglycerides; SAC significant alcohol consumption; WC waist circumference; AST aspartate aminotransferase.
Regarding S3 patients, once again, obesity (OR 1.33 (CI95 % 1.01–1.76), p = 0.03), SAC (OR 1.73 (CI95 % 1.17–2.57), p = 0.006) and abnormal WC (OR 1.54 (CI95 % 1.16–2.02), p = 0.002) were independent factors associated with non-obtain IQR-CAP <30 dB/m. For non-obtain an IQR<20 dB/m, in S3 patients, abnormal WC and AST >33 UI/L showed independent association, meanwhile for non-obtain IQR<10 dB/m no independent associations were observed (Table 4).
4DiscussionFrom the different non-invasive methods to diagnose MASLD, the use of TE has several advantages since it is simple to perform, operator-independent, provides an immediate result, and is suitable for repeated assessments [28]. Although head-to-head comparisons have shown that CAP is inferior to magnetic resonance imaging-based proton density fat fraction, TE will likely remain as a commonly non-invasive test used in clinical practice due to its lower cost and higher availability [29]. Thus, it is important to identify which factors are associated with CAP reliability when it is used as a non-invasive screening method for liver steatosis detection.
Nowadays, there is conflicting evidence regarding CAP measurement reliability recommendations since it has been observed that several factors could influence its values, like BMI, IQR value, and probe size used. The strongest probable quality marker is the IQR value, which measures the variability in the difference of CAP measurements. Wong et al. [30] stratified IQR values into three groups (<20, 21–39, and ≥40 dB/m) and determined the AUROCs of these in 0.86, 0.89, and 0.76, respectively. A significant difference was observed between IQR ≤40 dB/m and ≥40 dB/m (0.90 vs. 0.77, respectively, p = 0.004). Another study found no difference in CAP performance when IQR was ≥30 dB/m or ≥40 dB/m [31]. Despite these opposite results, IQR-CAP <40 dB/m has been established as a reliability criterion since there is no significant variability in AUROCs. Due to this, we decided to evaluate if there are factors associated with non-obtain lower IQR-CAP in liver steatosis detection by TE.
Different studies, including patients with NAFLD and other viral liver diseases, have shown that BMI is an independent factor that could affect CAP reliability [32–35]. This independent association has only been observed in NAFLD patients by Chen et al. [36]. In our study, we evaluated TE studies from patients who underwent liver steatosis screening, then, according to our results, the presence of obesity and liver steatosis are factors associated with non-obtain lower IQR-CAP values (<30 dB/m and <20 dB/m); however, when an IQR-CAP <10 dB/m was achieved, there are not factors associated with non-obtain this value.
Being overweight and obese are common characteristics in patients with MASLD; our results show that in overweight patients, SAC is an independent factor for non-obtain IQR <20 dB/m; due to this, evaluation of alcohol consumption in these patients should be necessary in liver steatosis screening by TE. In the patient with obesity scenario, we observed that in IQR-CAP <30 dB/m, there are no independent factors associated with non-obtain this value. On the other hand, when IQR-CAP is stricter (<20 dB/m and <10 dB/m), the presence of metabolic abnormalities such as glucose, AST and the presence of LSM >8 kPa were factors associated with non-obtain lower values of IQR-CAP therefore, in the detection of steatosis by TE in overweight and obese patients and, an IQR-CAP <20 dB/m or <10 dB/m should be achieved.
We found a MASLD prevalence of 34 % in a mean adult population in the fifth decade of life, without a significant difference according to gender. This percentage is higher than international studies for Hispanic subgroups that report a prevalence of 29 % to 33 % [37,38]. As we mentioned before, the presence of steatosis and BMI>25 kg/m2 could affect the reliability of CAP measurements; according to our observations, steatosis and BMI are influenced by each other; in patients with S1, the presence of obesity shows significant association with non-obtain IQR-CAP <30 dB/m, meanwhile, in S2 patients obesity and SAC were independently associated with non-obtain IQR-CAP <30, and obesity and HbA1c ≥5.7 % shows significant association with non-obtain IQR-CAP <20. With respect to S3, obesity, SAC, and WC have a significant independent association to non-obtain an IQR-CAP <30 dB/m; WC and AST showed an independent association with non-obtain an IQR-CAP <20 dB/m. With these results, it seems to be that when S1 was determined by a CAP value of 268 dB/m, there are more factors associated that could affect the reliability based on IQR-values; in the meta-analysis of Cao et al. [17], they proposed that in patients with obesity, modifying the CAP cut-off value for liver steatosis diagnosis could be considered; however, it is important to note that this recommendation was based on studies with European and Asian populations.
Liver steatosis estimation by CAP has some limitations, majorly in terms of diagnostic accuracy compared with biopsy or magnetic resonance; nonetheless, its good performance as a noninvasive screening method has been observed. When liver steatosis is estimated by CAP, the basic reliability criteria, such as correct probe size selection and obtaining an IQR-CAP <40 dB/m, should be accomplished in all patients; however, in scenarios of overweight/obesity and the presence of steatosis, stricter IQR-CAP values could provide more reliable measurements.
According to our results, in liver steatosis detection, the use of the correct probe and an IQR-CAP <40 dB/m as only reliability markers could be not enough since other factors could affect the reliability of liver steatosis estimation, and it should be considered, especially in those patients with obesity, metabolic abnormalities, and SAC.
5ConclusionsIn steatosis detection by CAP, anthropometric characteristics of obesity, significant alcohol consumption, glucose impairments, and minimal liver function test alterations were independent factors associated with non-obtaining lower values of IQR-CAP
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributionsE.JH. and I.LM.: Study concept and design, J.L.RF. acquisition of data, E.JH., I.LM. analysis and interpretation of data, J.L. RF and E. JH. drafting of the manuscript, E.JH., I.LM., G.CN., and M.U. critical revision of the manuscript for important intellectual content, M.U. study supervision. All authors read and approved the final manuscript.
The authors thank the Gastroenterology and Obesity Unit, Medica Sur Clinic & Foundation, for providing access to transient elastography results.