Background and aims. Autoimmune hepatitis (AIH) is a chronic inflammatory condition of the liver in which the immunological mechanisms involved in tissue destruction and/or repair are still unclear. Different pro-inflammatory cytokines have been shown to play a determinant role in AIH pathogenesis. Here, we aim to compare the circulating levels of pro-and anti-inflammatory cytokines such as IL-6, TNF-α, IL-17A/F, IL-21, IL-22, IL-23, and IL-10 in patients with type 2 AIH compared to patients with type 1 AIH and healthy controls (HC). Fourty-six Mexican patients with AIH were recruited in our study. Patients were classified as type 1 or 2 AIH based on immune serological markers. Fourty-four serum samples from healthy individuals were included as controls. Serum cytokine levels were determined by ELISA technique.
Results. Compared to healthy controls, serum levels of IL-17F, IL-21, IL-23, IL-10, IL-6, and TNF-α, but not IL-17A and IL-22, were significantly increased in AIH patients. When patients were grouped by aminotransferase activity, a biomarker of active disease, a positive correlation between serum IL-17F and alanine transaminase (rs: 0.4739; P = 0.0009) and aspartate transaminase (rs: 0.4984; P = 0.0004) levels was found. A cytokine signature profile associated with type 2 AIH was characterized by high serum IL-21 (type 1 AIH: 0.66 pg/mL; type 2 AIH: 331.1 pg/mL; P = 0.0042) and IL-22 (type 1 AIH: 0.1 pg/mL; type 2 AIH: 55.26 pg/mL; P = 0.0028) levels.
Conclusions. We show for the first time, differential regulation of certain pro-inflammatory cytokines associated with disease progression and AIH type in Mexican patients.
Autoimmune hepatitis (AIH) is a chronic inflammatory liver condition accompanied by immunological intolerance to hepatic components. This disease is associated more frequently with young women from different ethnicities.1 Clinical manifestations are commonly characterized by hypergammaglobulinemia, interface hepatitis, aminotransferase elevation, and serum autoantibodies.2 AIH is divided into two types based on autoantibody profile: type 1 is defined by seropositivity for anti-nuclear (ANA) and/or anti-smooth muscle (SMA) antibodies and constitutes 80% of AIH cases,3 while type 2 is characterized by high titers of anti-liver kidney microsomal antibody type 1 (anti-LKM-1). Type 2 AIH is rare in adults, with a prevalence of 4% the USA.3,4 Other major differences between types 1 and 2 include mean age of onset (type 1, 10 years-old; type 2, 6.5 years-old) and female to male ratio (type 1, 3:1; type 2, 9:1).5 AIH patients classified as type 2 frequently present with a more severe disease and worse clinical course. Whereas clinical and laboratory criteria used to classify AIH cases are well established, little is known about differences in immunological mediators and cytokine production associated with each type of AIH. Given its increased prevalence, the majority of published information regarding immunological mediators of AIH pathology has been obtained from type 1 AIH. Pro-inflammatory cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-8, play a major role in type 1 AIH.6 Moreover, patients with type 1 AIH also have higher levels of regulatory cytokines, such as IL-10, IL-4, and transforming growth factor-β (TGF-β), than patients with other chronic hepatic diseases.7,8 Recently, a significant increase in plasma IL-17 and IL-23 levels in AIH patients was described. Furthermore, a high percentage of Th17 cells have also been found in patients with type 1 AIH compared to healthy controls (HC) or patients with chronic hepatitis B.9 While patients with type 1 AIH respond well to standard prednisone and azathioprine treatment, the increased severity of type 2 AIH symptoms is most likely associated with resistance to immunosuppressive treatments and an increased risk of cirrhosis progression.1,5 Here, we analyzed circulating levels of pro and anti-inflammatory cytokines such as IL-6, TNF-α, IL-17A/F, IL-21, IL-22, IL-23, and IL-10 in patients with type 2 AIH compared to patients with type 1 AIH and HC.
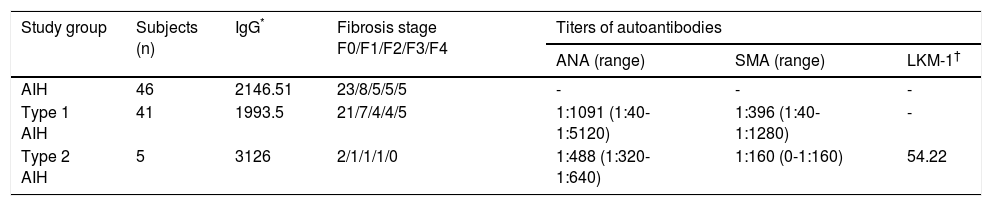
Material and MethodsPatients and samplesWe recruited forty-six Mexican patients with AIH and 44 HC by the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (México, D.F.). Diagnosis of AIH was made according to the revised scoring system proposed by the International Autoimmune Hepatitis Group (IAIHG). A definite diagnosis of AIH based on this revised scoring system required a pretreatment score exceeding 15 and/or 17 points or more after treatment as previously described.10,11 AIH diagnosis was confirmed by liver biopsy. Fibrotic stages (Scheuer score system), titers of autoantibodies and IgG levels at moment of diagnosis are shown in table 1. We only included Mexican mestizos, defined as individuals that for 2 generations, including their own, were born in México and were the progeny of the autochthonous inhabitants of the region and individuals of Caucasian or Black African origin that arrived in America in the sixteenth century. The Mexican population is principally composed of mestizos, persons whose proportionate genetic composition is 56% native American, 40% Caucasian (European ancestors, mainly of Spanish descent), and 4% Black African.12 Written informed consent to participate in the study was obtained from each participant after a full explanation of the study was provided in accordance with the guidelines of the Ethics Committee of the Instituto de Investigaciones Médico-Biológicas, Universidad Veracruzana (Veracruz, México). The institutional ethics review board approved the study protocol.
Histological findings, titers of autoantibodies and IgG levels of AIH patients at moment of diagnosis.
| Study group | Subjects (n) | IgG* | Fibrosis stage F0/F1/F2/F3/F4 | Titers of autoantibodies | ||
|---|---|---|---|---|---|---|
| ANA (range) | SMA (range) | LKM-1† | ||||
| AIH | 46 | 2146.51 | 23/8/5/5/5 | - | - | - |
| Type 1 AIH | 41 | 1993.5 | 21/7/4/4/5 | 1:1091 (1:40-1:5120) | 1:396 (1:40-1:1280) | - |
| Type 2 AIH | 5 | 3126 | 2/1/1/1/0 | 1:488 (1:320-1:640) | 1:160 (0-1:160) | 54.22 |
Data shown as means unless noted otherwise.
Blood samples from patients with AIH were collected at one single time point during immunosuppressive treatment, in a sterile vacuum tube that contained an anticoagulant (EDTA). The tubes were centrifuged at 3,000 rpm for 5 min to obtain plasma or serum then 500 μl aliquots of each was labeled and frozen at -70 °C for later use.
Classification criteria for type 1 and type 2 AIHSerum samples from AIH patients were tested for ANA, SMA, and anti-LKM-1 antibodies. Patients were classified according to antibody positivity: type 1 AIH patients were ANA and/or SMA-positive, while type 2 AIH patients were anti-LKM-1-positive. ANA and SMA antibodies were detected by indirect immunofluorescence using cryosectioned specimens of rat liver, kidney, and stomach. Serial dilutions of serum were prepared starting from 1:40. Type 1 AIH patients showed high titers of ANA (mean, 1:1,091; range, 1:40-1:5,120) and SMA antibodies (mean, 1:396; range, 1:40-1:1,280). For type 2 AIH, a commercial enzyme-linked immunosorbent assay (ELISA) kit was used according to the manufacturer’s instructions (INOVA QUANTA Lite® LKM-1 ELISA, San Diego, CA, USA). Following the kit interpretation criteria, samples were classified as Negative: ≤ 20 U, Equivocal: 20.1-24.9 U, and Positive: ≥ 25 U. Mean anti-LKM-1 antibody levels were 54.22 U (range, 28.9-101.3 U).
Biochemical assaysBiochemical studies to determine the levels of ALT and AST in serum of patients were carried out using a commercial ADVIA® Chemistry Systems kit (Munich, Germany). Bilirubin levels were determined by reflectance photometry on reactive strips and read in a Reflotron® Plus system (Roche Diagnostics, UK). Serum cytokine levels were determined using commercial ELISA kits according to the manufacturer’s instructions. IL-17A, IL-17F, and IL-23 were measured using an eBioscience kit (San Diego, CA, USA), while IL-21, IL-22, IL-10, IL-6, and TNF-α kits were obtained from Peprotech Inc. (Rocky Hill, NJ, USA).
Statistical analysisThe normal distribution of variables was analyzed by the Kolmogorov-Smirnov test using SigmaPlot 10.0 software. The Mann-Whitney U-test was used to compare nonparametric variables. The Kruskal-Wallis test was used for comparisons between more than two groups. Intergroup comparisons were performed using Dunn’s multiple comparison test; nonparametric correlations were performed with the Spearman test. A P < 0.05 was considered statistically significant. The data were generated using GraphPad Prism 6.01 software and expressed as medians.
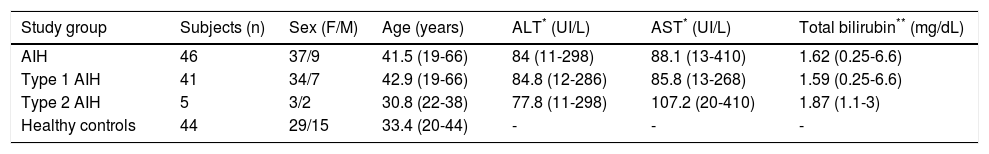
ResultsDemographic characteristics and treatment regimensThe female:male ratio of the AIH patients was 37:9, mean patient age was 41.5 years-old (range, 19-66 years-old), mean aspartate transaminase (AST) level was 88.1 IU/L (range, 13-410 IU/L), mean alanine transaminase (ALT) level was 84 IU/L (range, 11-298 IU/L), and mean total bilirubin was 1.62 mg/dL (range, 0.25-6.6 mg/dL) (Table 2).
Demographic and laboratory features of AIH patients.
| Study group | Subjects (n) | Sex (F/M) | Age (years) | ALT* (UI/L) | AST* (UI/L) | Total bilirubin** (mg/dL) |
|---|---|---|---|---|---|---|
| AIH | 46 | 37/9 | 41.5 (19-66) | 84 (11-298) | 88.1 (13-410) | 1.62 (0.25-6.6) |
| Type 1 AIH | 41 | 34/7 | 42.9 (19-66) | 84.8 (12-286) | 85.8 (13-268) | 1.59 (0.25-6.6) |
| Type 2 AIH | 5 | 3/2 | 30.8 (22-38) | 77.8 (11-298) | 107.2 (20-410) | 1.87 (1.1-3) |
| Healthy controls | 44 | 29/15 | 33.4 (20-44) | - | - | - |
Data shiown as means unless noted oherwise.
Ten patients included in our study showed an overlapping syndrome (primary biliary cirrhosis or PBC). There were not cases of primary sclerosing colangitis as another overlapping syndrome. Thirteen patients (28.26%) had concurrent immune disorders, including 4 patients with systemic lupus erythematosus (SLE), 3 patients with rheumatoid arthritis (RA), 3 patients with ulcerative colitis (UC), 1 patient with Sjogren’s syndrome, 1 patient with vitiligo and 1 patient with autoimmune hemolytic anemia (AIHA).
Of the 46 AIH patients cohort, 33 patients (71.73%) were treated with conventional treatment regimens, including 8 (17.39%) patients who received prednisone (PDN) alone (doses between 10-30 mg/per day) and 26 (56.52%) patients who received PDN in combination with azathioprine (AZA) (PDN doses between 2.5-30 mg/per day; AZA doses between 50-100 mg/per day). 12 patients (26.09%) had other treatment schemes (OT): ursodeoxycholic acid (UDCA), AZA in combination with UDCA or AZA alone. Information about the time of evolution with the therapy and percentage of patients under specific immunosuppressive treatment is shown on table 3.
Immunosuppressive treatment of AIH patients.
| Study group | Subjects (n) | Time under treatment (mean in months) ± SD | PDN, n (%) | PDN/AZA, n (%) | OT, n (%) |
|---|---|---|---|---|---|
| AIH | 46 | - | 8 (17.39) | 26 (56.52) | 12 (26.09) |
| Type 1 AIH | 41 | 40.43 ± 38.07 | 8 (19.51) | 21 (51.21) | 12 (29.26) |
| Type 2 AIH | 5 | 29.80 ± 29.41 | 0 | 5 (100) | 0 |
PDN: prednisone. AZA: azathioprine. UDCA: ursodeoxycholic acid. OT: others treatments (ursodeoxycholic acid, AZA/UDCA, AZA).
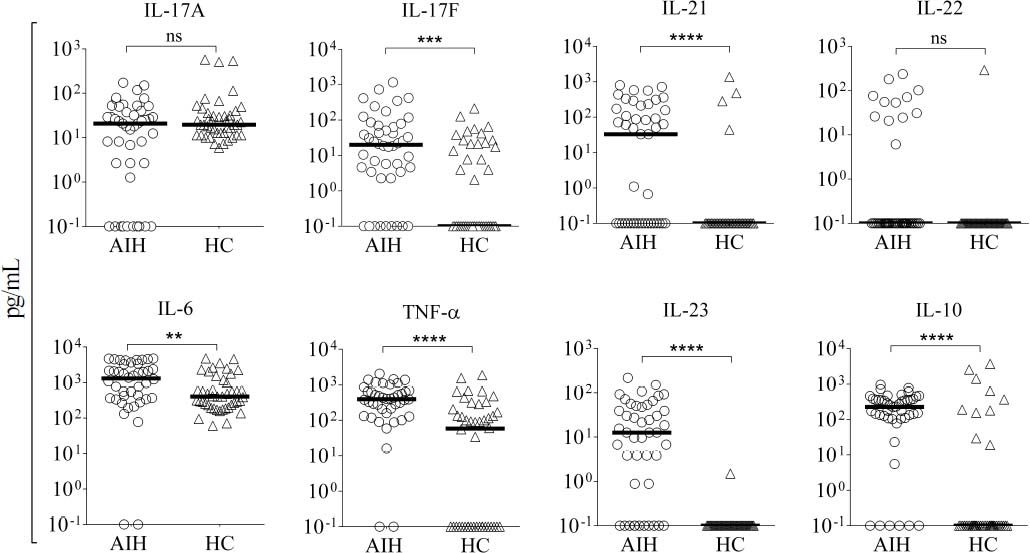
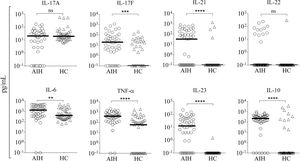
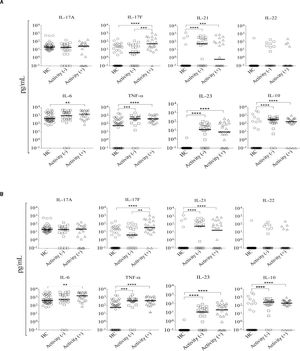
Little is known about how immunosuppressive treatment of patients with AIH can down-regulate key inflammatory mediators, and few studies have addressed which cytokines can be regulated by corticosteroid therapy. To investigate how the standard therapeutic regimen influences the inflammatory response in Mexican AIH patients, the serum levels of IL-17A, IL-17F, IL-23, IL-21, IL-22, IL-10, IL-6, and TNF-α were determined. Higher serum concentrations of IL-17F (AIH median, 19.96 pg/ mL; HC median, 0.1 pg/mL), IL-21 (AIH median, 32.85 pg/mL; HC median, 0.1 pg/mL), IL-23 (AIH median, 12.63 pg/mL; HC median, 0.1 pg/mL), IL-6 (AIH median, 1311 pg/mL; HC median, 403.40 pg/mL), IL-10 (AIH median, 224.9 pg/mL; HC median, 0.1 pg/mL), and TNF-α (AIH median, 391.40 pg/mL; HC median, 58 pg/mL) were detected in AIH patients. There were no significant differences in IL-17A (AIH median, 20.72 pg/mL; HC median, 19.58 pg/mL) and IL-22 (AIH median, 0.1 pg/mL; HC median, 0.1 pg/mL) serum levels between Mexican AIH patients and HC (Figure 1).
Serum levels of pro-inflammatory cytokines in autoimmune hepatitis (AIH) vs. healthy controls (HC). Comparison of the serum levels of IL-17A, IL-17F, IL-21, IL-22, IL-6, TNF-α, IL-23, and IL-10 in AIH patients (n = 46) and HC (n = 44). Median serum levels for each cytokine are shown by a horizontal bar. Statistical significance was analyzed by Mann-Whitney U-test. Cytokine levels are represented in log10 concentration (pg/mL); ** p = 0.0096, *** p = 0.0004, ****p < 0.0001.
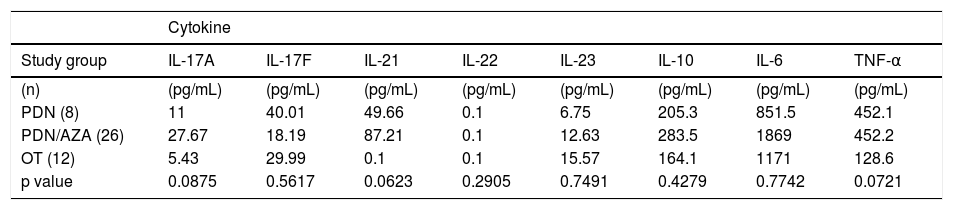
To know how the specific immunosuppressive therapy affects the serum levels of the studied cytokines, we classify AIH patients according the treatment received (PDN alone, PDN/AZA or OT). We compared and classified the patients as follow: A first group with 8 patients under PDN treatment, a second group including 26 patients with a PDN/AZA treatment scheme and a third group with 12 patients under OT. There were no significant differences between cytokines of each group of treatment (Table 4).
Serum levels of pro-inflammatory cytokines according to immunosuppressive therapy.
| Cytokine | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study group | IL-17A | IL-17F | IL-21 | IL-22 | IL-23 | IL-10 | IL-6 | TNF-α |
| (n) | (pg/mL) | (pg/mL) | (pg/mL) | (pg/mL) | (pg/mL) | (pg/mL) | (pg/mL) | (pg/mL) |
| PDN (8) | 11 | 40.01 | 49.66 | 0.1 | 6.75 | 205.3 | 851.5 | 452.1 |
| PDN/AZA (26) | 27.67 | 18.19 | 87.21 | 0.1 | 12.63 | 283.5 | 1869 | 452.2 |
| OT (12) | 5.43 | 29.99 | 0.1 | 0.1 | 15.57 | 164.1 | 1171 | 128.6 |
| p value | 0.0875 | 0.5617 | 0.0623 | 0.2905 | 0.7491 | 0.4279 | 0.7742 | 0.0721 |
Comparisons of cytokines according to immunosuppressive therapy were calculated using Kruskal-Wallis test.PDN: prednisone. PDN/AZA: prednisone/azathioprine. OT: other treatment.
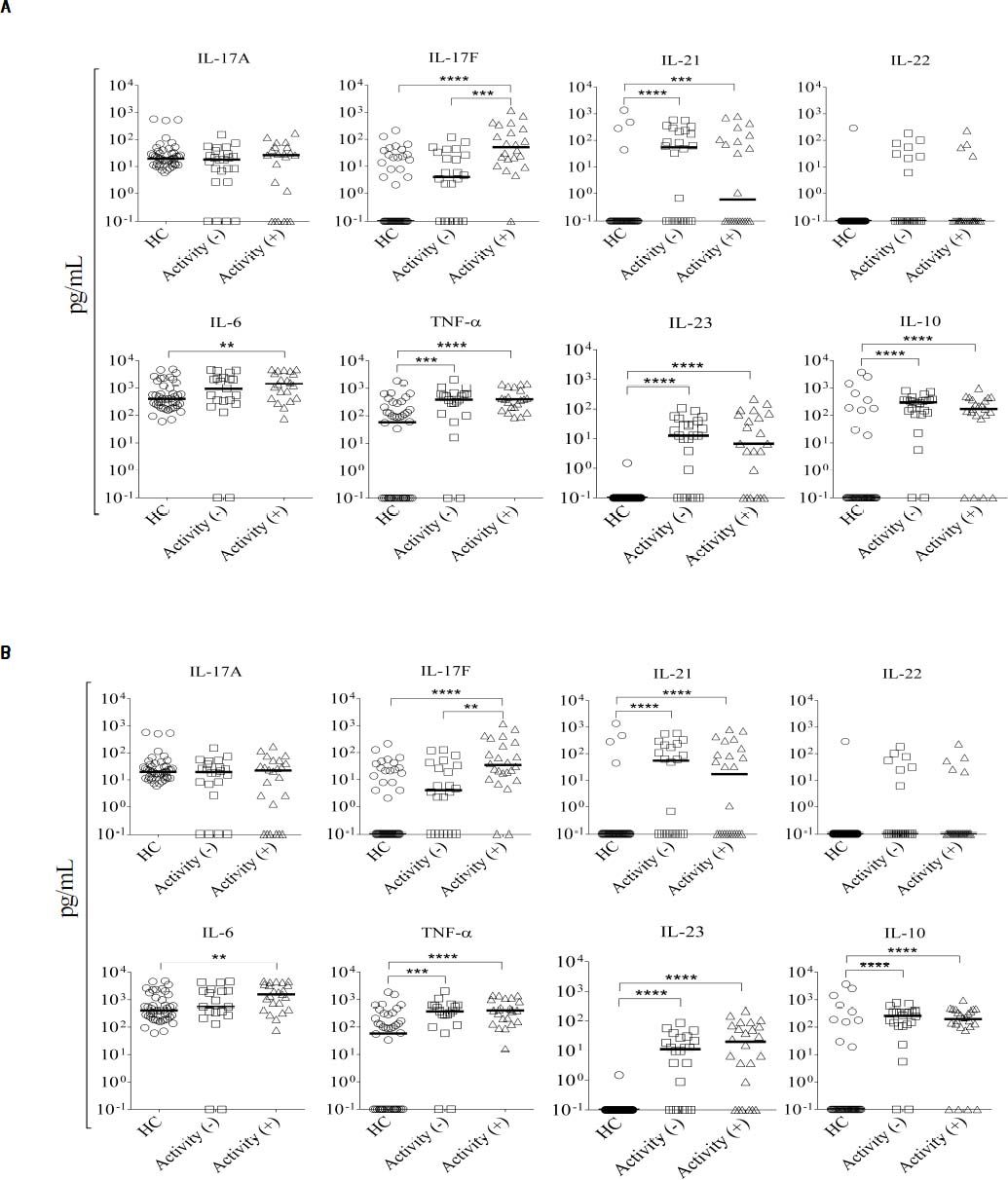
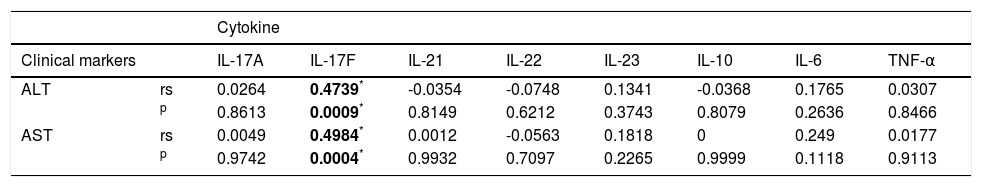
Aminotransferases (ALT or AST) are clinical markers of hepatic damage commonly used to evaluate the response to treatment. Therefore, we investigated the association between serum cytokine levels and these markers of liver damage in Mexican AIH patients. Serum IL-17F le vels significantly correlated with serum ALT and AST levels in AIH patients, while IL-17A, IL-21, IL-22, IL-23, IL-10, IL-6, and TNF-α levels did not (Table 5). Next, we divided the patients into two groups based on their aminotransferase activity: patients with aminotransferase activity (> 60 UI/L of ALT or AST) and without activity (≤ 60 UI/L of AALT or AST). Twenty-two patients had both ALT and AST activity (> 60 UI/L), while 24 patients had no aminotransferase activity (≤ 60 UI/L). In our study, biochemical remission was defined as the complete normalization or marginal elevation (less than two-fold elevation) of aminotransferase levels, complete normalization of bilirubin and gamma-globulins. Comparison of serum cytokine levels between these groups showed that IL-17F levels were higher in patients with ALT activity than in patients without it (P = 0.0004); no significant differences were found for the other measured cytokines (Figure 2A). Serum IL-17F levels were also significantly increased in patients with AST activity compared to those without aminotransferase activity (P = 0.0034; Figure 2B).
Correlation between cytokines and liver damage markers.
| Cytokine | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical markers | IL-17A | IL-17F | IL-21 | IL-22 | IL-23 | IL-10 | IL-6 | TNF-α | |
| ALT | rs | 0.0264 | 0.4739* | -0.0354 | -0.0748 | 0.1341 | -0.0368 | 0.1765 | 0.0307 |
| p | 0.8613 | 0.0009* | 0.8149 | 0.6212 | 0.3743 | 0.8079 | 0.2636 | 0.8466 | |
| AST | rs | 0.0049 | 0.4984* | 0.0012 | -0.0563 | 0.1818 | 0 | 0.249 | 0.0177 |
| p | 0.9742 | 0.0004* | 0.9932 | 0.7097 | 0.2265 | 0.9999 | 0.1118 | 0.9113 | |
Comparisons of cytokines and clinical states were calculated using the Spearman’s rank correlation coeficient. ALT: alanine transaminase. AST: aspartate transaminase. rs: Spearman’s rank correlation.
Serum concentration of cytokines according to aminotransferase activity. We classified 22 patients as having aminotransferase activity, while 24 did not: A. ALT. B. AST. Serum levels of IL-17A, IL-17F, IL-21, IL-22, IL-6, TNF-α, IL-23, and IL-10 were measured in autoimmune hepatitis (AIH) patients. Comparison of serum cytokine levels and aminotransferase activity groups was determined by Mann-Whitney U-test. Results are expressed as median values. Cytokine levels are represented in log10 concentration (pg/mL); **p < 0.001, *** p < 0.0001. HC: healthy controls.
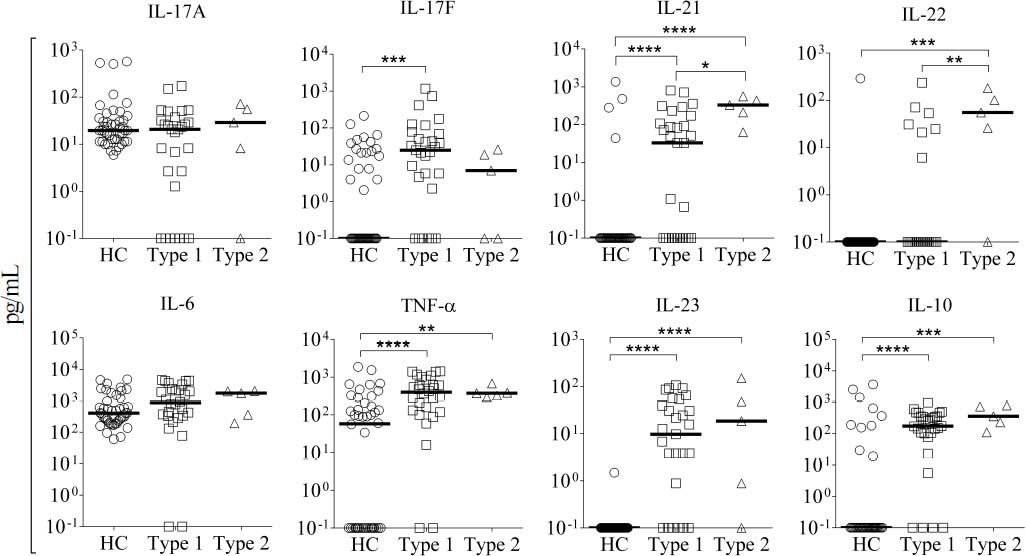
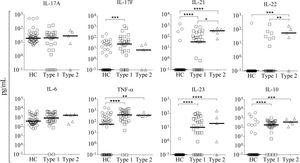
In order to evaluate if there is a difference in the pattern of cytokine production associated with type 1 or 2 AIH, patients were classified by autoantibody profile. A total of 41 patients were classified as type 1 AIH, and 5 patients were classified as type 2 AIH. Interestingly, we found that IL-21 and IL-22 serum levels were significantly elevated in type 2 but not type 1 AIH patients (IL-21: type 2 = 331.1 pg/mL, type 1 = 0.66 pg/mL, P = 0.0042; IL-22: type 2 = 55.26 pg/mL, type 1 = 0.1pg/mL, P = 0.0028). These results suggest that the pharmacological therapy used for AIH fails to selectively downregulate production of IL-21 and IL-22 to normal values, at least in type 2 AIH patients (Figure 3). In contrast, type 1 AIH patients tended to have higher serum concentration of IL-17F (22.32 pg/mL) than type 2 patients (6.98 pg/mL), although the difference was not statistically significant. There were no significant differences in the median levels of other cytokines (IL-17A, IL-6, TNF-α, IL-23, and IL-10) between type 1 and 2 patients (Figure 3).
Serum cytokine levels in patients according to AIH type. We classified 41 patients as type 1 and 5 patients as type 2 AIH. The serum levels of IL-17A, IL-6, TNF-α, IL-23, and IL-10 did not significantly differ between AIH type and HC. The type 1 group had higher serum concentration of IL-17F than the type 2 group, while IL-21 and IL-22 were higher in type 2 than type 1 patients. Results are represented as median values. Statistical significance was analyzed by Kruskal-Wallis and Dunn’s multiple comparison tests. Cytokine levels are represented in log10 concentration (pg/mL); * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. HC: healthy controls.
AIH is a complex and multifactorial pathology involving interactions between genetic background and environmental and immunological factors which trigger an inflammatory response through auto-reactive T-cells, inducing the progressive destruction of hepatocytes leading to liver failure if left untreated. Both T-cells and inflammatory cytokines have been implicated in the pathogenesis and severity of AIH. In this study, we investigated the relationship between disease type, clinical outcome, and circulating cytokines (IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-10, IL-6, and TNF-α) during corticosteroid treatment in Mexican patients with AIH. Importantly, we present new evidence regarding the dynamic regulation of IL-17F, IL-21, IL-22, and IL-23 in AIH. These cytokines are primarily produced by activated T-cells in several autoimmune diseases, including RA, experimental autoimmune encephalitis, and PBC, and enhance the inflammatory response within tissue.13,14
A second important observation from our study is the persistence of elevated serum levels of IL-17F, IL-21, IL-23, IL-6, TNF-α, and IL-10 in patients with AIH, even though all patients in our study were under immunosuppressive therapy (Figure 1). Overall, these heightened levels did not correlate with aminotransferase (AST or ALT) activity, with the exception of IL-17F. The percentage of patients in remission phase classified by serum aminotransferase levels was 52.17%, whereas 47.83% of patients showed residual aminotransferase activity (Figure 2A and 2B). Interestingly, our results are in contrast to previous observations reported by Kamijo, et al. in Japanese AIH patients.15 In our study, the majority of Mexican AIH patients produced high concentration of pro-inflammatory cytokines even after 6 months at least of corticosteroid treatment and despite reaching the remission phase. In the Japanese population, the levels of pro-inflammatory mediators IL-12p40, IL-17F, IL-18, IL-23, IP-10, MIP-1α, and MIP-1β returned to normal after more than 6 months with corticosteroid therapy. Thus, although similar cytokines were elevated in both people groups, the immunological response after standard immunosuppressive therapy may differ by ethnicity. Differences in the clinical response to corticosteroid therapy associated with ethnicity have been described previously.16–19 However, other variations between the study by Kamijo and present results could explain these discrepancies, notably, the time of sample collection. In our study, blood samples were collected at the beginning of the remission phase, whereas the study by Kamijo measured cytokines 6 months after remission onset. Furthermore, higher concentration of proinflammatory cytokines in Mexican AIH patients vs. HC suggests standard pharmacological therapy fails to downregulate cytokine production back to normal levels in our study population.
A third interesting observation was the fact that there were high serum concentrations of IL-17F in Mexican AIH patients. In fact, serum IL-17F levels were associated with clinical outcome d uring the treatment phase. We found that AIH patients with positive aminotransferase activity produced higher concentrations of IL-17F than patients without aminotransferase activity (clinically classified in remission; Figure 2A and 2B). IL-17F serum levels positively correlated with serum ALT and AST levels (Table 5). However, no significant differences were observed when we compared the serum levels of IL-17A, IL-21, IL-22, IL-23, IL-10, IL-6, and TNF-α in AIH patients with or without aminotransferase activity. While there is some evidence in the literature for the potential role of the IL-17A isoform in liver disease, little is known about the role of IL-17F. IL-17A stimulates multiple types of nonparenchymal liver cells to secrete pro-inflammatory cytokines and chemokines promoting liver inflammation.9,20,21 Likewise, IL-17F is also a pro-inflammatory cytokine that interacts with both TNF-α and IL-6 and may be important in both amplification of inflammation and fibrosis in the liver.22 Our data suggest that IL-17F is associated with AIH severity and may represent a suitable biomarker of active disease.
Finally, our study demonstrates an association between elevated levels of IL-21 and IL-22 with type 2 AIH in patients undergoing immunosuppressive therapy. A previous study has suggested there are different immunological responses depending on the type of AIH.6 This idea is well supported by our current results which show that specific immunological mediators, such as IL-21 and IL-22, correlate with type 2 but not type 1 AIH. IL-21 is a pleiotropic cytokine implicated in the humoral and cellular response that induces proliferation and differentiation of B-cells into plasma cells, enhancing immunoglobulin production and isotype class switching, in addition to promoting activation of cellular responses mediated by CD8+ T-cells and the development of immunological memory.23–26 Recently, a number of studies have shown that IL-21 is involved in the pathogenic mechanisms of many autoimmune diseases, such as celiac disease (CD), RA, SLE, and type 1 diabetes mouse model. This cytokine has been associated with tissue damage by inducing CD4+ T-cells to produce IL-17.27–29 However, little is known about the participation of IL-21 in autoimmune liver disease. Pan, et al. showed that the levels of IL-21 expression in the liver tissues of patients with chronic active hepatitis B were significantly associated with increased inflammation and fibrosis.30 Furthermore, IL-21 may contribute to the fibrogenesis of hepatitis B virus (HBV)-associated liver cirrhosis by activating hepatic stellate cells. Different lines of evidence support the idea that IL-22 plays an important role in the pathogenesis of many autoimmune diseases, such as SLE, psoriasis, CD, RA, and UC.31–34 Moreover, in viral human liver diseases, IL-22 was shown to play a pathological role in exacerbating chronic liver inflammation and injury by recruiting hepatic Th17 cells in HBV-infected patients.35 These data suggest that IL-22 exhibits pro-inflammatory activity in different liver diseases. Understanding the different immunological responses elicited by type 1 and 2 AIH is important because there are significant differences in their clinical course. Whereas patients with type 1 AIH respond well to standard prednisone and azathioprine treatment, type 2 patients frequently present with more severe symptoms and clinical course, which is likely associated with resistance to the immunosuppressive treatment and increased risk of cirrhosis.1,18,19 Given the different regulation of IL-21 and IL-22 with AIH type, our study opens a very attractive field of research on these cytokines in the pathology of AIH.
AcknowledgmentsThis work was supported by Fondo Sectorial en Investigación Básica SEP-CONACyT Project ID 134884. Thomas-Dupont P, Izaguirre-Hernández IY, Sánchez-Vargas LA, Maldonado-Rentería MJ, and Hernández-Flores KG were recipients of a Ph.D. Scholarship from CONACyT, México No. 279061, 278911, 279133, 290733, and 279262, respectively. We are grateful to Drs. Antonio Velazquez and Irma Espinoza of the blood bank department from the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (México City, México) for their contributions and obtainment of HC samples.
Conflict of InterestsWe declare that the authors have not any financial or personal relationship with other people or organizations that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.