Introduction and aim. Liver disease is associated with cognitive dysfunction also at early stages, and minimal hepatic encephalopathy, affecting 20-70% of patients, is frequently under-recognized. The main purpose of this work was to demonstrate that a substantial number of patients, enrolled due to an acute confusional state in absence of a diagnosis of liver disease, suffers of hepatic encephalopathy.
Material and methods. Before a diagnosis of a well-compensated liver diseases was performed, 410 patients with an acute confusional state were enrolled in this study.
Results. Even in the presence of minimal alterations of hepatic function, the psychometric tests applied demonstrated early signs of cerebral frontal alteration. The alteration was associated with the severity of liver disease, paralleling the progression of the patient to minimal hepatic failure or chronic liver disease.
Conclusions. These psychometric tests are essential to detect early and subclinical frontal failure. Frontal dysfunction may be a useful tool in the follow-up of these patients.
Liver disease affects many people, both young and old, with different co-morbidities. Individuals with both chronic and acute liver failure may demonstrate cognitive deficits, including impairments in memory, attention, and psychomotor function.1–3 Such impairment has been associated with significant deterioration in quality of life.4-6 Patients with more severe disease display greater cognitive deficits (immediate memory and processing speed)1,2,7 indicating that patients with end-stage disease may require extra support with daily activities and health-care decision-making. Of notice the observation than even in minimal hepatic encephalopathy, cognition is impaired.8 The prognosis of minimal hepatic encephalopathy has recently been studied; 30% of patients developed overt encephalopathy9 within 2 years from the initial assessment.1,8 Other studies have also shown that this entity is an independent predictor of poor survival in patients with liver disease.1,10 Nevertheless, the importance of these subtle deficits has been underlined only recently, with the evidence of negative effects in patient outcome. Consequently the current consensus states that these disorders need to be diagnosed and treated.11–15
Minimal hepatic encephalopathy affects about 20-70% of patients with liver disease, depending on tests used and population studied.8,9,12 These mild deficits are often unrecognized during routine clinical examination,16 while they are detected in up to 84% of patients during neuropsychological testing.4 Indeed, it has been recognized that appropriate neuropsychological tests are able to highlight signs of cognitive impairment in patients who were felt to be free from hepatic encephalopathy.8,17,18
Despite that, when different neuropsychological tests have been used to identify this syndrome, different cognitive deficits emerged.3,8 Common findings were impairment of visuo-spatial functioning, attention and psychomotor speed,3,11,18 even if these observations are still under debate.19 In conclusion, there is a general lack of consistent studies, which accounts daily-living affecting cognitive impairment in minimal hepatic encephalopathy, and of specific tests, apt to detect it. These tests should be simple enough to be applied as every-day clinical visit, and sensitive enough to detect cognitive impasse.
The purpose of the present study was to identify a neuropsychological test that can be immediate, sensitive, related to clinical and laboratory parameters, easy to do, and well-accepted by patients, in order to assess a bedside detection of cognitive and/or behavioral alterations in liver patients. In particular, we aimed to screen the baseline neuropsychological performances of patients with liver diseases as compared to matched healthy controls, and to describe the correlations between clinical, haematological, and neuropsychological parameters. Finally, we repeated the same evaluations at 6 and 12 months in those patients with liver diseases that had an indication to undergo a hepatological follow-up and/or treatment.
Material and MethodsCharacteristics of subjects and exclusion criteriaThe study was conducted in accordance with the Declaration of Helsinki and with the Ethics Guidelines of the Institute. Informed consent in writing was obtained from each patient.
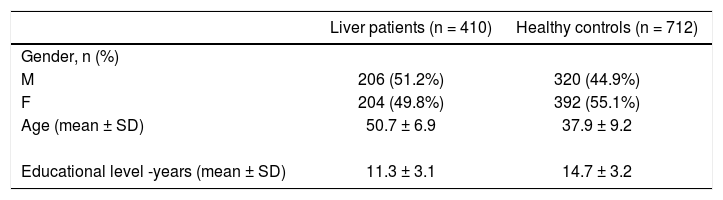
410 patients with diagnosis of acute confusional state, not comprised by other clinical conditions, such as declared cognitive impairment, loss of consciousness, epileptic seizure, head or neck chronic pain, migraine, etc. were included in the present study. The patients have been studied in Neurological Clinic, have been diagnosed as patients with an early-stage liver disease and referred to the Liver Center of the University of Trieste (from January 1st, 2011 to June 1st 2014). We enrolled also 712 healthy subjects, who were selected among patients’ relatives, students, researchers and other members of the hospital staff. The demographic and clinical characteristics of patients and controls are described in table 1.
Patients with previously diagnosed psychiatric diseases or CNS disorders were excluded from the study. All patients underwent neuroimaging (346 brain CT and 64 brain MRI). Exclusion criteria were the evidence of non-lacunar infarcts, normal pressure hydrocephalus, cortical hemispheric large vessel stroke or lobar haemorrhage, Wernike encephalopathy, Korsakoffs disease, subdural haematoma, meningitis or viral encephalitis.
Clinical and neuropsychological variables and study designFor both patients and controls, we performed a standardized baseline assessment, including a detailed clinical history, a physical examination, and a neurological and neuropsychological evaluation. Furthermore, patients with liver diseases underwent laboratory tests. The main variables analyzed were:
- •
Hepatic assessment. Child-Pugh sub-scores,20–21 ammonium, bilirubin, and albumin levels, PT prolongation (INR), presence of ascites and overt encephalopathy.
- •
Hepatic encephalopathies. West Haven Criteria (The West-Haven Classification).
- a)
Stage 0. MHE (previously known as subclinical HE). Lack of detectable changes in personality or behavior. Minimal changes in memory, concentration, intellectual function, and coordination. Asterixis is absent.
- b)
Stage 1. Trivial lack of awareness. Shortened attention span. Impaired addition or subtraction. Hypersomnia, insomnia, or inversion of sleep pattern. Euphoria, depression, or irritability. Mild confusion. Slowing of ability to perform mental tasks. Asterixis can be detected.
- c)
Stage 2. Lethargy or apathy. Minimal disorientation. Inappropriate behavior. Slurred speech. Obvious asterixis. Drowsiness, lethargy, gross deficits in ability to perform mental tasks, obvious personality changes, inappropriate behavior, and intermittent disorientation, usually regarding time.
- d)
Stage 3. Somnolent but can be aroused, unable to perform mental tasks, gross disorientation about time and place, marked confusion, amnesia, occasional fits of rage, present but incomprehensible speech.
- e)
Stage 4. Coma with or without response to painful stimuli. However, the terms that limit each stage of the classification are not clearly defined, and the metric characteristics of the stage are unknown. It is for this reason that other scales such as the Clinical Hepatic Encephalopathy Staging Scale (CHESS) have been proposed.22
- a)
The presence or absence of the nine items on the CHESS score may be helpful in eliminating interobserver variability and in making a distinction between the various grades of encephalopathy. This staging scale, however, requires further validation.5
- •
Executive functions. Ten Point Clock Test (TPCT).24
- •
Attention, judgment, and analogical reasoning. Frontal Assessment Battery (FAB)24 composed by the sub-items: similarities (3-0), lexical fluency (3-0), motor series (3-0), conflictual instructions (3-0), go/no-go (3-0), prehension (3-0).
- •
Insight. Clinical Insight Rating Scale (CIR),25 that provides a measure of 4 comprising items (awareness, cognitive deficit, disease progression, and functional deficit).
- •
Behaviour. Neuropsychiatric Inventory (NPI).26
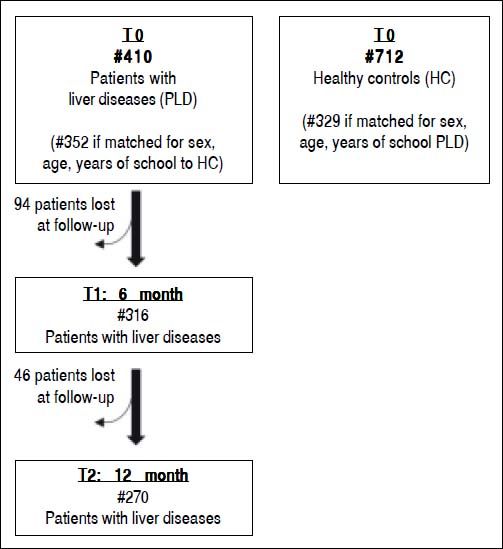
Out of the 410 liver patients evaluated at baseline, 316 (77.1%) and 270 (65.9%) were re-evaluated after the 6-months and 12-months, respectively. A group of patients has been lost at follow-up because of the low severity of liver disease or scarce compliance (Figure 1).
Statistical analysisUsing coarsened exact matching27 we matched 410 patients with liver diseases analyzed at baseline with 712 healthy subjects. On the basis of sex (same), age (same) and years of school (≤ 6 vs. ≤ 10 vs. ≤ 13 vs. ≤ 8 vs. ≤ 22 years) we were able to match 352 patients with liver diseases to 329 controls (Figure 1). Such matching was performed to reduce confounding due to factors known to affect psychometric performance. To further reduce such confounding, we controlled for sex, age and years of school in all analyses, not only those comparing patients with liver diseases and matched controls but also those involving liver patients moly with liver disease. Values of psychometric scores of patients and matched controls were estimated and compared by using a multivariable linear regression model with robust confidence intervals to relax the assumption of homoscedasticity of residuals, which was clearly violated in some cases, e.g. for FAB sub-scores. Such regression model included disease (0 = none; 1 = cirrhosis), gender (0 = female; 1 = male), age (years) and school (years) as predictors. The p-value for the between-group (patients with liver diseases vs matched liver patients) comparison is that associated to the Wald test of the disease term in the above model. Spearman’s rho with Bonferroni’s correction was used to evaluate the association between the different psychometric tests in patients with liver diseases.
To better explore the relationship between FAB, CIR and NPI, we employed linear regression with robust confidence intervals and multivariable fractional polynomials to model non-linear predictors. The same model was used to evaluate the relationship between FAB and continuous biochemical indexes (ammonium, albumin, INR, bilirubin) and dichotomous clinical signs (ascites and hepatic encephalopathy). Akaike information criterion (AIC) was used to compare different predictors of FAB.
Patient clinical and neuropsychological variables were assessed at baseline and compared to the follow-up data (at 6 and 12 months) using the related-samples Wilcoxon signed rank test (or related-samples McNemar test for dichotomic variables).
Statistical analysis was performed using Stata and the user-written CEM command.28
ResultsAfter a complete clinical assessment, we were able to diagnose hepatic encephalopathy in 237 (57.8%) of the 410 patients with liver diseases, initially not recognised to have any declared cognitive alteration and without overt previously diagnosis of liver failure.
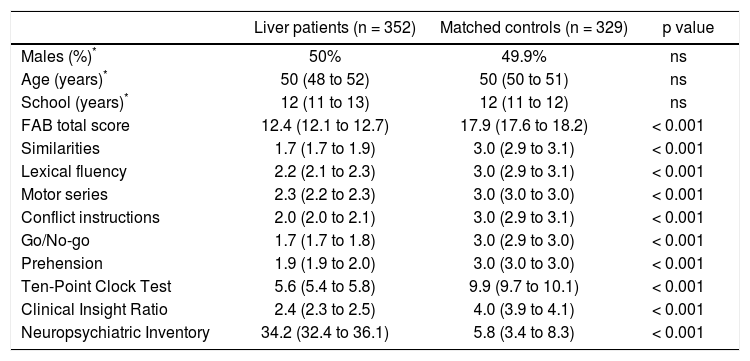
The comparison of baseline psychometric measurements between the selected 352 patients with liver diseases and the 329 matched controls is given in table 2. Despite the initial absence of a clinical suspect of cognitive and behavioral alteration, liver patients performed significantly worse than matched healthy controls in all tests (FAB total score -similarities, lexical fluency, motor series, conflict instructions, go/no-go, prehension-, Ten-Point Clock tests, Clinical insight ratio; Neuropsychiatric Inventory; all p < 0.001).
Comparison of patients with liver diseases and matched controls.
| Liver patients (n = 352) | Matched controls (n = 329) | p value | |
|---|---|---|---|
| Males (%)* | 50% | 49.9% | ns |
| Age (years)* | 50 (48 to 52) | 50 (50 to 51) | ns |
| School (years)* | 12 (11 to 13) | 12 (11 to 12) | ns |
| FAB total score | 12.4 (12.1 to 12.7) | 17.9 (17.6 to 18.2) | < 0.001 |
| Similarities | 1.7 (1.7 to 1.9) | 3.0 (2.9 to 3.1) | < 0.001 |
| Lexical fluency | 2.2 (2.1 to 2.3) | 3.0 (2.9 to 3.1) | < 0.001 |
| Motor series | 2.3 (2.2 to 2.3) | 3.0 (3.0 to 3.0) | < 0.001 |
| Conflict instructions | 2.0 (2.0 to 2.1) | 3.0 (2.9 to 3.1) | < 0.001 |
| Go/No-go | 1.7 (1.7 to 1.8) | 3.0 (2.9 to 3.0) | < 0.001 |
| Prehension | 1.9 (1.9 to 2.0) | 3.0 (3.0 to 3.0) | < 0.001 |
| Ten-Point Clock Test | 5.6 (5.4 to 5.8) | 9.9 (9.7 to 10.1) | < 0.001 |
| Clinical Insight Ratio | 2.4 (2.3 to 2.5) | 4.0 (3.9 to 4.1) | < 0.001 |
| Neuropsychiatric Inventory | 34.2 (32.4 to 36.1) | 5.8 (3.4 to 8.3) | < 0.001 |
Liver patients and matched controls were compared after controlling for sex, age, and years of school. Values are means and robust 95% confidence intervals estimated from linear regression (see Statistical analysis for details).
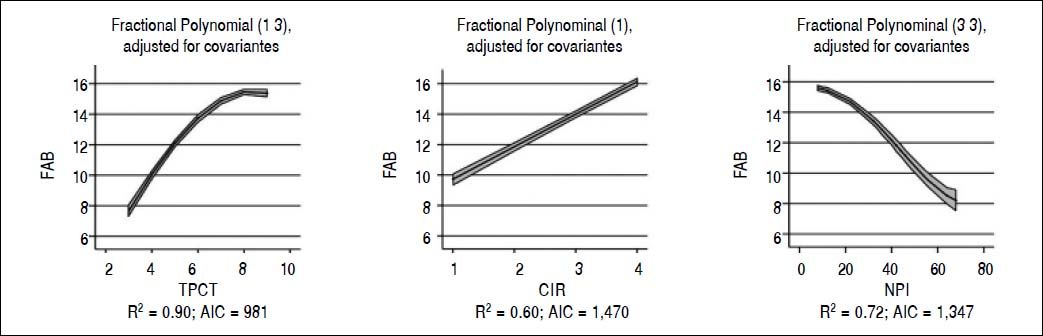
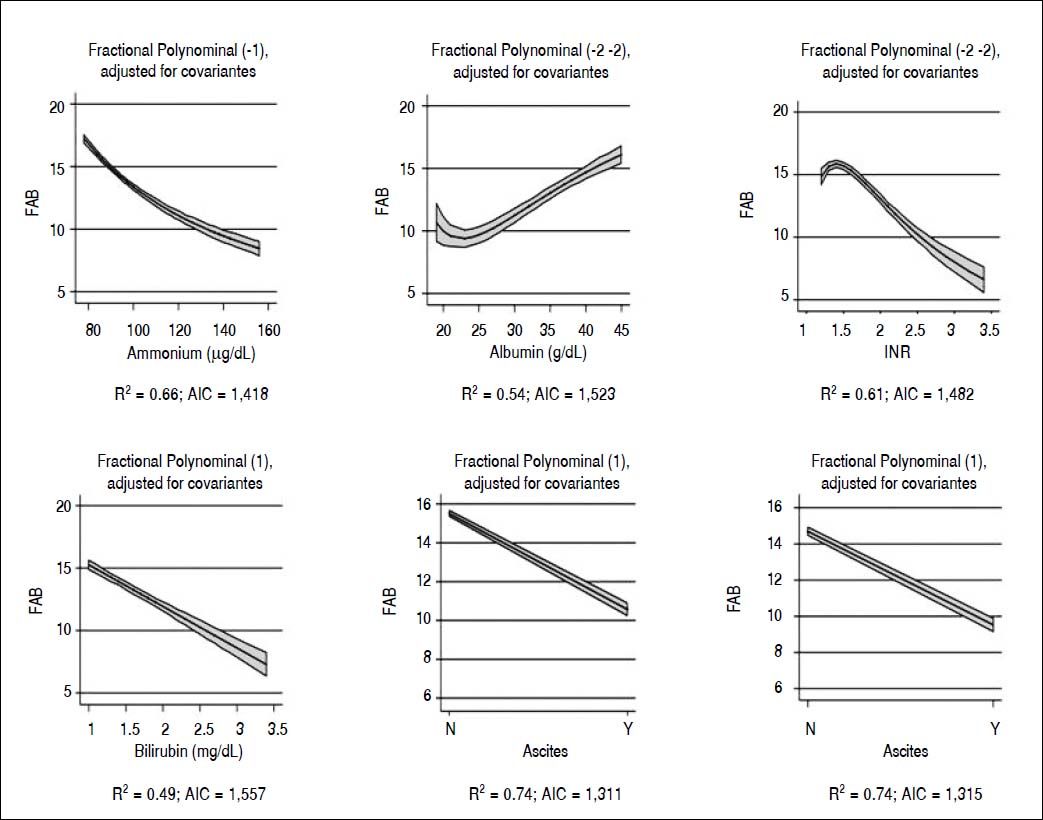
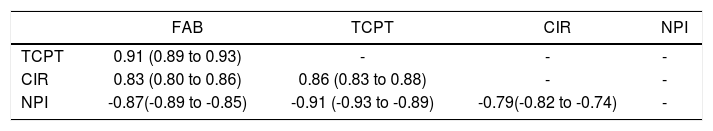
When we analyzed the association between all psychometric tests performed at baseline; a strong association was found among all the tests and in particular between FAB and TCPT and between NPI and CIR (p < 0.001 for both) (Table 3). Considering FAB as outcome (Figure 2), CIR and NPI explained the 60 and 72% of its variance, respectively (Figure 2, central and right panel, respectively). Nevertheless, the best association was observed between FAB and TPCT, as determined both by the highest R2 (90) and lowest AIC (Figure 2, left panel). We also analyzed the association between FAB and biochemical indexes/clinical signs of liver disease (Figure 3). The presence/absence of ascites were the strongest predictors of the FAB score (Figure 3, central lower panel), emphasising the relevance of clinical observations to guide the subsequent neuropsychological evaluation.
Association between psychometric tests in patients with liver diseases.
| FAB | TCPT | CIR | NPI | |
|---|---|---|---|---|
| TCPT | 0.91 (0.89 to 0.93) | - | - | - |
| CIR | 0.83 (0.80 to 0.86) | 0.86 (0.83 to 0.88) | - | - |
| NPI | -0.87(-0.89 to -0.85) | -0.91 (-0.93 to -0.89) | -0.79(-0.82 to -0.74) | - |
Liver patients: n = 352. Values are Spearman’s rho and 95% confidence intervals; p < 0.001 for all test after Bonferroni’s correction.
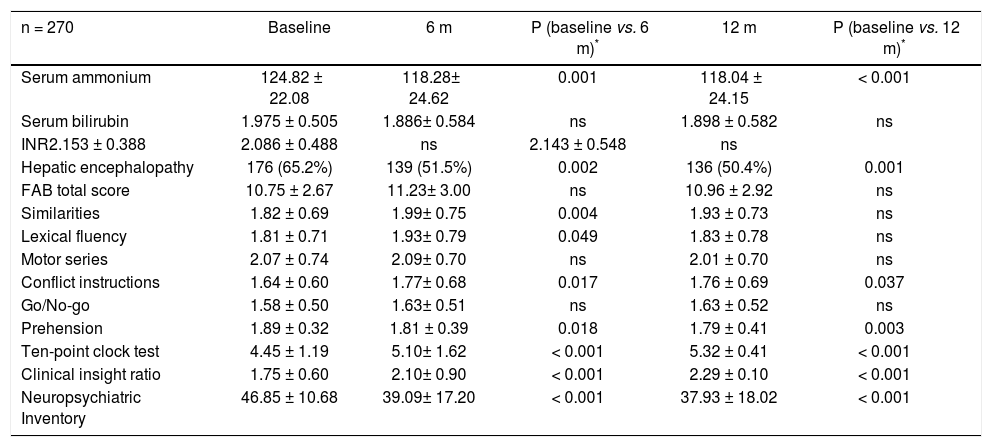
The clinical and neuropsychological characteristics of the subset of patients with liver diseases with a prolonged follow-up and their evolution are reported in tables 4 and 5. The biochemical indices of liver functions (6 and 12 m: serum ammonium and ascites: both p<0.001) improved significantly over the time together with the improvement in the TPCT performance (both 6 and 12 m: p < 0.001), an increased insight level (CIR; both 6 and 12 m: p < 0.001) and a better performance in the NPI (both 6 and 12 m: p < 0.001). Despite FAB total score remained unchanged, there was an improvement of the similarities (p < 0.005), lexical fluency (p < 0.05), conflict instructions (p < 0.05), and prehension (p < 0.05) in the first 6 months, confirmed at 12 months (conflict instructions and prehension p < 0.05 and p < 0.005, respectively), indicating a general clinical improvement.
Patient with liver disease characteristics at 6 and 12-months follow-up.
| n = 270 | Baseline | 6 m | P (baseline vs. 6 m)* | 12 m | P (baseline vs. 12 m)* |
|---|---|---|---|---|---|
| Serum ammonium | 124.82 ± 22.08 | 118.28± 24.62 | 0.001 | 118.04 ± 24.15 | < 0.001 |
| Serum bilirubin | 1.975 ± 0.505 | 1.886± 0.584 | ns | 1.898 ± 0.582 | ns |
| INR2.153 ± 0.388 | 2.086 ± 0.488 | ns | 2.143 ± 0.548 | ns | |
| Hepatic encephalopathy | 176 (65.2%) | 139 (51.5%) | 0.002 | 136 (50.4%) | 0.001 |
| FAB total score | 10.75 ± 2.67 | 11.23± 3.00 | ns | 10.96 ± 2.92 | ns |
| Similarities | 1.82 ± 0.69 | 1.99± 0.75 | 0.004 | 1.93 ± 0.73 | ns |
| Lexical fluency | 1.81 ± 0.71 | 1.93± 0.79 | 0.049 | 1.83 ± 0.78 | ns |
| Motor series | 2.07 ± 0.74 | 2.09± 0.70 | ns | 2.01 ± 0.70 | ns |
| Conflict instructions | 1.64 ± 0.60 | 1.77± 0.68 | 0.017 | 1.76 ± 0.69 | 0.037 |
| Go/No-go | 1.58 ± 0.50 | 1.63± 0.51 | ns | 1.63 ± 0.52 | ns |
| Prehension | 1.89 ± 0.32 | 1.81 ± 0.39 | 0.018 | 1.79 ± 0.41 | 0.003 |
| Ten-point clock test | 4.45 ± 1.19 | 5.10± 1.62 | < 0.001 | 5.32 ± 0.41 | < 0.001 |
| Clinical insight ratio | 1.75 ± 0.60 | 2.10± 0.90 | < 0.001 | 2.29 ± 0.10 | < 0.001 |
| Neuropsychiatric Inventory | 46.85 ± 10.68 | 39.09± 17.20 | < 0.001 | 37.93 ± 18.02 | < 0.001 |
Values are means ± standard deviation.
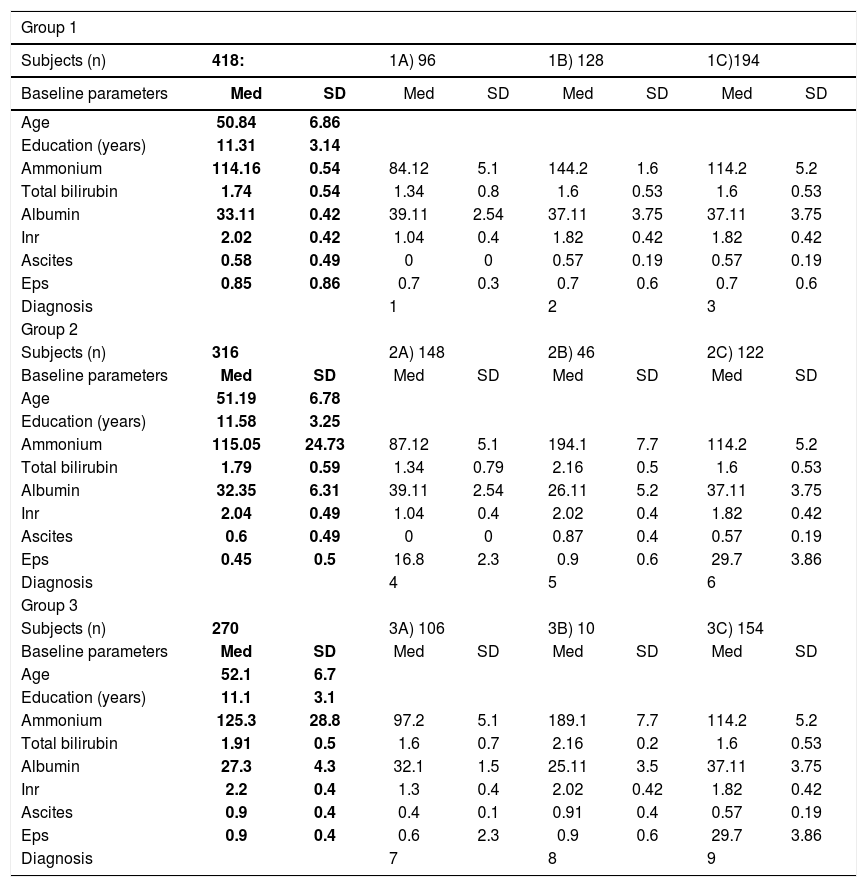
Clinical characteristics and diagnosis of patient with liver disease at 6 and 12-months follow-up.
| Group 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subjects (n) | 418: | 1A) 96 | 1B) 128 | 1C)194 | ||||
| Baseline parameters | Med | SD | Med | SD | Med | SD | Med | SD |
| Age | 50.84 | 6.86 | ||||||
| Education (years) | 11.31 | 3.14 | ||||||
| Ammonium | 114.16 | 0.54 | 84.12 | 5.1 | 144.2 | 1.6 | 114.2 | 5.2 |
| Total bilirubin | 1.74 | 0.54 | 1.34 | 0.8 | 1.6 | 0.53 | 1.6 | 0.53 |
| Albumin | 33.11 | 0.42 | 39.11 | 2.54 | 37.11 | 3.75 | 37.11 | 3.75 |
| Inr | 2.02 | 0.42 | 1.04 | 0.4 | 1.82 | 0.42 | 1.82 | 0.42 |
| Ascites | 0.58 | 0.49 | 0 | 0 | 0.57 | 0.19 | 0.57 | 0.19 |
| Eps | 0.85 | 0.86 | 0.7 | 0.3 | 0.7 | 0.6 | 0.7 | 0.6 |
| Diagnosis | 1 | 2 | 3 | |||||
| Group 2 | ||||||||
| Subjects (n) | 316 | 2A) 148 | 2B) 46 | 2C) 122 | ||||
| Baseline parameters | Med | SD | Med | SD | Med | SD | Med | SD |
| Age | 51.19 | 6.78 | ||||||
| Education (years) | 11.58 | 3.25 | ||||||
| Ammonium | 115.05 | 24.73 | 87.12 | 5.1 | 194.1 | 7.7 | 114.2 | 5.2 |
| Total bilirubin | 1.79 | 0.59 | 1.34 | 0.79 | 2.16 | 0.5 | 1.6 | 0.53 |
| Albumin | 32.35 | 6.31 | 39.11 | 2.54 | 26.11 | 5.2 | 37.11 | 3.75 |
| Inr | 2.04 | 0.49 | 1.04 | 0.4 | 2.02 | 0.4 | 1.82 | 0.42 |
| Ascites | 0.6 | 0.49 | 0 | 0 | 0.87 | 0.4 | 0.57 | 0.19 |
| Eps | 0.45 | 0.5 | 16.8 | 2.3 | 0.9 | 0.6 | 29.7 | 3.86 |
| Diagnosis | 4 | 5 | 6 | |||||
| Group 3 | ||||||||
| Subjects (n) | 270 | 3A) 106 | 3B) 10 | 3C) 154 | ||||
| Baseline parameters | Med | SD | Med | SD | Med | SD | Med | SD |
| Age | 52.1 | 6.7 | ||||||
| Education (years) | 11.1 | 3.1 | ||||||
| Ammonium | 125.3 | 28.8 | 97.2 | 5.1 | 189.1 | 7.7 | 114.2 | 5.2 |
| Total bilirubin | 1.91 | 0.5 | 1.6 | 0.7 | 2.16 | 0.2 | 1.6 | 0.53 |
| Albumin | 27.3 | 4.3 | 32.1 | 1.5 | 25.11 | 3.5 | 37.11 | 3.75 |
| Inr | 2.2 | 0.4 | 1.3 | 0.4 | 2.02 | 0.42 | 1.82 | 0.42 |
| Ascites | 0.9 | 0.4 | 0.4 | 0.1 | 0.91 | 0.4 | 0.57 | 0.19 |
| Eps | 0.9 | 0.4 | 0.6 | 2.3 | 0.9 | 0.6 | 29.7 | 3.86 |
| Diagnosis | 7 | 8 | 9 | |||||
Values are means ± standard deviation. 1. Acute confusional state, acute hepato-hepaty (due to acute, occasional drugs or alcohol abuse). 2. Hepatic insufficiency ≥ stage II from: EBV, Mycoplasma, etc. (5); HCV infection (34); HBV and HCV (16); drugs abuse (30); autoimmune hepatitis -first diagnosis (5); chronic alcoholism -first clinical evidence (38). 3. Minimal hepatic insufficiency from: EBV, Mycoplasma (12); HCV -first diagnosis-(42); HBV + HCV (34); autoimmune hepatitis -first diagnosis (8); chronic alcoholism, drugs use (102). 4. Minimal hepatic disease from autoimmune hepatitis (8); Mycoplasma infection (17); drug withdrawal (12); HCV (32); HBV+HCV (16); chronic moderate alcohol abuse (79). 5. Hepatic insufficiency ≥ stage II from: chronic immunodepression (12); HCV (4); HBV + HCV (12); chronic alcoholism, abuse of alcohol and drugs (18). 6. Hepatic insufficiency stage I-II: autoimmune hepatitis (5), HCV (40); HBV + HCV (22); chronic drugs abuse (10); chronic alcoholism (40). 7. Minimal autoimmune hapatitis (8); drug or alcohol withdrawal (60), HCV (26); HBV + HCV (12). 8. Liver failure > stage III: HBV-and HCV-related (6); chronic alcoholism or drugs abuse (4). 9. Hepatic insufficiency stage I-II: drug withdrawal (8); HCV (50); HBV + HCV (30); chronic drug abuse (10); autoimmune hepatitis (5); chronic alcoholism (52).
There is no reason to consider hepatic encephalopathy as an all-or-nothing phenomenon and a continuous scale of impairment seems more likely to explain the pathogenesis of minimal hepatic encephalopathy.8,11,12 It is now generally agreed that neuropsychological tests offer the best option for the diagnosis of a minimal subclinical hepatic encephalopathy. Nevertheless, there is a considerable debate about which tests should be used, when and by whom should be performed, and how the results should be interpreted.5,8 Unfortunately, a “gold standard” neuropsychological test or battery to evaluate common cognitive deficits in patients with liver diseases is still missing.29 In general, any battery of neuropsychological tests should assess a broad range of cognitive functions; in liver dysfunction it should probably include tests analyzing psychomotor speed, visuopraxis, attention, and concentration.5,8,30 Some authors suggest not to include language and memory tests in minimal hepatic encephalopathy assessment,3 while others disagree.5
In this study we have performed a complete neuropsy-chological evaluation sensitive enough to detect altered behavior and cognitive impairment in an early liver-disease population not diagnosed with cognitive problems.
To make the burden of the operator and patient less demanding, all the selected tests were rapid, easy to perform, and well accepted by the patient (voluntarily performing all the evaluated the test, in a total time of 8 min). In particular, we applied FAB, TPCT, CIR, and NPI which allow to evaluate attention, judgment, and analogical reasoning, executive functions, awareness of the subject on his situation, and behavior as a whole, respectively.
When our short neuropsychological assessment has been applied, patients with liver diseases performed all psychometric tests significantly worse than healthy controls (Table 2). This result is particularly interesting because these patients did not have either a severe form of liver disease or a suspected cognitive deficit. After a complete clinical assessment, we diagnosed overt hepatic encephalopathy in a high percentage of the patients and most important, frontal impairment, together with a low level of awareness and insight has been detected in the subgroup with minimal hepatic encephalopathy not interfering with their daily living.
Our study demonstrates a strong association among all psychometric tests, in particular between FAB and TCPT, and NPI and CIR (p < 0.001) (Table 5). Since the association between FAB and TPCT was the strongest (Figure 2) and FAB is a much more comprehensive evaluation of frontal function than TPCT, FAB should be considered the test of choice for a rapid (4 minutes) and sensitive evaluation of cognitive alteration in minimal hepatic encephalopathy. Finally, insight and behavioral alterations seem to minimally interfere with FAB and TPCT scores, as demonstrated by the lower variance of CIR and NPI to FAB. When the association of FAB with both biochemical and clinical indices of liver disease was considered, this test correlates with all the considered parameters, and in particular with the presence/absence of ascites and hepatic encephalopathy (Figure 3). Taken together, these observations offer decisive elements to select a cognitive test not easily modifiable by behavior alterations, and well-related to clinical variables of liver disease.
Our psychometric evaluations detected an improvement of cognitive performances at 6 and 12 months follow-up (Tables 4 and 5), that reflects the parallel improvement of the liver parameters obtained after a proper treatment of the underlying liver disease. More in detail (Table 5), this subgroup of patients diagnosed with a more severe disease at the baseline that required a prolonged follow-up and a subsequent treatment, showed a better performance in some FAB sub-items (conflict instructions and prehension), TPCT, CIR, and NPI (Table 4), stressing the importance to recognise and treat properly the underlying liver condition to obtain a recovery of the cognitive performance of patients, even in early stages of disease.
ConclusionsIn conclusion, we suggest the use of FAB test to detect cognitive impasse in patients with minimal hepatic encephalopathy or overt hepatic encephalopathy, yet not suspected. FAB has been demonstrated to correlate with the other tests applied (TCPT, CIR, and NPI) and with biochemical and clinical parameters of liver disease. Most importantly, this is a brief and easy test, requiring only a piece of paper and a pencil, which can be used as a pocket-system in every-day practice.
Abbreviations- •
AIC: akaike information criterion.
- •
CIR: clinical insight rating scale
- •
CEM: coarsened exact matching.
- •
CNS: central nervous system.
- •
CT: computerized tomography.
- •
FAB: frontal assessment battery.
- •
MRI: magnetic resonance imaging.
- •
NPI: neuropsychiatric inventory.
- •
PT: prothrombin time.
- •
TPCT: ten point clock test.
Authors declare that they have no competing interests of financial support to declare.