Background and aim. The lack of information about hepatocellular carcinoma (HCC) in Brazil weakens health policy in preventing deaths from the illness. The aim of this study was to establish the cumulative incidence and the risk factors for hepatocellular carcinoma development in patients under a surveillance program.
Material and methods. 884 patients with compensated cirrhosis were prospectively followed up for at least five years, from August 1998 until August 2008, with at least one annual ultrasonography liver examination and serum alpha fetoprotein (AFP) measurement.
Results. Among 884 patients, 72 (8.1%) developed a tumor with a median follow up of 21.4 months. In the hepatocellular carcinoma group, hepatitis C virus infection was the major etiological factor (65.3%), 56.9% (41/72) were male and the mean average age was 57 ± 10 years. The annual incidence of hepatocellular carcinoma was 2.9%. 79.2% (57/72) of HCCs were detected within Milan Criteria, and the mean survival time was 52.3 months, significantly higher than for those outside Milan, with a mean time of 40.6 months (p = 0.0003).
Conclusion. The annual incidence of HCC among this large series of Brazilian cirrhotic patients was around 2.9% with a detection rate of 8.1%, or a cumulative incidence rate over five years of 14.3%. The three variables related to HCC risk were low serum albumin [HR: 0.518 (0.46-0.78)], high AFP > 20 ng/mL [HR: 3.16 (1.86-5.38)], and ethnicity (Brazilian-East Asian descendants vs. other mixed Brazilian ethnicities) [HR: 2.86 (1.48-5.53)].
The incidence of hepatocellular carcinoma (HCC) is rising throughout the world, ranging from 2 to 3% of cirrhosis in Western up to 6 to 11% in East Asian countries.1 Men are affected three times as often as women.2
The major risk factors for HCC include chronic infections with the hepatitis B (HBV) or C (HCV) virus. However, alcohol abuse, non-alcoholic steatohepatitis (NASH) and other causes of cirrhosis can be considered other risk factors for HCC.2 Indeed, 70-90% of HCCs develop in cirrhotic patients.3,4 In addition, the risk of HCC in patients with cirrhosis depends on the activity and duration of the inflammatory process underlying the cirrhotic process. The leading cause of HCC varies according to environmental factors.
However, to date, the incidence of HCC and major risk factors in Brazil are unknown. We conducted this prospective study with the aim of determining the incidence and risk factors for HCC in a large cohort of Brazilian patients with cirrhosis, with long-term follow up.
Material and MethodsPatientsThe current study was performed with outpatients from the University of São Paulo School of Medicine, the largest referral center for hepatology in Brazil, from January 1998 to August 2008.
Study designWe initially enrolled 1,375 cirrhotic patients recruited during 5 years, classified as follows according to the etiology:
- •
Hepatitis C, defined by anti-HCV (+) and HCV-RNA (+).
- •
Hepatitis B, defined by HBsAg (+) and HBV-DNA (+).
- •
Alcohol abuse, by an intake of more than 60 g of ethanol/day (females) or more than 80 g of ethanol/day (males), for more than 10 years.
- •
NASH, by the presence of metabolic syndrome, diagnosed as reported elsewhere,5 or by biopsy and miscellaneous (other factors, including cryptogenic cirrhosis and viral co-infection).
To ensure that patients involved in this study were initially free of tumor, we excluded 491 patients whose last ultrasonography (US) was more than 6 months prior to inclusion. Whenever clinically indicated, histological diagnosis from 14 Gauge needle biopsy was adopted. The presence of underlying cirrhosis was assessed histologically in 312/884 patients (35.3%) or by clinically significant portal hypertension in 572/884 patients (64.7%).
Thus, our study sample was composed of a cohort of 884 patients aged 17-84 years with compensated cirrhosis who were tumor, ascites and jaundice free at time zero.
The liver ultrasonography exams were performed by the same operator (DCPV).
Follow up and tumor stagingPatients were followed up for at least 60 months or until death up to August 2008 for HCC tumor development with US examinations and serum AFP levels. The patients were followed up by ultrasonography exams alone and AFP levels were checked at entry and at time of diagnosis. When we took patient adherence issues into account, most of our sample (74.7%) received their US scans within 12 months. A sub analysis was also performed of the 660 patients in this subset, while the other 224 patients were unable to complete regular surveillance.
We registered a minimum of four checkpoints for each patient:
- •
Pretest time (1,375 patients enrolled).
- •
Time zero or Baseline (those who fulfilled the inclusion criteria).
- •
Diagnosis time (period of development of the HCC), and
- •
Final time (final US, or censored data (which occurs if a patient withdraws from a study, i.e. is lost from the sample before the final outcome is observed), or time of mortality in relation to the time zero checkpoint).
It is relevant to note that many patients were also evaluated in between checkpoints 2 and 3, checkpoints 3 and 4, or checkpoints 2 and 4; since these examinations did not correspond to a checkpoint within the study, they were computed but not considered.
Any presumed HCC lesion detected by US at time of diagnosis was confirmed by at least one of the following diagnostic criteria: positive histology, or two imaging techniques compatible with HCC (computed tomography and magnetic resonance with arterial enhancement and washout), or one of the previously mentioned imaging results (any technique) suggestive of HCC plus serum AFP levels > 400 ng/mL.6
Incidence and risk factorsAdditional data recorded included: gender, age, ethnic origin, etiology of cirrhosis, size and number of nodules, presence of macroscopic tumorous thrombosis, time of follow up, interval between US exams, and blood laboratory analysis. Laboratory analysis included: platelet count, albumin, bilirubin, creatinine, glucose, international normalized ratio (INR) and serum AFP. Laboratory data were obtained at time zero and at time of diagnosis from patient medical records. We also calculated the mathematical model for end-stage liver disease (MELD) score for all the patients.7 Detected tumors were also classified as within or outside the Milan Criteria.8
The cumulative annual incidence included all 884 patients (812 HCC-free patients and 72 HCC patients). However, some patients were censored at the time of liver transplantation (OLT) (1.4%), developed other tumors (0.7%), died due to causes unrelated to liver cirrhosis (4.6%) or were lost before follow up (22.2%).
Statistical analysisPearson χ2, Fisher exact, Mann-Whitney, and Student-t tests were used to evaluate differences in demographic, clinical, and etiologic features of the patient groups and tumor characteristics.
Survival time was calculated using the Kaplan-Meier method and survival curves were compared using the log-rank test. The survival times of HCC patients were analyzed starting from the time of enrollment.
The association between different variables and the development of HCC over time was assessed using univariate Cox analysis. Multivariate analysis with a backward stepwise method and Cox proportional hazards regression analysis were performed with variables that attained a p value < 0.1 in a univariate analysis. In order to avoid the common problems of over fitting and collinearity, several different models were created with variables that were statistically significant in a univariate analysis (p < 0.1) or were clinically relevant. The modeling strategy used in this study was based on a reduction in the likelihood ratio (- 2LL) of the different models developed and the number of variables in each model. The lower the value of - 2LL, the more explanatory power the model has over variability in the data. A p value of 0.05 or less was considered statistically significant.
Statistical analyses were made using the “R” program and its extensions and SPSS 18, Copyright IBM Corporation 2010, IBM Corporation, Route 100, Somers, NY 10589, U.S.A.
Ethical considerationsThis study was approved by the Institutional Ethics Review Board and patients signed an informed consent form of their own free will.
ResultsStudy populationAs shown in table 1, the majority of the 884 compensated cirrhotic patients included in this study were men, with a mean age of 55. Between 1998 and 2008, 72 (8.1%) patients developed HCC (HCC patients) with a median of diagnosis time of 21.4 months (a minimum of 6 months and maximum of 59.0 months). Thirty-six of these patients were still alive at the end of the study.
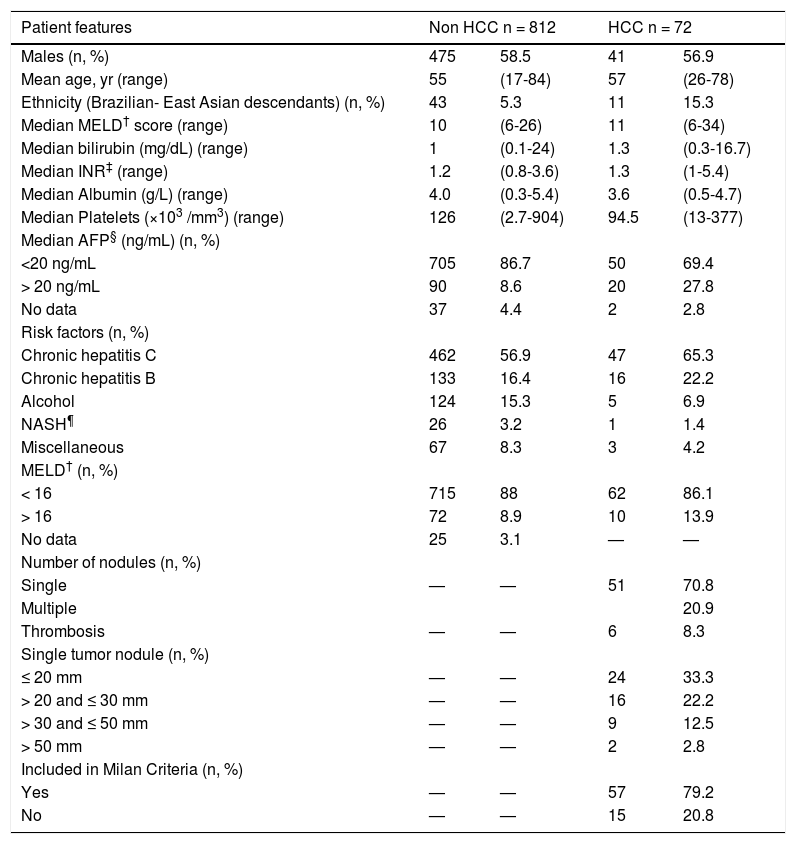
Epidemiologic and clinical features of a) 812 HCC-free patients at time zero for HCC surveillance and b) 72 patients at the time of diagnosis of HCC (HCC patients).
| Patient features | Non HCC n = 812 | HCC n = 72 | ||
|---|---|---|---|---|
| Males (n, %) | 475 | 58.5 | 41 | 56.9 |
| Mean age, yr (range) | 55 | (17-84) | 57 | (26-78) |
| Ethnicity (Brazilian- East Asian descendants) (n, %) | 43 | 5.3 | 11 | 15.3 |
| Median MELD† score (range) | 10 | (6-26) | 11 | (6-34) |
| Median bilirubin (mg/dL) (range) | 1 | (0.1-24) | 1.3 | (0.3-16.7) |
| Median INR‡ (range) | 1.2 | (0.8-3.6) | 1.3 | (1-5.4) |
| Median Albumin (g/L) (range) | 4.0 | (0.3-5.4) | 3.6 | (0.5-4.7) |
| Median Platelets (×103 /mm3) (range) | 126 | (2.7-904) | 94.5 | (13-377) |
| Median AFP§ (ng/mL) (n, %) | ||||
| <20 ng/mL | 705 | 86.7 | 50 | 69.4 |
| > 20 ng/mL | 90 | 8.6 | 20 | 27.8 |
| No data | 37 | 4.4 | 2 | 2.8 |
| Risk factors (n, %) | ||||
| Chronic hepatitis C | 462 | 56.9 | 47 | 65.3 |
| Chronic hepatitis B | 133 | 16.4 | 16 | 22.2 |
| Alcohol | 124 | 15.3 | 5 | 6.9 |
| NASH¶ | 26 | 3.2 | 1 | 1.4 |
| Miscellaneous | 67 | 8.3 | 3 | 4.2 |
| MELD† (n, %) | ||||
| < 16 | 715 | 88 | 62 | 86.1 |
| > 16 | 72 | 8.9 | 10 | 13.9 |
| No data | 25 | 3.1 | — | — |
| Number of nodules (n, %) | ||||
| Single | — | — | 51 | 70.8 |
| Multiple | 20.9 | |||
| Thrombosis | — | — | 6 | 8.3 |
| Single tumor nodule (n, %) | ||||
| ≤ 20 mm | — | — | 24 | 33.3 |
| > 20 and ≤ 30 mm | — | — | 16 | 22.2 |
| > 30 and ≤ 50 mm | — | — | 9 | 12.5 |
| > 50 mm | — | — | 2 | 2.8 |
| Included in Milan Criteria (n, %) | ||||
| Yes | — | — | 57 | 79.2 |
| No | — | — | 15 | 20.8 |
Most patients, 79.2% (57/72), were classified within the Milan Criteria and 70.8% (51/72) displayed single nodules, 33.3% (24/72) of which were less than 20 mm in diameter (Table 1). The median size of all tumors was 21 mm (8-92 mm, SD 8.45 mm). HCC diagnosis was confirmed by histology in 33.3% of the cases, the use of two imaging methods in 59.7%, and by imaging plus serum AFP levels in 7%. HCV was the major etiological factor of HCC, in 65.3% of patients, as well as cirrhosis in 56.9% of patients at baseline. In Brazil, the cut-off MELD score to list a patient for liver transplantation is 16. Thus, at time zero, 82 patients (9.3%) had MELD scores > 16, whereas in the HCC group, 10 (13.9%) had MELD scores > 16 (p = 0.327).
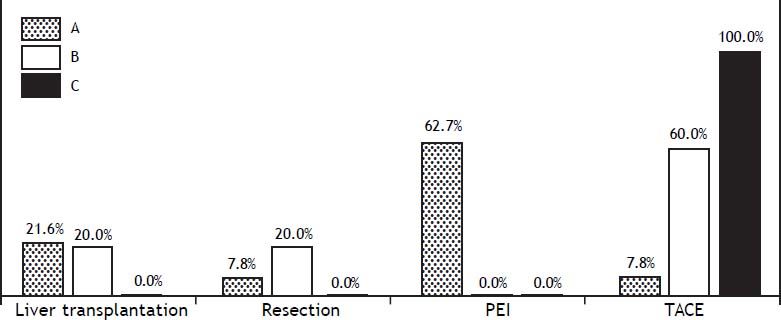
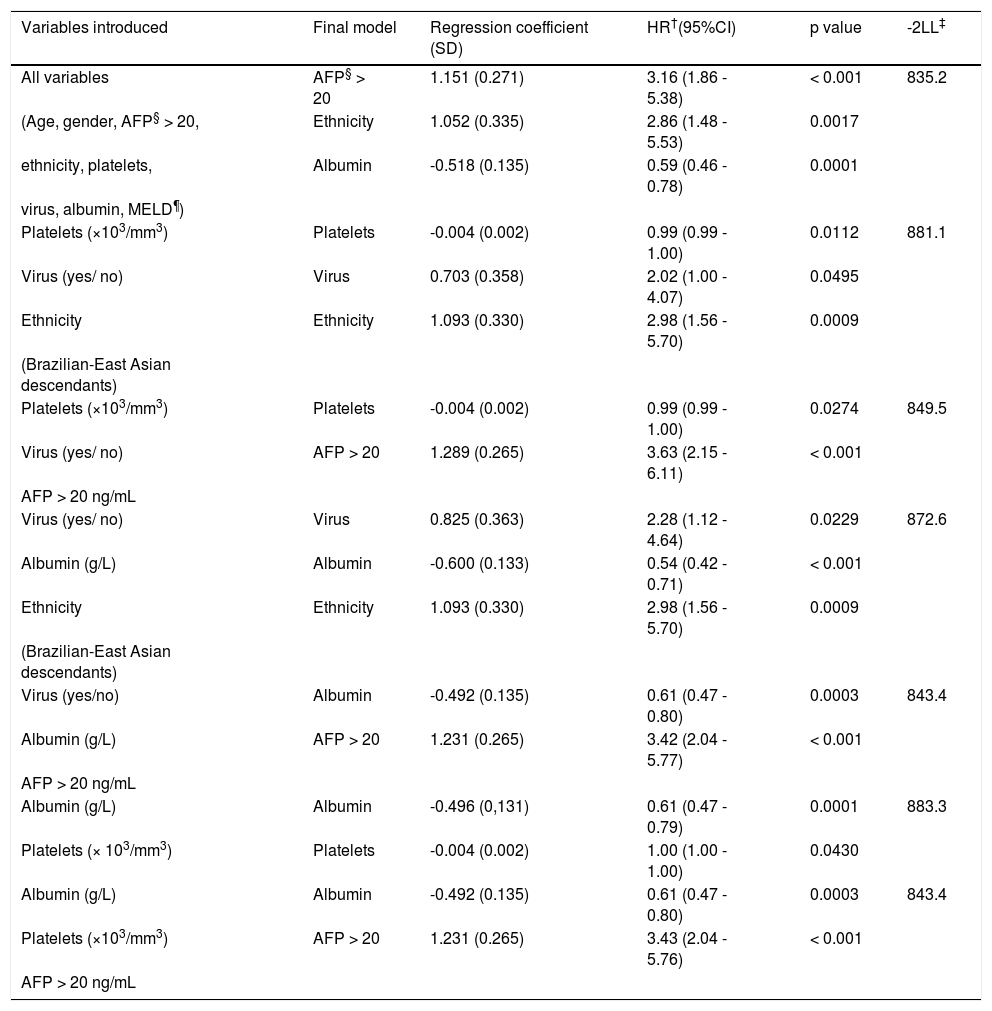
Barcelona Clinic Liver Cancer staging (BCLC) was adopted in the HCC patients and was distributed as follows: BCLC-A (79.2%), BCLC-B (12.5%) and BCLC-C (8.3%). The treatments performed were (Figure 1):
- •
16.6%: liver transplantation (LT).
- •
7.0%: resection.
- •
44.5%: percutaneous ethanol injection (PEI).
- •
12.5%: transcatheter arterial chemoembolization (TACE); and
- •
19.4%: palliative care.
Approximately 10% of HCC-free patients and 27.8% of HCC patients had initial AFP levels above 20 ng/mL (reference value for increased risk of HCC development) (Table 1).
One hundred and ninety-seven out of the 884 (22.2%) were lost before follow up. The mean MELD value of these patients was MELD 11.4 at entry.
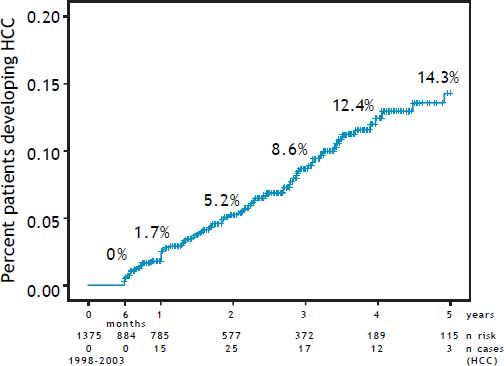
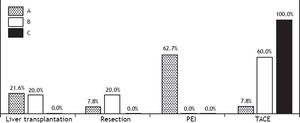
Incidence and risk factorsThe annual incidence of HCC was 2.9%. The cumulative incidence increased from 1.7% at year one to 5.9% at year two, 8.6% at year three, 12.4% at year four and 14.3% at year five (Figure 2). HCV patients presented the highest overall incidence rate with 16.9%, followed closely by HCB group with 15.3%. The smallest rate was found in the NASH with 4 % and ethanol group with 5.7% of HCC over five years and finally, the miscellaneous etiology group with 7.7% in the same period. 79.2% (57/72) of HCCs were detected within Milan Criteria, and the mean survival time was 52.3 months, significantly higher than for those outside Milan, with a mean time of 40.6 months (p = 0.0003).
Cumulative annual incidence rates of the HCC patients under surveillance. A. Pre-test time = 1375 cirrhotic patients initially recruited (1998-2003) at the University of São Paulo School of Medicine. B. Time zero = 884 cirrhotic patients who were selected after 6 months for being hepatocellular carcinoma, ascites and jaundice free. The annual calculated cumulative incidence is plotted in the graph. The follow up time was from 1998 till 2008 and was always calculated with the numerator the n cases HCC in the time n divided by the patients at risk in the time n-1.
Among the adherence group, 55/660 (8.3%) developed HCC over the five year period, and the cumulative incidence rate was 13.9%, of this group 80% fell within the Milan criteria and 20% outside. The overall mean survival curve of HCC patients over five years within the Milan Criteria was 52,6 compared to 38,5 months outside Milan Criteria, p < 0.001.
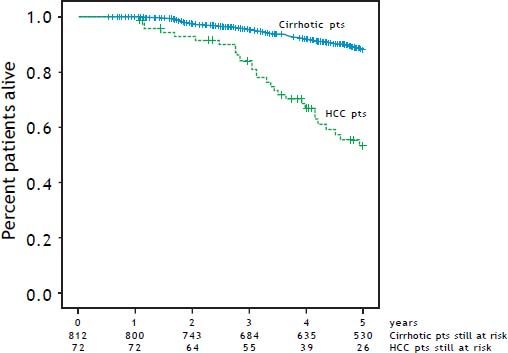
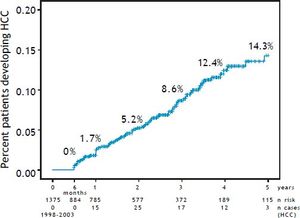
Figure 3 compares the cumulative survival rates of HCC-free and HCC patients (patients that will have HCC) under the present prospective surveillance program (p < 0.001). The 5-year survival rate of HCC-free patients was 80%, whereas the 5-year survival rate of HCC patients was 53.2%.
In a univariate analysis, 6 of 8 variables showed statistical significance (p < 0.05): serum albumin, platelet count, MELD score, ethnicity (mixed Brazilian ethnicity or Brazilian-East Asian descendants), viral etiology (yes/no), and serum AFP (> 20 ng/mL). Age and gender were considered control variables. The factors related to higher annual incidence of HCC were: East Asian ethnicity, serum albumin level and serum AFP dosage > 20 ng/mL, which remained independent predictors of the development of HCC during follow up in a multivariate analysis. This model had the lowest likelihood ratio (-2LL) with the smallest number of variables (Table 2).
Risk factors related to a higher incidence of HCC found in this cohort of patients: seven modeling strategies.
| Variables introduced | Final model | Regression coefficient (SD) | HR†(95%CI) | p value | -2LL‡ |
|---|---|---|---|---|---|
| All variables | AFP§ > 20 | 1.151 (0.271) | 3.16 (1.86 - 5.38) | < 0.001 | 835.2 |
| (Age, gender, AFP§ > 20, | Ethnicity | 1.052 (0.335) | 2.86 (1.48 - 5.53) | 0.0017 | |
| ethnicity, platelets, | Albumin | -0.518 (0.135) | 0.59 (0.46 - 0.78) | 0.0001 | |
| virus, albumin, MELD¶) | |||||
| Platelets (×103/mm3) | Platelets | -0.004 (0.002) | 0.99 (0.99 - 1.00) | 0.0112 | 881.1 |
| Virus (yes/ no) | Virus | 0.703 (0.358) | 2.02 (1.00 - 4.07) | 0.0495 | |
| Ethnicity | Ethnicity | 1.093 (0.330) | 2.98 (1.56 - 5.70) | 0.0009 | |
| (Brazilian-East Asian descendants) | |||||
| Platelets (×103/mm3) | Platelets | -0.004 (0.002) | 0.99 (0.99 - 1.00) | 0.0274 | 849.5 |
| Virus (yes/ no) | AFP > 20 | 1.289 (0.265) | 3.63 (2.15 - 6.11) | < 0.001 | |
| AFP > 20 ng/mL | |||||
| Virus (yes/ no) | Virus | 0.825 (0.363) | 2.28 (1.12 - 4.64) | 0.0229 | 872.6 |
| Albumin (g/L) | Albumin | -0.600 (0.133) | 0.54 (0.42 - 0.71) | < 0.001 | |
| Ethnicity | Ethnicity | 1.093 (0.330) | 2.98 (1.56 - 5.70) | 0.0009 | |
| (Brazilian-East Asian descendants) | |||||
| Virus (yes/no) | Albumin | -0.492 (0.135) | 0.61 (0.47 - 0.80) | 0.0003 | 843.4 |
| Albumin (g/L) | AFP > 20 | 1.231 (0.265) | 3.42 (2.04 - 5.77) | < 0.001 | |
| AFP > 20 ng/mL | |||||
| Albumin (g/L) | Albumin | -0.496 (0,131) | 0.61 (0.47 - 0.79) | 0.0001 | 883.3 |
| Platelets (× 103/mm3) | Platelets | -0.004 (0.002) | 1.00 (1.00 - 1.00) | 0.0430 | |
| Albumin (g/L) | Albumin | -0.492 (0.135) | 0.61 (0.47 - 0.80) | 0.0003 | 843.4 |
| Platelets (×103/mm3) | AFP > 20 | 1.231 (0.265) | 3.43 (2.04 - 5.76) | < 0.001 | |
| AFP > 20 ng/mL |
To investigate the incidence of HCC in cirrhotic patients and the risk factors associated with it in Brazil; we prospectively evaluated a cohort of 884 cirrhotic patients using US, AFP levels, and clinical parameters in a follow up lasting 5-10 years.
Examination of the incidence of HCC in cirrhotic patients revealed remarkable gender and age variations in Brazil. In our cohort, from the largest Brazilian public referral center receiving patients from all over the country, men were more likely to be affected than women, a finding similar to that reported in studies performed in the USA, Japan, and Italy.3 Likewise, HCV was the main etiology of the cirrhosis and HCC. This observation is supported by Carrilho, et al.4 who reported HCV as the most common etiology of liver cirrhosis and HCC development in Brazil. Prior to this, Brazil was considered to have a low prevalence of HCC and HBV was thought to be the most common cause of liver disease.9
Studying an Italian cohort, Sangiovanni, et al.10 identified the mean age of diagnosis of HCC patients as 61 years. In a study in Taiwan, age at diagnosis varied between 50 and 79 years.11 In the United States more than 60% of all HCC patients are 65 or older.12 In a recent Japanese nationwide survey, the mean age of incidence of HCC was 66.6 years.13 In our cohort the mean age of a patient with cirrhosis was 55 and HCC development 57 years, suggesting a younger age of the onset of cirrhosis and HCC development in the Brazilian population. The earlier emergence of cirrhosis and HCC observed in Brazil may be due to restricted access to appropriate antivirals for treating HCV-positive patients in comparison with the treatments employed in more developed countries. Another explanation could relate to the prevalence and early acquisition time of the major HCC risk factors (HCV, HBV, and alcohol induced liver disease) in Brazil in comparison to developed countries, where they tend to be much later.14 In any case, studies have suggested an increase in the incidence rates of HCC in all age groups among all populations. It has further been suggested that carcinogenic factors may have affected various generations of birth cohorts early in life and led to increased HCC risk.15
Our results show that the annual incidence of HCC of the population in Brazil is 2.9% (Figure 2). A similar rising trend and incidence rate (3%) was reported by Sangiovanni, et al.10 while studying a Milanese cohort. Indeed, our sample showed the same proportions of etiology (HCV, HBV, alcohol) as Sangiovanni’s work. HCC incidence in the United States is also rising: in the period 1981-1985 it was 9.6 per 100,000 whereas by 1991-1995 the incidence was 15.2 per 100,000 (16). In Japan, HCC has reached almost 40/ 100,000 population with 80% caused by HCV infection.17
We found that approximately 50% of HCC patients survived for 5 years, a higher number than suggested by Sangiovanni, et al.10 This could be due to differences and more recent advances in the therapeutic strategies employed with these patients. In addition, the Milan criteria analysis showed a significant difference in relation to survival at 60 months, i.e. 52.3 compared to 40.6 months (p = 0.0003). Although we could not perform a rigid surveillance program in all patients, we have shown herein that, in real life conditions, maintaining the patients under screening even under sub-optimal conditions was also very helpful, still yielding rather early detection. On the other hand, clinical outcomes are still poor when compared with other tumor types, the morbidity and mortality rate of patients with HCC is rising.18
The detection rate of small HCC tumors nowadays is comparable with some Japanese centers,17 since we detected tumor nodules with a mean size of 26 mm in 92% of our HCC group. Indeed, we performed research in 2005 (unpublished) on patients outside the surveillance programme. Only 47.3% of the HCC patients were classified as within Milan criteria. Also, during three different periods, the statistics are as follows: from 1988-1993 only 31,7% of HCC were detected within Milan criteria; from 1993-1998 this ratio increased to 61.8% and finally from 1998-2005, with the maturity of the surveillance program among the cirrhotic patients of the Gastroenterology Department, the best numbers were achieved, with 85.7% of HCC patients within Milan criteria.
We observed that 8.3% of patients developed tumors in the advanced stages, as suggested by the presence of thrombus. We think that these rates will occur in a population even when patients are submitted to frequent and effective surveillance programs. Among our research group, Kikuchi, et al.19 reported 3 cases with vascular invasion in 74 nodules smaller than 30 mm.
The detection rate of HCC was 8.1% (72/884). In a multivariate analysis, 3 variables were related to HCC risk including low serum albumin, high AFP > 20 ng/mL, and ethnicity (Brazilian-East Asian descendants versus other mixed Brazilian ethnicities). According to the model strategy adopted, from a total of seven models, five of them presented serum albumin as a common variable and predictor of HCC development. This result is in agreement with Ripoll, et al.,20 who reported that low albumin levels are indicative of the severity of liver disease, poor liver function and a greater risk of developing HCC. We calculated an annual incidence of HCC among a large series of Brazilian cirrhotic patients of around 2.9%/year with frequency of 8.1%. Therefore, AFP measurement, while not yet recommended by the European guidelines,21 remains in common clinical practice for surveillance of patients at risk of HCC. Singal, et al.22 explored the benefit of adding AFP measurement with US for the detection of early HCC in a metaanalysis. The same group published more recently that patterns of AFP over time are independently associated with HCC.23 Nonetheless, as was mentioned, in our cohort only 7% and 55% of the patients with HCC had AFP > 400 and > 20 ng/mL, respectively, at the time of diagnosis. Thus, AFP levels alone rather than as a factor in a dynamic evaluation may, in fact, be a weak tool for HCC diagnosis. However, it may also be very helpful in selecting patients at a higher risk of HCC development or with poor prognoses, because we found higher AFP levels in patients outside the Milan Criteria (albeit without significant differences between the groups). Further studies on the relevance of using AFP in risk surveillance for HCC development and prognosis are warranted. Finally, the ethnicity variable caught our attention, because 50.0% (8/16) of patients with HBV infection and HCC were Brazilian-East Asian descendants with a family history of HCC.
The present study, a pioneer in Latin American populations, showed that the mean annual incidence of HCC among this large series of Brazilian cirrhotic patients is around 2.9%/year with a frequency of 8.1%. The factors related to a higher annual incidence of HCC were: East Asian ethnicity, serum albumin level, and serum AFP dosage > 20 ng/mL, which remained independent predictors of the development of HCC during the follow-up in a multivariate analysis. Also, 79.2% of the HCC patients were detected within the Milan Criteria, and the mean survival curve was 52.3 compared to 40.6 months for those outside the Milan Criteria.
Abbreviations- •
2LL: likelihood ratio.
- •
BCLC: Barcelona clinic liver cancer.
- •
HBV: hepatitis B.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C.
- •
HR: hazard ratio.
- •
INR: international normalized ratio.
- •
MELD: model for end-stage liver disease.
- •
NASH: non-alcoholic steatohepatitis.
- •
OLT: liver transplantation.
- •
TACE: transcatheter arterial chemoembolization.
- •
US: ultrasonography.
We are deeply indebted to Dr. Mauro Tomoyuki Taniguchi for offering data from the Sao Paulo Municipality obituary service, undergraduate student Larissa Aragão for collecting histology data, Aline Siqueira Ferreira for revising the manuscript and the Alves de Queiroz Family Fund for Research for its continuing sponsoring support.
Grant SupportThe study was supported in part by Alves de Queiroz Family Fund for Research.