Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptoralpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016; 150: 1147-59.
Comment:Nonalcoholic steatohepatitis (NASH), the severe histological form of nonalcoholic fatty liver disease (NAFLD),1 is a chronic liver disease that has been neglected in the past but fortunately, is currently given full attention not only by health care providers but scientist involved in pharmacological research. This is justified by both, the alarming increasing disease prevalence and the knowledge gained from its natural history that may eventually end in liver cirrhosis and the development of hepatocellular carcinoma.1,2
NASH represents a challenge to physicians as not only do they need to optimize the available tools to perform an accurate diagnosis of the disease stage, namely liver fibrosis, but to offer patients a safe and effective therapy. While the first line of treatment of NAFLD is -or should desirably be- lifestyle intervention, including weight loss and physical activity/exercise, clinical experience shows that complete success is not achievable to satisfactory levels in a significant proportion of patients with associated comorbidities.3,4 Pharmacological intervention needs then to be implemented, and for being it successful should be targeted to “treat” the pathophysiological abnormalities associated with NAFLD and NASH. The ideal drug then should be effective in treating not only the liver disease but the associated risk factors, such as insulin resistance, abnormal circulating lipid profiles, arterial hypertension and cardiovascular outcomes. How many pills are so needed to achieve these goals? At least, a combination of 3 or 4 drugs is for instance currently required to treat the constellation of risk factors associated with the Metabolic Syndrome (MetS) and the associated systemic inflammatory state. In addition, there is no “one” pill that could demonstrate today to be effective in improving the complex NASH-liver phenotype, which includes liver cell injury, a mixed inflammatory lobular infiltrate, hepatocellular ballooning, and fibrosis.5 At any rate, the ideal drug to treat NASH patients with MetS should be a “magic pill” designed to treat all phenotypes in one. From the pathophysiological perspective, the ideal “all-in-one pill” should be then designed to target multiple molecular pathways.
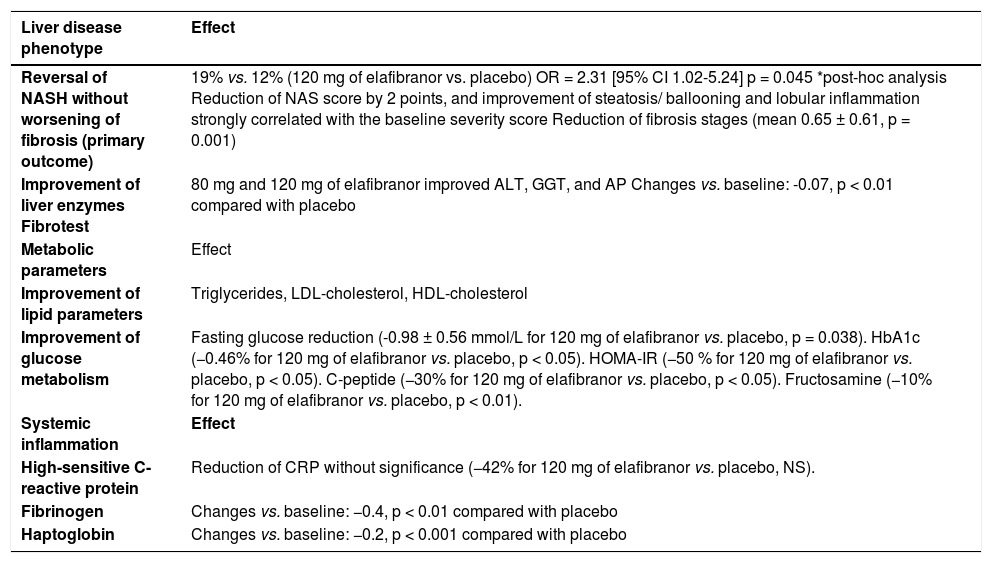
Ratziu, et al. of the GOLDEN-505 Investigator Study Group recently reported the efficacy and safety of elafibranor in a large (sample size n = 276, placebo n = 92, elafibranor 80 mg n = 93, and elafibranor 120 mg n = 91) international phase II randomized trial of patients with NASH.6 The results of the use of elafibranor on the liver-related traits, including histological and biochemical parameters, and also on the metabolic-associated risk factors are certainly promising as summarized in table 1. Moreover, safety assessment showed that elafibranor is relatively well tolerated and relatively free of serious adverse events, being 120 mg the ideal dose to be administered orally once a day.
Summary of the effects of elafibranor on NASH and MetS-associated phenotypes.
| Liver disease phenotype | Effect |
|---|---|
| Reversal of NASH without worsening of fibrosis (primary outcome) | 19% vs. 12% (120 mg of elafibranor vs. placebo) OR = 2.31 [95% CI 1.02-5.24] p = 0.045 *post-hoc analysis Reduction of NAS score by 2 points, and improvement of steatosis/ ballooning and lobular inflammation strongly correlated with the baseline severity score Reduction of fibrosis stages (mean 0.65 ± 0.61, p = 0.001) |
| Improvement of liver enzymes Fibrotest | 80 mg and 120 mg of elafibranor improved ALT, GGT, and AP Changes vs. baseline: -0.07, p < 0.01 compared with placebo |
| Metabolic parameters | Effect |
| Improvement of lipid parameters | Triglycerides, LDL-cholesterol, HDL-cholesterol |
| Improvement of glucose metabolism | Fasting glucose reduction (-0.98 ± 0.56 mmol/L for 120 mg of elafibranor vs. placebo, p = 0.038). HbA1c (−0.46% for 120 mg of elafibranor vs. placebo, p < 0.05). HOMA-IR (−50 % for 120 mg of elafibranor vs. placebo, p < 0.05). C-peptide (−30% for 120 mg of elafibranor vs. placebo, p < 0.05). Fructosamine (−10% for 120 mg of elafibranor vs. placebo, p < 0.01). |
| Systemic inflammation | Effect |
| High-sensitive C-reactive protein | Reduction of CRP without significance (−42% for 120 mg of elafibranor vs. placebo, NS). |
| Fibrinogen | Changes vs. baseline: −0.4, p < 0.01 compared with placebo |
| Haptoglobin | Changes vs. baseline: −0.2, p < 0.001 compared with placebo |
Several considerations deserve be highlighted about the results of elafibranor for the treatment of NASH. First and the most exciting point is that the overall effects could be defined as of “broad spectrum” targeting not only the liver disease but the MetS-associated abnormalities. Regarding the liver disease, it would appear however, that elafibranor “improve” or “reverse” rather than “cure definitely”the main histological lesions. Nevertheless, previous experience with other drugs for the treatment of MetS-associated diseases suggests that to “cure” the disease with a short-term therapy is hardly achievable.
It is worth to note that remarkable improvement of the liver phenotype was achieved in patients that had histological scores of severe disease, and that was attributed by the authors to the activation of the drug molecular targets.6 Outstandingly, there was also a significant reversion of the histological markers of disease progression, such as hepatocellular ballooning.7 One may argue that the effect of elafibranor on the improvement of hepatocellular ballooning is indeed a consequence of the improvement of glucose metabolism as there is replicated evidence in human studies showing that ballooning is significantly associated with abnormal glycemic control.8,9 As well, the improvement of the steatosis score might be explained as a consequence of the correction of the circulating lipid abnormalities. Nevertheless, for those who support the “liver-centric” approach of the development of the MetS, there are robust arguments to endorse the idea that the improvement of the liver phenotype indeed “orchestrated” the systemic metabolic changes. For example, extended data of the elafibranor trial presented at the International Liver Congress (EASL 2016) showed that the effects on glucose metabolism but not circulating lipids were more pronounced in patients that had a NAS score (NAFLD activity score) higher than 6.10
Second, it is still unknown whether elafibranor will be equally effective in Non- Caucasian populations as the elafibranor trial included almost exclusively Caucasian subjects. In addition, is still unknown if the use of elafibranor will be equally effective and safe in treating affected children.
The third comment is about how long should be NASH patients treated with elafibranor. If more than 52 weeks are needed to reverse the liver disease, how safe is the long-term activation of the PPARs? Ratziu, et al. reported that 5 patients in the elafibranor group vs. 0 patients receiving placebo showed a mild but statistically, significant increase in the serum levels of creatinine, and also 2 patients in the elafibranor developed renal impairment.6 At this point, it should be mentioned that elafibranor is a synthetic ligand of PPARa and PPAR5, of which the “suprapharmacological” concentrations are still unknown and off-target effects are difficult to predict. On the other hand, it was shown that elafibranor does not equally activate both PPARs and it would appear that the EC50 (half maximal effective concentration) is not the same for the activation of both receptors.11 In fact, GFT505 and its main active circulating metabolite, GFT1007, show 5 fold more affinity for human PPARα than PPAR5 in vitro.11 Although circulating levels of the drug may be saturating on both receptors, the two last points are relevant as for instance, previous studies have shown ligand-induced renal toxicity associated with the use of aleglitazar12 (a ligand of PPARα) and tesaglitazar13 (a dual PPARα/γ ligand). Finally, long-term follow-up of patients treated with elafibranor must be guaranteed as the ligand-mediated effect/s, including toxicity, of prolonged activation of PPARδ by an exogenous agent are not fully characterized. In addition, close monitoring of the development of carcinogenesis is needed as for instance, it was shown in vitro that PPARα activation is associated with increased proliferation of a human cancer breast cell line.14 Furthermore, there is evidence on the development of lesions that could potentially predispose to colon cancer after activation of PPARδ.15 A particularly important point should be finally added about in vitro observations suggesting the potential enhanced hepatic stellate cell proliferation by ligand activation of PPAR5.16
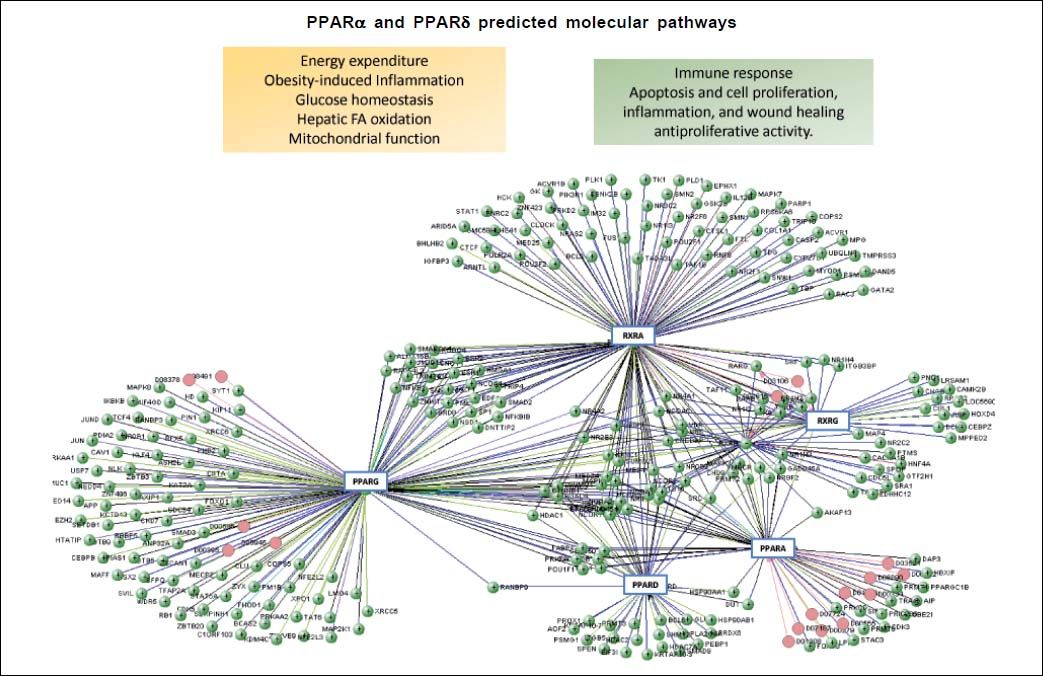
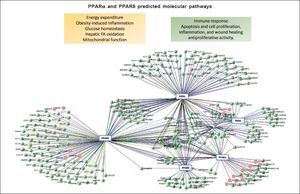
What are the molecular targets of elafibranor?Elafribranor, also known as GFT505, is a drug compound designed to target peroxisome proliferator-activated receptors (PPARs), more specifically elafibranor is a dual ligand of PPARα and PPARδ. The PPARs are ligandactivated transcription factors that belong to the Super-family of nuclear hormone receptors, which clusters a myriad of genes involved not only in different metabolic processes but cellular functioning. Other members of this superfamily are the steroid hormone receptors, vitamin D3 receptor, retinoid acid receptors (RARs and RXRs) and thyroid hormone receptors (THRs) (Figure 1).
Computational prediction of protein-protein Interaction was performed by the blolnformatic resource VIsANT 3.0, a Web-based platform that integrates and displays biological interactions based on KEgG pathways and expression data.28Figure 1 also depicts major effects associated with activation of PPARa and PPARS on cellular and metabolic function. Currently, several pharmaceutical forms of fibrates (pink circles) are well-known as agonists of PPARa.
While PPARs are all involved in the regulation of glucose and fat metabolism, they do differ not only in the range of target tissues but expression patterns. For example, PPARδ is ubiquitously expressed; nevertheless, its biggest expression is in the skeletal muscle, gastrointestinal tract, and kidney. In addition, while PPARδ binds lipid-derived substrates, it has preference for poly-unsaturated fatty acids; once activated, PPARδ regulates the peroxisomal beta-oxidation of fatty acids mostly in skeletal muscles thereby, ameliorating and controlling insulin resistance. Elafibranor, however, seems to act reducing gluconeogenesis in the liver.17 Also, PPARδ regulates cardiac mitochondrial biogenesis.18 On the contrary, PPARα, which is highly expressed in the liver, is activated by the endogenous ligand 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine, oleoyl-ethanolamide- a naturally occurring lipid that regulates satiety-, and leukotriene B4, among other ligands. Synthetic known ligands of PPARα are fenofibrate, clofibrate, and gemfibrozil, which have been largely used in the past to treat circulating lipid disorders.
Why is the effect of elafibranor suggestive of being an attractive drug candidate for the treatment of NASH? Just because of the potential benefits of the activation of the PPARs.
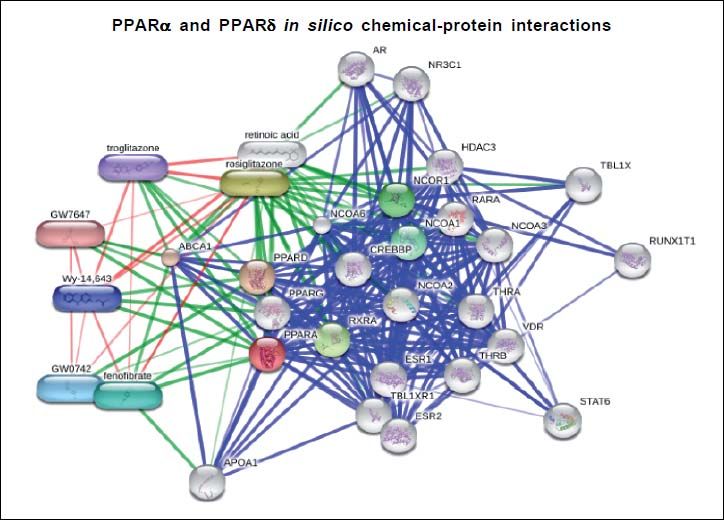
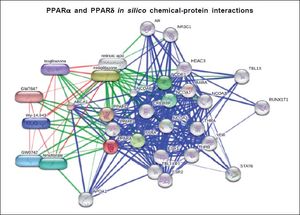
Figure 1 depicts the interaction network of genes and proteins associated with the ligand-activated effect of PPARα and PPARδ. A large node of genes is associated with the RXR (retinoid X receptor) that heterodimerize with PPARs after they translocate into the nucleus. Furthermore, several chromatin state modifiers, such as HDACs (histone deacetylases), are close related targets, which play an important role in the pathophysiology of NAFLD as part of the spectrum of epigenetic modifiers of the disease.19–22 Altogether, there is plenty of evidence on the role of nuclear receptors in the development of NAFLD.23 Then, it not surprising that PPARGC1A (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha, also known as PGC-1α), for instance, is represented in the interactome.24 Moreover, figure 2 shows an in silico prediction of PPARα and PPARδ chemical-protein interactions; endogenous and synthetic PPARs ligands are depicted.
Protein-protein interactions are shown in blue, chemicalprotein interactions in green, and interactions between chemicals in red. Input: PPARα and PPARδ. PPARα (proliferator-activated receptor δ), PPARS (proliferator-activated receptor δ). Predicted functional chemical partners: rosiglitazone (score: 0.998); fenofibrate (score 0.995); GW0742 (PPARδ agonist, score: 0.995); Wy-14,643 (a synthetic thiacetic acid, score: 0.995); troglitazone (antidiabetic and anti-inflammatory drug, member of the drug class of the thiazolidinediones, score 0.991); GW7647 (score: 0.991); retinoic acid (tretinoin, score 0.997). The prediction was performed by the web resource STITCH 4.0, which explores known and predicted interactions between protein and chemicals. STITCH contains interactions for between 300,000 small molecules and 2.6 million proteins from 1,133 organisms.
Results on the potential use of the rs738409 variant in PNPLA3 (patatin-like phospholipase domain containing 3), a multifunctional enzyme that has both triacylglycerol lipase and acylglycerol O-acyltransferase activity,25 as a candidate for future pharmacogenetic studies were recently reported. As expected,26 the variant was associated with the baseline histological severity of NASH; nevertheless, the treatment response to elefibranor could not be predicted by the association with the minor-disease associated G allele.27 This observation, although disappointing at first sight, is plausible as a priori. The first line of candidate genes to be targeted for pharmacogenetic studies should be those that are involved in the direct pharmacological effect of elafibranor (Figure 1) or in the drug transformation or degradation, which should be further investigated.
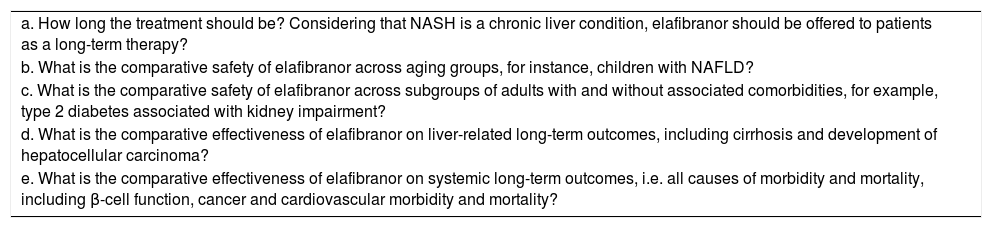
Identifiable unresolved clinical questionsThe GOLDEN-505 trial has opened an important and remarkable door into the safety and effective therapy of NASH with “one pill” approach. The initial study tested two elafibranor doses and also showed a strong interaction between the histological severity of NASH at baseline and the treatment response. As a proof of principle, the GOLDEN-505 trial showed that fibrosis is not aggravated by the drug; on the contrary, the results on the possibility of fibrosis reversal are promising. Nevertheless, future studies on elafibranor for the treatment of NASH should be able to answer unresolved questions as highlighted in table 2.
Identifiable unresolved clinical questions on the use of elafibranor for the treatment of NASH.
| a. How long the treatment should be? Considering that NASH is a chronic liver condition, elafibranor should be offered to patients as a long-term therapy? |
| b. What is the comparative safety of elafibranor across aging groups, for instance, children with NAFLD? |
| c. What is the comparative safety of elafibranor across subgroups of adults with and without associated comorbidities, for example, type 2 diabetes associated with kidney impairment? |
| d. What is the comparative effectiveness of elafibranor on liver-related long-term outcomes, including cirrhosis and development of hepatocellular carcinoma? |
| e. What is the comparative effectiveness of elafibranor on systemic long-term outcomes, i.e. all causes of morbidity and mortality, including β-cell function, cancer and cardiovascular morbidity and mortality? |
The authors have no conflict of interest to declare.