Background/Aims. The main objective of this study was to describe the profile of patients who were benefitted in a collective effort to perform liver biopsies in Bahia, Brazil.
Methods. A cross-sectional study was conducted with a sample composed of all the patients who were submitted to liver biopsy during a collective effort carried out in Bahia between July 2007 and November 2009. At the time of the procedure, data on the age and gender of patients and the reason for performing the biopsy were recorded. Data on the degree of fibrosis and the presence of co-morbidities. Following statistical analysis, the frequency of the liver diseases that led to the biopsy procedure was described, and the profile of the patients was stratified into groups according to the most prevalent etiologies.
Results. Of the 550 patients evaluated, 55.3% were men and 44.7% women. Mean age was 46.63 ± 11.59 years and there was no statistically significant difference in age between males and females. Of the 550 patients, 72% had hepatitis C and the mean age of these patients was 48.49 ± 10.1 years, significantly higher than the mean age of the patients with hepatitis B (40.41 ± 12.43 years). Furthermore, 70.7% of the patients with hepatitis C were between 41 and 60 years of age. The most frequent fibrosis grade was F2 (44%) and the prevalence of advanced fibrosis was 27.7%. Overall, 85 patients, most of them men, had some degree of iron overload. With respect to the safety of the biopsy procedure, severe complications occurred in only two patients.
Conclusion. Hepatitis C is the predominant liver disease that demanded liver biopsy. The profile of the patients who benefitted from this collective effort is similar to that of patients in the rest of the country. Moreover, non-Ultrasonography guided liver biopsy is safe and the collective effort to carry out liver biopsies in Bahia was found to be a viable venture.
Biopsy is the most specific diagnostic tool for staging liver disease as well as for defining etiologies.1,2 It is also useful for identifying specific diseases whose diagnosis depends on histopathology, and additionally, it has been used to evaluate liver graft after transplantation.3 Furthermore, the method takes on singular importance when the objective is to identify a concomitant morbidity that may often affect prognosis of the primary disease such as when steatosis is associated with hepatitis C.4-6 Finally, biopsy may also be useful in defining treatment. According to the Brazilian guidelines for the treatment of hepatitis C, liver biopsy is mandatory in most patients.7 It is a low-risk procedure, but mild to severe complications has been frequently reported.8
In several centers, hepatitis C is the principal indication for liver biopsy because of the requirement to perform this assessment prior to defining treatment.9,10 More recently, non-invasive methods have largely replaced liver biopsy in Europe, but liver biopsy remains as the gold standard to define prognosis and fibrosis stage.
In Brazil, the treatment of hepatitis C and B, as well as other liver diseases such as autoimmune hepatitis, primary biliary cirrhosis and Wilson’s disease, is provided by the public healthcare system. However, in the majority of cases, implementation of treatment depends on a liver biopsy. Since the public healthcare system still lacks hospital beds, even in day-hospitals, patients with liver disease in many Brazilian states have to wait several months in line to undergo this procedure within a public Hospital. Up to mid-2007, the largest referral center in the state of Bahia, Brazil, performed an average of four liver biopsies per month. In the 2005, 284 new cases of hepatitis C were registered at this center in order to be evaluated for treatment.11 Therefore, many patients for whom treatment was indicated remained untreated despite the availability of good outpatient care and access to medication. This phenomenon is also observed in many other etiologies of liver diseases.
To solve this dilemma, a collective effort was organized in 2007 to perform liver biopsies in Bahia. An agreement between the Ministry of Health, the Bahia State Health Department and the University Hospital of the Federal University of Bahia permitted the number of liver biopsies to be increased to thirty per month, thus enabling more than 500 patients to undergo biopsy within an 18-month period. This project met the demand of liver disease referral centers within the city of Salvador, Bahia, Brazil.
MethodsA descriptive study was performed involving a series of cases in which specimens were obtained during a collective effort program. The study sample was composed of all the patients who underwent a liver biopsy procedure within the collective effort liver biopsy program conducted in Bahia between July 2007 and November 2009.
Data referring to the gender and age of the patients and the reason for performing the biopsy were collected from the patients’ charts. The biopsy results, including information on the degree of fibrosis and on iron overload, were obtained from the biopsy reports issued by our referral center for liver pathology (FIOCRUZ, Bahia).
Statistical analysis included calculating the mean age and the gender distribution of all patients. Next, the primary diseases that were the reason for performing the procedure were grouped into six predominant categories.
The biopsies were performed by the Percutaneous technique, not guided by ultrasonography, although all patients were submitted to an ultrasonographic evaluation within the 90 day-period preceding the biopsy. The reason for this protocol was to rule out liver masses, gallbladder anatomic variants and ascite.
The Patients with clinical and laboratorial diagnosis of Cirrhosis or with Ultrasonography and/or Endoscopy compatible with portal hypertention were not submitted to liver biopsy. According to Brazilian statements, these patients can be treated without a histopathologic diagnosis.
Student’s t-test was used to compare mean ages, while the chi-square test was used for the dichoto- mous variables.
All the analyses were carried out using the SPSS statistical software program, version 15. Significance was defined as p-values < 0.05 (p < 0.05).
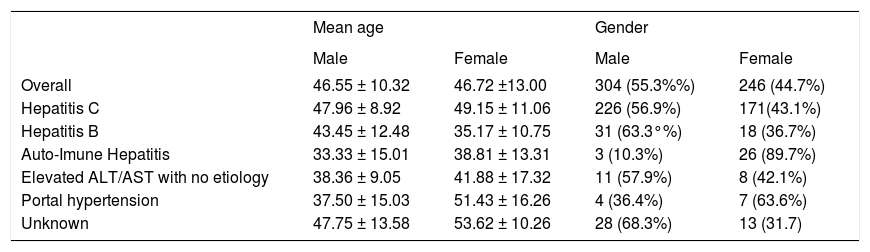
ResultsOf the 550 patients evaluated, 304 (55.3%) were men and 246 (44.7%) women. Mean age of patients was 46.63 ± 11.59 years. There was no significant difference in mean age between males and females, but it was different when stratified in function of the etiology of liver disease that motivated the indication of biopsy (Table 1).
Demographic characteristics of patients.
| Mean age | Gender | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Overall | 46.55 ± 10.32 | 46.72 ±13.00 | 304 (55.3%%) | 246 (44.7%) |
| Hepatitis C | 47.96 ± 8.92 | 49.15 ± 11.06 | 226 (56.9%) | 171(43.1%) |
| Hepatitis B | 43.45 ± 12.48 | 35.17 ± 10.75 | 31 (63.3°%) | 18 (36.7%) |
| Auto-Imune Hepatitis | 33.33 ± 15.01 | 38.81 ± 13.31 | 3 (10.3%) | 26 (89.7%) |
| Elevated ALT/AST with no etiology | 38.36 ± 9.05 | 41.88 ± 17.32 | 11 (57.9%) | 8 (42.1%) |
| Portal hypertension | 37.50 ± 15.03 | 51.43 ± 16.26 | 4 (36.4%) | 7 (63.6%) |
| Unknown | 47.75 ± 13.58 | 53.62 ± 10.26 | 28 (68.3%) | 13 (31.7) |
With respect to the frequency of the etiologies of liver diseases in the patients submitted to biopsy, 396 (72%) had hepatitis C, 49 (8.9%) had hepatitis B, 29 (5.3%) had autoimmune hepatitis, 19 (3.5%) had elevated ALT/AST levels of undefined cause and 11 (2%) had portal hypertension of undefined cause, while in 46 cases (8.3%) the reason for biopsy was related to other causes and in 5 cases or the cause was unknown (Figure 1).
When the patients with hepatitis C were analyzed separately, it was found that 226 patients (56.9%) were men and 171 (43.2%) women. Mean overall age of these patients was 48.49 ± 10.1 years, with a mean age of 47.96 ± 9.92 years in the case of the men and 49.15 ± 11.06 years for the women. There was a statistically significant difference when the mean age of these patients was compared with the mean age of the patients with other etiologies (41.74 ± 13.69 years), particularly those with hepatitis B for whom mean age was 40.41 ± 12.43 years (Figure 2).
When the patients were separated into four quar- tiles according to age-group, 70.7% of the patients with hepatitis C were between 41 and 60 years of age. In contrast, in the patients with other etiologies of liver disease, only 43.8% of them were in this same age-group (Figure 3).
Among the patients with hepatitis C, 170 (42.9%) had F2 fibrosis, making this the most common grade of fibrosis. In this same group, 107 patients (27.0%) had advanced fibrosis (METAVIR F3 or F4). The majority of the patients with advanced fibrosis was male, 65 men (60.7%) compared to 42 women (39.3%) (p = 0.4).
On the other hand, the difference between the mean age of patients with advanced fibrosis (51.43 ± 8.45 years) and that of the patients with mild/moderate fibrosis (47.34 ± 10.18 years) was statistically significant (p = 0.001) (Figure 4).
Of all the 550 patients, 85 (15.5%) had some degree of iron overload. These patients consisted predominantly of men (n = 71; 83.5%). Curiously, 61 (70.6%) of the patients with iron overload had a diagnosis of hepatitis C; however, there was no difference in the prevalence of iron overload between the group of patients with hepatitis C (n = 61; 15.4%) and those with other etiologies (n = 24; 15.7%). With respect to the degree of iron overload, 27 (45.8%) had grade 2. There was no statistically significant difference in age-group between the patients with hepatitis C and iron overload (49.89 ± 8.31 years) and those with hepatitis C and no iron overload (48.24 ± 10.38 years).
Of the patients with hepatitis C and iron overload, 52 (85.24%) were male with a mean age of 48.88 ± 8.3 years compared to a mean age of 55.67 ± 5.67 years for the women (p = 0.023) (Figure 5). In this group, there was an association between gender and iron overload with a likelihood ratio of 26.30 (p = 0.01). Likewise, in the group of patients as a whole, the correlation between gender and iron overload resulted in a likelihood ratio of 35.65 (p = 0.001).
With respect to the safety of the procedure, only two patients (0.36%) suffered major complications that required surgical management. These consisted of one case of hemorrhage and one case of biliary peritonitis. No deaths were recorded.
DiscussionSince this collective effort was a pioneering project, there are no similar series in the literature with which to make a direct comparison of the present results. Nevertheless, the present sample most probably reflects the population seen in hepatology referral centers around the country with respect to the predominant liver diseases, gender and age.
The majority of the patients with an indication for liver biopsy were male. This finding may be justified by the fact that men are more likely to be exposed to factors that are related to a higher risk of hepatitis C and to a more severe disease course. In addition these patients usually have more probability of co-morbidities such as: alcohol consumption and Metabolic Syndrome.
In Brazil, specifically in the northeastern region, illegal intravenous drug use is not a common cause of hepatitis C virus transmission. In this region, the main cause is the use of intravenous stimulants and vitamin complexes, a common practice among young people in the 70s and 80s, principally among young males who practiced sports.12 This may be the reason why the majority of hepatitis C carriers are male and older than 40. Then, the mean age of hepatitis C patients in the present study is consistent with the period during which this risk factor prevailed.
With respect to the frequency of the different pathologies, the results of the present study are in agreement with data published in the literature. There was a vast predominance of patients with hepatitis C among these patients submitted to biopsy. As discussed previously, this finding reflects the situation in hepatology referral centers all over this country, where hepatitis C is the principal reason for liver biopsies.
The mean age of the subgroup of patients with a diagnosis of hepatitis C was similar to that found in a study in which 4996 patients from all over Brazil were evaluated.13 On the other hand, it was slightly higher than the mean age of 43.03 years found in Spain14 and lower than that of 54 years found in Chile.15 Although the Spanish studies concluded that the prevalence of hepatitis C in the 45-65 year age-group was lower than that found in either the younger or older age-groups,16 in the present study the opposite was true. In agreement with the findings of another study carried out in Brazil, the great majority of patients with hepatitis C were in the 41-60 year age-group.13 Once again, these data are coherent with the principal risk factor in this area. Contamination among males in the 1970s and 1980s resulted from the use of over-the-counter medication (vitamin complexes and stimulants) early in life by individuals of 15-25 years of age who were initiating sports activities.
Another important finding was that patients with hepatitis C were significantly older than those with other etiologies, particularly the patients with hepatitis B. The slow rate of progression of this disease may, perhaps, justify this finding.17 As a result of the slow rate of progression and the few symptoms, the patient tends to seek medical care only at a later stage, which may justify the more advanced age of the patients. On the other hand, a similar finding would be expected in the patients with hepatitis B; however, these patients are younger than those with hepatitis C. The epidemiological idiosyncrasies of the population in this study may hold an explanation for this finding.
The majority of the patients submitted to biopsy already have a moderate degree of fibrosis at the time of the procedure. The natural disease course, in which the majority of patients have no fibrosis or only mild fibrosis, corroborates with the hypothesis that the patients evaluated here were diagnosed later than would be ideal.17 Probably for this reason, the patients with more advanced fibrosis were older than those with less advanced fibrosis.
The natural history of hepatitis C appears kaleidoscopic.18 Fibrosis is known to progress gradually and in a non-linear manner.19 Progression to cirrhosis depends on factors in the host and on external co-factors; therefore, various studies have been conducted to attempt to identify predictors of a faster rate of liver fibrosis progression, however, the time of the disease is indisputably important. Unfortunately, it was impossible to evaluate co-factors of fi- brogenesis such as the use of medication or alcohol or the metabolic syndrome, among others, in this study. On the other hand, it is highly probable that these patients became infected at 15-25 years of age, hence at an early age. This characteristic would lead us to expect more patients with less advanced stages of fibrosis. Therefore, the present findings strongly suggest that co-morbidities such as metabolic syndrome or alcohol use may be present in these patients.
The effect of the patient’s age at the time of infection is well known. Being over 50 years of age at the time of infection and being male are factors associated with a greater rate of fibrosis progression.6,20,21 An analysis involving 2,235 patients compared those who became infected prior to 20 years of age with those who became infected after the age of 50, and found that these latter patients had an additional three points in their fibrosis rate.21 The age at which the patient became infected is a major factor involved in accelerating the rate of fibrosis progression.19 The explanation is in the patient’s immune response as well as in the increase in fibrogenesis and the reduction in fibrolysis. In addition, the liver of older patients is more susceptible to lesions and less capable of regeneration.3
In an attempt to annul the effect of other possible confounding variables, a study was conducted in 2004 using a complex simulation model.22 The results of this study corroborated with previous data, confirming that the rate of progression to advanced stages of fibrosis was faster, both, in patients over fifty years of age, the longer time of infection and in male gender. Moreover, as a result of this analysis, which differed from analyses carried out previously, the authors concluded that age > 50 years at the time of infection had been overvalued as a predictor of fibrosis and that, in part, this aspect was due to other characteristics simultaneously present in older patients. A similar fact was found with respect to gender, albeit to a lesser degree.
In another series of more severely ill patients, the prevalence of advanced fibrosis was 38.9%, confirming the existence of an inverse association between better immunological status and the rate of fibrosis progression.23
These findings enable the results of the present study to be evaluated with greater precision. The prevalence of advanced fibrosis (METAVIR F3 or F4) in the hepatitis C patients was notably higher than the prevalence found in another series studied in France with a similar number of patients. In the present population, 27.7% of the patients had advanced fibrosis compared to 19% in the French series.24 This result may be justified by the fact that the patients in the French sample were approximately seven years younger than those in the present study.
The excessive number of patients with iron overload unrelated to the HFe mutation in this region of the world has often been observed by referral centers in Brazil.
In hepatitis C, there is speculation that iron may be a co-factor in liver damage; therefore, iron overload may act as a co-morbid condition in a variety of forms. It has been proposed that this effect could be explained by an increase in the hydroxyl radicals that would lead to progressive fibrosis.25 The mechanisms that provoke this overload remain unknown, even when various factors known to trigger iron overload are excluded.26
In view of the possible importance of iron overload, the prevalence of this co-morbidity was evaluated in the present population. This is a controversial issue. Some investigators consider this event to be rare,26 while others have reported a frequency similar to that found in the present study.10 In this study, the interesting finding is that both the prevalence and the degree of iron overload were practically the same in the groups of patients with hepatitis C and in those without this etiology.
When the data were stratified according to gender, results were similar to those found in the literature.10 The majority of patients with hepatitis C and iron overload were male. The predominance of males among patients with iron overload has also been reported in other studies.26,27 Alcohol consumption may explain this finding; nevertheless, this variable was not evaluated in the present study. The iron overload seen in hepatitis C may be associated with a poorer prognosis of the disease, affecting its rate of progression and possibly leading to treatment failure.11 Patients with hepatitis C have lower serum levels of hepcidin, a protein synthesized in the liver and the principal regulator of cellular iron export, which would appear to be the most probable cause of iron overload in this liver disease.28 It has been suggested that the reduction in hepcidin levels results from the effect of the virus on the production of hepcidin by the liver; however, it is also possible that this deficiency occurs as a result of the inflammatory process caused by the disease itself.29 Age was not related to iron overload. This is in agreement with results reported by other investigators.10
With respect to safety, although some authors have stated that ultrasound-guided biopsy is safer,30 the low complication rate in the present series give strength to the hypothesis that, when performed by experienced professionals, liver biopsy does not have to be ultrasound-guided. This finding is important when the objective is to offer low cost medical care that is accessible to the entire population.
ConclusionThe results of the present study show that:
- •
Hepatitis C is the most frequent indication for liver biopsy in outpatient referral centers in Northeastern Brazil.
- •
The presence of advanced fibrosis and iron overload was found in similar proportions to those found by other authors.
- •
The complication rate with non-ultrasonography guided biopsy was low.
In addition, the study confirms the feasibility of a pilot project involving three major components of the public healthcare system in this state as a model for other states in Brazil and for other countries with similar characteristics.