Background and aim. The effect of preoperative transcatheter arterial chemoembolization (TACE) on the short- and long-term outcome of resectable hepatocellular carcinoma (HCC) is controversial. We conducted a retrospective evaluation of this aspect using data from our center.
Material and methods. A total of 656 consecutive patients who underwent curative resection of HCC were divided into two groups based on the preoperative TACE: the liver resection (LR) group (405 cases) and the TACE-LR group (183 cases). Overall and disease-free survival curves were constructed using the Kaplan-Meier method and compared with the log-rank test. The significance of differences in survival was tested using a log-rank test. Univariate and multivariate analyses were used to identify the factors that best predicted overall survival or tumorfree survival.
Results. Although the cost of LR showed no difference between groups, the overall cost was significantly higher in the combined group than in the LR group (P < 0.001). The complication rate after resection was also comparable between the two groups. In regard to long-term outcome, the 1-, 3-, and 5-year overall survival rates were 83.7, 68.9 and 57.5%, respectively, after direct liver resection and 80.9, 65.0 and 54.1%, respectively, after combined TACE and resection (P = 0.739). The 1-, 3-, and 5-year recurrence-free survival rates were also comparable between two groups (P = 0.205). Both univariate analysis and multivariate analysis showed that macro-vascular invasion was the factor that best predicted overall survival or tumor-free survival rate.
Conclusion. Preoperative TACE has comparable intraoperative and short-term outcomes but more overall cost due to repeated TACE, and the procedure did not significantly improve the overall or tumor-free survival rate. Preoperative TACE should not, therefore, be recommended as a routine procedure before resection for resectable HCCs particularly in cases due to underlying hepatitis B virus (HBV).
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor worldwide and the third leading cause of cancer-related death.1 These facts are especially important in China, a country that has a high prevalence of HBV infection. Liver transplantation, resection, and radiofrequency ablation (RFA) are three potential curative therapies for early stage HCC.2 However, the shortage of deceased donors3 and potential risk for living donors, including death,4 has limited the extensive use of liver transplantation for the treatment of HCC. Furthermore, the effectiveness of using RFA on small HCCs (< 3 cm) has also limited the use of transplantation in HCC cases.5 Thus, liver resection is the first choice for most HCC cases. However, due to the high rate of tumor recurrence after hepatic resection, the 5-year overall survival rate after liver resection is reported to be 40-50%,6 and post-operative survival of HCC patients remains unsatisfactory. Many preoperative methods such as transcatheter arterial chemoembolization (TACE), transcatheter arterial chemoinfusion (TACI), Radiofrequency abalation (RFA), and High Intensity Focused Ultrasound (HIFU), among others,7 have been introduced to improve the prognosis of liver resection for HCC. TACE was introduced during the late 1970s as a palliative treatment for patients with unresectable HCC.8 In Japan, TACE was introduced and performed extensively not only for palliation but also as a preoperative adjuvant, even for patients with resectable hepatocellular carcinoma (HCC), with the goal that this procedure may improve the survival rate.9 HCCs frequently invade the portal vein, leading to intrahepatic metastasis, and preoperative therapy may prevent intrahepatic recurrence due to portal vein invasion of the HCC tumor. However, from a surgical viewpoint, TACE before liver resection has both potential disadvantages and benefits. The effectiveness of preoperative TACE for improving postoperative long-term survival in cases of HCC remains controversial: some researchers believe that it may reduce the viability of HCC cells before surgery and reduce postoperative tumor recurrence.10,11 Many studies have failed to demonstrate a positive effect of preoperative TACE.12,13 However, some studies reported that preoperative TACE negatively affected the survival of patients after hepatic resection.14,15 Many of these studies were conducted many years ago, during the development of TACE and surgical techniques, and there are only a few recent reports on this subject. Therefore, the therapeutic benefits of preoperative TACE in patients with resectable HCC remain uncertain. In the present study, we evaluated the outcomes of TACE followed by liver resection (TACE-LR) compared to LR alone using longitudinal data from our center.
Material and MethodsBetween June 2005 and August 2008, a series of 656 consecutive patients underwent a curative resection of HCC at West China Hospital of Sichuan University. The preoperative diagnosis of HCC was based on the history of HBV or HCV infection, enhanced imaging techniques (CT or MRI) and alphafetoprotein (AFP) level. Histological criteria were used to confirm HCC diagnoses after resection; no preoperative biopsies were performed in our study due to the consideration of bleeding and needle track implantation metastases. The baseline characteristics (age, gender, BMI, underlying disease, Child score, MELD score, serum creatinine), tumor characteristics (tumor number, diameter of targets, AFP level, tumor differentiation), intra-operative data (surgical procedure, operative time, blood loss, blood transfusion rate, inflow occlusion), post-operative recovery (hospital stay days, cost for the LR, overall cost, complication), tumor recurrence and patients’ survival of these two groups were collected and compared. The primary endpoints of this study were overall survival rate and tumor free survival rate, and the secondary endpoints including operating time, blood loss, intraoperative blood transfusion, and inflow occlusion (either whole or hemi-), the overall hospital stay days, cost of LR, postoperative complications, and mortality.
The inclusion criteria in our study were as follows:
- •
Age from 18 to 70 years old.
- •
Liver function with Child score A or B.
- •
Without extra-hepatic metastasis.
- •
All targets able to be R0 resected, and
- •
All HCCs confirmed by histological examination.
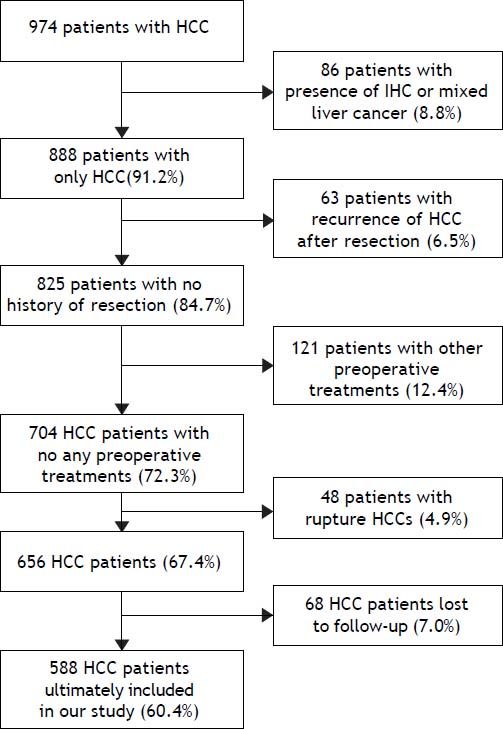
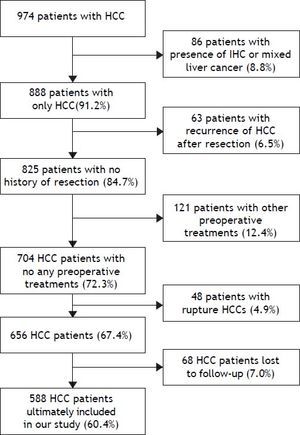
On the basis of inclusion and exclusion criteria, 588 patients (60.4%) were enrolled in this retrospective study (Figure 1). At last, 386 cases were excluded from our present study, and a total of 588 cases were ultimately included in our study. These cases were divided into two groups: a liver resection (LR) group (405 cases) and a TACE-LR group (183 cases) according to whether preoperative TACE was performed.
All of these 588 cases received routine and regular follow-up at our outpatient department. Patients were monitored for recurrence based on serum AFP levels each month through the first year and every three months thereafter; ultrasonography was performed once every two months in the first year and every three months thereafter, and abdominal enhanced imaging including CT or MRI was conducted when a possible recurrent lesion was found or when a continuously increasing AFP level was observed.
Preoperative TACE protocolPreoperative TACE is performed for four reasons: detecting of latent intrahepatic metastatic foci (32 cases), trying to down-stage tumors (57 cases), providing sufficient time of therapy for damaged liver function (76 cases), be willing to improve overall survival and disease-free survival rates after curative resection (18 cases). Routine TACE was performed by experienced physicians. We routinely perform TACE through the femoral artery under local anesthesia, after the insertion of a catheter through the femoral artery. Hepatic arteriography was performed to collect information on the tumor number, type, location, size and arterial supply. The tip of the catheter was directed toward tumor-feeding arteries (left or right branches) for superselective embolization of all tumors detected by digital subtraction angiography. Then, an emulsified suspension consisting of cisplatin, mitomycin, epirubicin and lipiodol was injected into tumor vessels, and this was followed by embolization with a gelatin sponge.16 The injection was continued until stasis was confirmed in the feeding artery. Contraindications for the TACE procedure were severe renal dysfunction, complete obstruction of the trunk of the portal vein due to tumor thrombus and uncontrolled ascites.
Statistical analysisFor comparison between the clinicopathological features and preoperative treatments, all values were expressed as means and associated standard deviations. Continuous variables were compared using T-test, and nominal variables were compared using χ2 test. Overall and disease-free survival curves were obtained using the Kaplan-Meier method. The significance of differences in survival was tested using a log-rank test. The prognostic significance of various clinicopathological factors with respect to overall and tumor-free survival rates was investigated by univariate and multivariate analyses. P-values < 0.05 were regarded as statistically significant. Data analysis was performed using SPSS software (Version 17.0) for Windows XP.
ResultsTaceThe TACE surgery group received TACE 1.6 ± 0.5 times prior to resection. Preoperative TACE was performed once in 72 patients, twice in 62 patients, three times in 35 patients, and 4-6 times in 14 patients, the indicator to accept another TACE was the obvious presence of arterial blood supply in the imaging scan. The mean waiting time from the first TACE treatment to resection was 135 days, and the time from the last TACE treatment to resection was 26 days. The main reasons for preoperative TACE included confirmation of the diagnosis if the imaging scan (CT or MRI) were not clear (n = 52, 28.4%), down-staging of the tumors if the tumor burden were too risk for radical therapies (n = 68, 37.1%) and palliative control of HCC when resection, LT or RFA was temporarily impossible due to the destoryed liver function (n = 63, 34.4%).
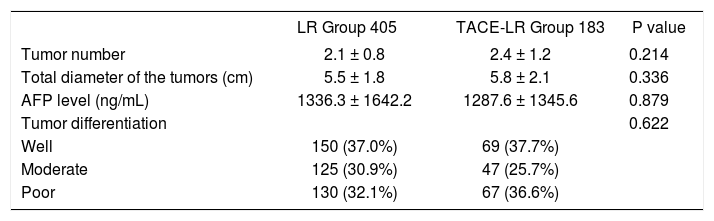
Baseline characteristics of the patients and tumorsThe clinicopathological characteristics of patients with or without preoperative TACE are shown in table 1. The mean age is similar between the two groups, and the difference in the gender distribution between the two groups was not statistically significant. The most common cause of HCC was HBV infection (84.7%). Although the liver function, including MELD score and Child score, was worse in the TACE-LR group, this difference was not significant (P > 0.05). However, for the TACE-LR group, the pre-TACE liver function (MELD score and Child score) was much worse than in the cases of patients who accepted liver resection for HCC (P < 0.05). The time for resection from the diagnosis of HCC is 4.6 ± 2.7 months in the TACE-LR group, and 0.4 ± 0.1 months in the LR group (P < 0.001). There was no difference between the two groups for the patients’ BMI, preoperative renal function (serum creatinine), and other characteristics. In regard to tumor characteristics (Table 2), although the tumor number in the TACE-LR group was more than that in the LR group and the diameter of the tumors in the TACE-LR group was larger than that in the LR group, these differences were not significantly different (all P > 0.05). The pre-LR AFP levels in the two groups were similar, and the tumor differentiation confirmed by the pathological examination in the two groups was also not significantly different (P = 0.622).
Baseline demographics for the two groups.
| LR Group 405 | TACE-LR Group 183 | P value | |
|---|---|---|---|
| Age | 53.2 ± 6.3 | 53.8 ± 7.1 | 0.722 |
| Gender (M:F) | 338:67 | 160:23:00 | 0.216 |
| BMI (kg/m2) | 23.2 ± 1.9 | 23.2 ± 2.1 | 0.914 |
| Underlying liver disease | 0.530 | ||
| HBV | 345 (85.2%) | 153 (83.6%) | |
| HCV | 22 (5.4%) | 8 (4.4%) | |
| HBV and HCV | 3 (0.7°%) | 2 (1.1%) | |
| Alcoholic cirrhosis | 15 (3.7%) | 4 (2.2%) | |
| No underlying liver disease | 20 (4.9%) | 16 (8.7%) | |
| MELD score (Pre-TACE) | 8.0 ± 3.6 | 8.8 ± 4.2/12.5 ± 5.3 | 0.113/ 0.014 |
| Child score (Pre-TACE) | 0.167 / 0.008 | ||
| A (5-6) | 278 (68.6%) | 115 (62.8%)/73 (39.9%) | |
| B (7-9) | 127 (31.4%) | 68 (37.2%)/67 (36.6%) | |
| C (≥ 10) | 0 | 0 /43 (23.5%) | |
| Serum creatinine (μmol/L) | 74.3 ± 23.8 | 68.2 ± 25.5 | 0.376 |
Tumor characteristics of two patient groups.
| LR Group 405 | TACE-LR Group 183 | P value | |
|---|---|---|---|
| Tumor number | 2.1 ± 0.8 | 2.4 ± 1.2 | 0.214 |
| Total diameter of the tumors (cm) | 5.5 ± 1.8 | 5.8 ± 2.1 | 0.336 |
| AFP level (ng/mL) | 1336.3 ± 1642.2 | 1287.6 ± 1345.6 | 0.879 |
| Tumor differentiation | 0.622 | ||
| Well | 150 (37.0%) | 69 (37.7%) | |
| Moderate | 125 (30.9%) | 47 (25.7%) | |
| Poor | 130 (32.1%) | 67 (36.6%) |
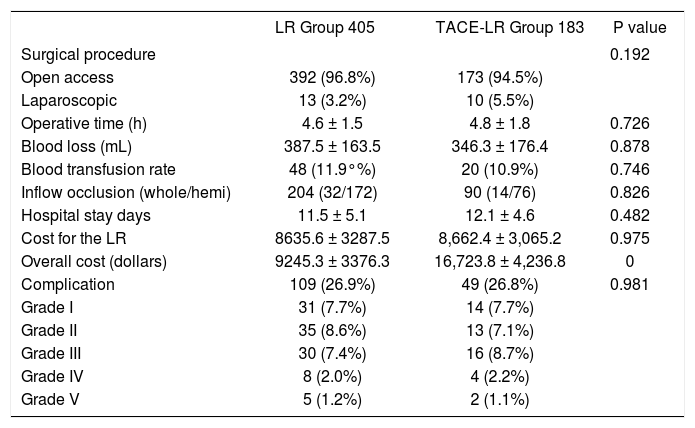
Table 3 shows the surgical procedures and immediate results of the two groups. A majority of the patients (96.1%) in the two groups accepted liver resection with an open access procedure, and no difference was observed between two groups (P = 0.192). Other intraoperative parameters, including operating time, blood loss, intraoperative blood transfusion, and inflow occlusion (either whole or hemi-), did not differ significantly between the two groups. The postoperative recovery data analysis, including the overall hospital stay days and cost of LR, also showed no significant differences between the two groups. However, the overall cost, including the LR cost, TACE cost, and the cost in the out-patient department in the TACELR group, was significantly higher than that in the LR group (P < 0.001). The postoperative complications for all patients in our study were classified using the Clavien system. The overall complication rate was 26.9% in the LR group, which was comparable with the 26.8% rate observed in the TACE-LR group (P = 0.981). The major complication (grade III-V) rate in the LR group was 10.6 and 12.0% in the TACE-LR group, which was not significant. The mortality (grade V) was 1.2% in the LR group and 1.1% in the TACE-LR group. No mortality was observed in the TACE-LR group after TACE but before resection.
Intraoperative data and postoperative recovery comparison between the two groups.
| LR Group 405 | TACE-LR Group 183 | P value | |
|---|---|---|---|
| Surgical procedure | 0.192 | ||
| Open access | 392 (96.8%) | 173 (94.5%) | |
| Laparoscopic | 13 (3.2%) | 10 (5.5%) | |
| Operative time (h) | 4.6 ± 1.5 | 4.8 ± 1.8 | 0.726 |
| Blood loss (mL) | 387.5 ± 163.5 | 346.3 ± 176.4 | 0.878 |
| Blood transfusion rate | 48 (11.9°%) | 20 (10.9%) | 0.746 |
| Inflow occlusion (whole/hemi) | 204 (32/172) | 90 (14/76) | 0.826 |
| Hospital stay days | 11.5 ± 5.1 | 12.1 ± 4.6 | 0.482 |
| Cost for the LR | 8635.6 ± 3287.5 | 8,662.4 ± 3,065.2 | 0.975 |
| Overall cost (dollars) | 9245.3 ± 3376.3 | 16,723.8 ± 4,236.8 | 0 |
| Complication | 109 (26.9%) | 49 (26.8%) | 0.981 |
| Grade I | 31 (7.7%) | 14 (7.7%) | |
| Grade II | 35 (8.6%) | 13 (7.1%) | |
| Grade III | 30 (7.4%) | 16 (8.7%) | |
| Grade IV | 8 (2.0%) | 4 (2.2%) | |
| Grade V | 5 (1.2%) | 2 (1.1%) |
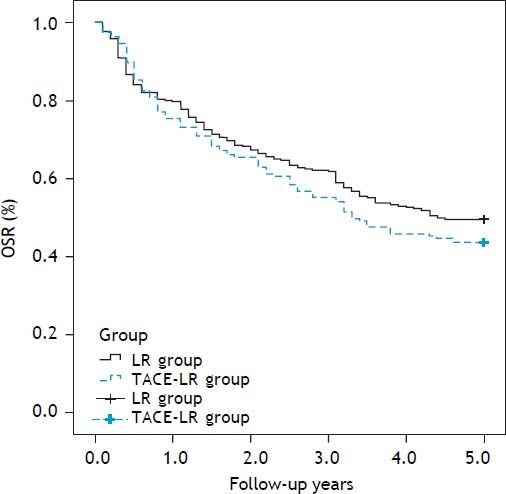
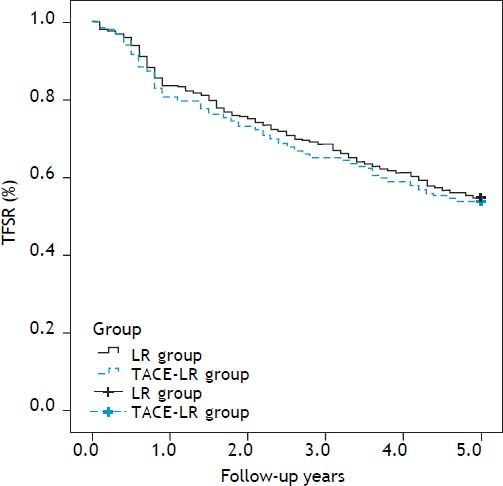
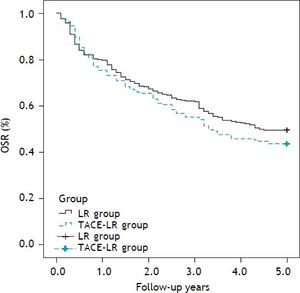
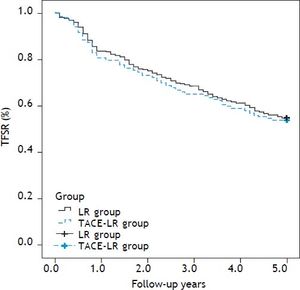
Overall and recurrence-free survival rates during a follow-up period of at least 5 years after resection were compared between two groups. The 1-, 3-, and 5-year overall survival rates were 83.7, 68.9 and 57.5%, respectively, after direct liver resection and 80.9, 65.0 and 54.1%, respectively, after combined TACE and resection. No difference in the survival curve was observed between the two groups (Figure 2) (P = 0.739). The 1-, 3-, and 5-year recurrence-free survival rates were 79.5, 61.7, and 49.6%, respectively, in the direct LR group and 76.0, 55.7 and 43.7%, respectively, in the combined group. No significant differences were observed between the two groups (Figure 3) (P = 0.205). The most common site for tumor recurrence or metastasis was the liver (79.6%), followed by intra-abdominal lymph node (32.8), lung (24.5%), bone (10.2%), and other locations (4.7%). In one month after resection, the most common cause of death was complication from surgery (7 cases, 77.7%). However, in one year after resection, tumor progression was the primary overall cause of death in 192 cases (73.7%): 132 cases (79.0%) in the LR group and 60 cases (61.9%) in the TACE-LR group. Liver failure related to liver cirrhosis was the second most common cause of death, and this ratio did not change during the one-year follow-up after liver resection.
The overall survival rate (OSR) comparison after resection between two groups: The 1-, 3-, and 5-year overall survival rates were 83.7, 68.9 and 57.5%, respectively, after direct liver resection and 80.9, 65.0 and 54.1%, respectively, after combined TACE and resection group, no significantly difference was observed between two groups (P = 0.739). LR: liver resection. TACE-LR: combined transcatheter arterial chemoembolization and liver resection.
Tumor free survival rate (TFSR) comparison after resection between two groups: The 1-, 3-, and 5-year recurrence-free survival rates were 79.5, 61.7, and 49.6%, respectively, in the direct LR group and 76.0, 55.7 and 43.7%, respectively, in the combined group, there was also no significantly difference between two groups (P = 0.205). LR: liver resection. TACE-LR: combined transcatheter arterial chemoembolization and liver resection.
The various clinicopathological factors included in the univariate and multivariate analyses in our study included gender, age (< 50; > 50), AFP level (< 400 ng/mL; > 400 ng/mL), tumor size (< 5 cm; > 5 cm), macro-vascular invasion, micro-vascular invasion, tumor differentiation grade, surgical procedure (open access vs. laparoscopic), blood loss (< 400 mL; > 400 mL), blood transfusion (yes or no), inflow occlusion (yes or no), liver cirrhosis (yes or no), Child score (Child grade A or grade B) and preoperative TACE. Univariate analysis revealed that a preoperative serum AFP level of more than 400 ng/mL, poor tumor differentiation, macro-vascular invasion and blood loss > 400 mL were the three most important predictive factors for overall survival rate. Multivariate analysis showed that an AFP level > 400 ng/mL and macro-vascular invasion may both influence overall survival after resection. However, preoperative TACE was not shown to influence overall survival rate after liver resection. For tumor-free survival rate, univariate analysis showed that tumor size > 5 cm, pre-operative TACE, and macro-vascular invasion were the three most important predictive factors for overall survival rate. However, the multivariate analysis of these three factors showed that only macro-vascular invasion may adversely influence tumor-free survival.
DiscussionThe theory behind TACE is based on the principle that primary HCC is supplied almost exclusively (90%) by the hepatic arteries. The obstruction of the feeding arteries can induce tumor ischemic necrosis. A combination of TACE with chemotherapy can drastically increase the local concentration of the chemotherapeutic agent and may improve the benefit of therapy.17 Preoperative TACE is performed for four reasons:8,18 the first is to improve the detection of latent intrahepatic metastatic foci, the second is to increase the resectability of HCCs by down-staging tumors that are either initially borderline resectable or unresectable, the third is to provide sufficient time of therapy in cases of damaged liver function, and the fourth is to improve overall survival and disease-free survival rates after curative resection. In the present study, we evaluated the efficiency of preoperative TACE on the short- and long-term outcomes using data from a large cohort of patients.
Several retrospective studies have shown the efficacy of preoperative TACE9.18,19 The main theory for using preoperative TACE was that HCC tumors frequently invade the portal vein (30-50% in patients with an HCC of 3-5 cm in diameter), leading to intrahepatic metastasis, which is the most common cause of postoperative recurrence in the liver.6,20 Intratumoral necrosis was found to weaken the adhesive potential of the tumor, subsequently facilitating the release of cancer cells from the primary tumor and their entry into the bloodstream.21 Controlling microsatellite lesions is also very important for improving postoperative survival. Some RCTs have also shown that TACE significantly improved overall survival compared with inactive treatment in patients with unresectable HCC;22 thus, this therapy was recommended as a standard intervention for patients with unresectable large/mutifocal HCC who do not have vascular invasion or extrahepatic metastasis.17 However, there are some researchers who do not support the use of preoperative TACE in the management of patients with resectable HCC.23 There are several reasons for why TACE is unsuitable for reducing the risk of postoperative tumor recurrence or for producing a better survival rate,17 but the main reason is that TACE mainly affects well-differentiated HCC without completely killing poorly differentiated cells,24 which harbor a high grade of malignancy and readily spread within the portal venous system; furthermore, hematogeneous and lymphatic dissemination of cancer cells can precede TACE treatment.25
Several studies have failed to show a positive effect of preoperative TACE.15,26 Meanwhile, other studies have also shown that there are disadvantages of preoperative TACE. In Paye’s report,17 operative time was significantly longer in the TACE group as compared to the no-TACE group, and this longer time was related to more difficult dissections in the TACE group due to inflammatory pedicles, perhepatic adhesions, or arterial thrombosis. However, in the present study, the mean operative time was comparable between the two groups, we found no significant difference in the postoperative complications between two groups (26.9 vs. 26.8%), and the overall complication rate was comparable with other reports.17 However, other studies have reported that preoperative TACE may decrease liver function and even lead to liver failure.27,28 Hepatic function impairment induced by TACE could be repaired easily in a non-cirrhotic liver, but hepatic function may gradually and progressively deteriorate due to TACE in some cirrhotic patients.14 For the 183 patients who received preoperative TACE in our study, liver function did not worsen due to the treatment, and no patient failed to accept LR due to poor liver function caused by the preoperative TACE. Sixty-three cases accepted TACE because temporary loss of liver function made it impossible to recover and accept liver resection. We also compared the intraoperative data including operative time, blood loss, transfusion rate, and inflow occlusion (whole/hemi-), and we found no difference between the two groups. Furthermore, the cost for liver resection was not significantly different between the two groups, but the overall cost was significantly higher in the combined group. The main cause of this additional expense was the repetitive TACE, but the preoperative TACE did not add cost to the liver resection. In an RCT study by Zhou, et al., it was shown that five patients lost the chance for a potentially curative liver resection because of progression of disease with metastases (4 cases) or liver failure (1 case) during the time interval between the last TACE and hepatic resection.29 However, when resection, LT or RFA is not possible, many more patients benefit from preoperative TACE upon confirmation of diagnosis, resulting in down-staging of the tumors and palliative control of HCC. Additionally, two randomized controlled studies of preoperative TACE for HCC resection30,31 showed that pre-LR TACE did not improve actual or disease-free survival after resection. In fact, a lower overall survival rate in the TACE group was observed in Wu, et al.’s report. The 1-, 3-, and 5-year overall survival rates in our study were 83.7, 68.9 and 57.5%, respectively, after direct liver resection and 80.9, 65.0 and 54.1%, respectively, after combined TACE and resection. The 1-, 3-, and 5-year recurrence-free survival rates were 79.5, 61.7, and 49.6%, respectively, in the direct LR group and 76.0, 55.7 and 43.7%, respectively in the combined group. No significant differences were observed between the two groups in either overall survival rate or tumorfree survival rate. Both univariate analysis and multivariate analysis showed that macro-vascular invasion was the most important factor for predicting overall survival or tumor-free survival rate. Additionally, our results are consistent with those of previous reports.12,30 The inclusion criteria for the radical therapy of HCC are not consistent such as the Milan criteria and the BCLC criteria.17,32–33 More works should be made to prove the effectivenes of these criteria in furture.
Our study has several limitations. First, although there were 583 patients in the study, all of the data were collected retrospectively from only one center in Sichuan providence in China. Additional, multicenter RCTs should be performed for a more thorough analysis. Second, most of the HCCs were caused by HBV infection; because Japanese and Western countries have high rates of HCV infection or alcohol use, further studies including those patients should be performed. So, larger sample size, the involvement of multiple centers and randomized trials should be considered in our future work.
Preoperative TACE does not improve long-term outcomes, including overall and tumor-free survival. When considering the overall costs, preoperative TACE should be avoided for patients with resectable HCC, particularly in cases of HBV-related HCC.
FundingNo funding.
Ethical ApprovalNot needed.
ContributorsWang Wentao proposed the study. Lei Jianyong and Zhong Jinjing performed research and wrote the first draft. Lei Jianyong and Zhong Jinjing collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. Wang WT is the guarantor. Lei Jianyong and Zhong Jinjing contribute equally to this study and should be the Co-first author.
Competing InterestLei Jianyong and Zhong Jinjing contribute equally to this study and should be the Co-first author. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.