Background and rationale. Fatty liver (FL) and abdominal visceral fat (AVF) are strongly associated with systemic inflammation, however, it has not been defined if each one is independently involved, and if the insulin resistance is associated. To investigate if FL, AVF and insulin resistance are independently or additively associated with the high-sensitivity C-reactive protein (hs-CRP) in subjects without coronary artery disease we included 491 men and 553 women.
Material and methods. All had anthropometric and plasma biochemical measurements, FL and AVF assessments by computed tomography.
Results. The FL prevalence was 35.6% in men and 28.0% in women, p < 0.01. The prevalence of obesity, metabolic syndrome and homeostasis model assessment of insulin resistance (HOMA-IR) was significantly higher in FL compared to non FL subjects. FL and AVF accounted for 21 and 17%, respectively, to hs-CRP plasma levels. FL, AVF ≥ P75 and HOMA-IR ≥ P75 were independently and additively associated with plasma hs-CRP. The risk of hs-CRP ≥ 3 mg/L increased progressively in men from 1.36 (0.5-3.86) through 3.58 (1.32-9.7) in those with 1 or 3 factors respectively. In women from 2.25 (1.2-4.2) to 4.67 (2.3-9.4), respectively. In conclusion, both the FL and hs-CRP ≥ 3 mg/L occur in 1 of every 3 non CAD subjects. In men, FL and AVF ≥ P75 were associated with 3.6 times the risk of hs-CRP ≥ 3 mg/L, while in women, these factors were independently and additively associated with a 4.7 times higher risk of systemic inflammation.
Fatty liver (FL) disease is the most common hepatic condition in adult population. Depending on the ethnic group, it affects from 24 to 42% of the overall population and from 70 to 90% of obese subjects.1 It is characterized by the hepatic triglyceride (TG) infiltration in individuals with less than 20 g of alcohol consumption per day and without other causes of liver disease.2 Several studies have shown that FL is associated with cardiometabolic risk factors.3–5
A recent study showed that FL identified by computed tomography was associated independently of visceral fat, with diabetes, hypertension, impaired fasting glucose, metabolic syndrome, high TG levels, as well as low concentrations of serum high-density lipoprotein cholesterol (HDLC) and adiponectin.6 In another study that used the hyperinsulinemic-euglycemic clamp, increased insulin resistance in liver, adipose tissue and muscles was observed in obese subjects with FL compared to obese subjects without fatty liver.7 In addition, recent studies have shown association of FL with coronary artery disease (CAD) even after adjusting for traditional risk factors.8,9 Altogether, these studies suggest that increased intrahepatic fat contributes to the pathogenesis of metabolic abnormalities and CAD. Nevertheless, it is unclear how the FL is associated with the development of CAD. One possible mechanism is the chronic inflammation state manifested by increased serum markers of systemic inflammation, among which C-reactive protein is one of the most widely studied for having a clear association with atherosclerosis.10
Several reports have shown the association of FL with C-reactive protein,11–14 however, the possible involvement of abdominal visceral fat (AVF) and insulin resistance, two pro-inflammatory factors closely related to FL, has not been considered in those studies. The main objective of this study was to investigate whether fatty liver is associated with the C-reactive protein concentration, independently of AVF, and insulin resistance, in men and women. Furthermore, we investigated whether the coexistence of increased AVF and insulin resistance increases that association.
Material and MethodsThe Genetics of Atherosclerotic Disease Study (GEA) was designed at the National Institute of Cardiology Ignacio Chávez, Mexico, to examine the genetic basis of CAD and to investigate the relationship between traditional and emerging risk factors with atherosclerosis in the adult Mexican mestizo population. The GEA study included 1,500 patients with premature CAD and a control group of 1,600 individuals with no personal or family history of premature CAD. The present study included 1,044 individuals of the control group, aged 30 to 75 years, without diabetes mellitus, alcohol consumption less than 20 g/day, no acute or chronic inflammatory processes, and no history or clinical evidence of renal failure or liver disease.2 Participants were recruited from blood bank-donors, and through brochures posted in social service centers. The GEA study was approved by the Ethics committee of the National Institute of Cardiology Ignacio Chávez and conducted according to the Declaration of Helsinki. All participants signed an informed consent form.
SubjectsQuestionnaires were applied to participants to obtain information regarding demographics, personal history of cardiovascular risk factors, physical activity, alcohol consumption, and medications. Weight was measured in kilograms (kg) and height was measured in centimeters (cm), using a calibrated scale and a wall-mounted height rod. Body mass index (BMI) was calculated using the weight (kg)/ height (m2) formula. Waist circumference was measured with a fiberglass tape, at a midpoint between the lower margin of the last rib and the iliac crest with a participant breathing out gently. After at least 5 min of rest, systolic and diastolic blood pressures were measured three times in a sitting position. The average of the last two consecutive measurements was used for the analysis.
SamplesAfter 10 h of fasting and 20 minutes in a sitting position, venous blood samples were collected. Glucose, total cholesterol, TG and HDL-C concentrations were determined in plasma with enzymatic methods (Roche Diagnostics GmbH, Mannheim, Germany) using a Hitachi 902 auto analyzer (Hitachi LTD, Tokyo, Japan).15 Low-density lipoprotein cholesterol (LDL-C) was calculated based on the Friedewald’s equation.16 The reproducibility and precision of lipids and lipoproteins measurements were periodically assessed by the Lipid Standardization Program of the Center for Disease Control and Prevention (LSP-CDC, Atlanta, GA. USA). The intra and inter-assay coefficients of variation were less than 3%. The serum insulin concentration was determined by radioimmunoassay (Human insulin RIA kit; Millipore, Cat. HI-14K St. Charles, Missouri, USA). The intra and inter-assay coefficients of variation were 2.1% and 6.8%, respectively. Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR) (Insulin IU/mL X Glucose mmol/22.5).17 High-sensitivity C-reactive protein (hs-CRP) concentration was determined by immunonephelometry (Cardiophase hsCRP, SIEMENS Healthcare Diagnostics Products GmbH, Marburg, Germany) on a BN ProSpec nephelometer according to the manufacturer’s procedures. Inter and intra-assay coefficients of variation were less than 6%.
TomographyComputed axial tomography (CAT) is a validated method to quantify AVF and abdominal subcutaneous fat (ASF) and to identify the presence of steato-sis.18–20 In this study, these measurements were performed using a 64-slice CAT scanner (Somaton Sensation, 64, Forcheim, Germany). Liver attenuation in CAT was calculated as the average of five measurements made in regions of interest of 1.0 cm2 in both lobes. Spleen attenuation measured in several regions of interest was used as an internal control for the standardization of hepatic attenuation. The presence of hepatic steatosis was defined as the liver/spleen attenuation ratio less than 1.0 Hounsfield units.21 To measure abdominal fat, a single topographic slice at the level of L4-L5 inter-vertebral disc space was performed. AVF and ASF areas were separated by manual tracing along the abdominal muscle wall. Total abdominal fat and AVF were quantified in cm3 and the ASF was calculated by subtracting the AVF area from total abdominal fat.15,16
Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg and/or use of antihypertensive medications. Overweight was defined as a BMI from 25.0 to 29.9 kg/m2 and obesity as a BMI ≥ 30 kg/m2. The presence of abdominal obesity was considered when waist circumference values were ≥ 80 cm in women and ≥ 90 cm in men.25 Fasting blood glucose from 100 to 125 mg/dL was considered impaired fasting glucose, whereas values ≥ 126 mg/dL or treatment with hypoglycemic agents were used to determine the presence of diabetes mellitus. Metabolic syndrome was defined based on the presence of 3 or more of the following factors:23
- •
Central obesity.
- •
Triglycerides ≥ 150 mg/dL.
- •
HDL cholesterol < 40 mg/dL in men and < 50 mg/dL in women.
- •
Fasting glucose ≥ 100 mg/dL.
- •
Systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg.
According to its association with increased cardiovascular risk, hs-CRP was considered high when it was ≥ 3 mg/dL.24 Physical activity was measured using the Baecke questionnaire.25 Total activity was obtained from the sum of the work exercise and leisure time activities. This questionnaire, has been validated in adult populations and provides reliable information.
In order to set the cut-off points to other coronary risk factors in our study population, a group of individuals with BMI < 30, no hypertension, hypertriglyceridemia, hypoalphalipoproteinemia or diabetes mellitus was selected. The 75th percentile (P75) for risk factors in the sub-sample was as follows: insulin = 15.20 µU/mL in men, and 16.97 µU/mL in women; HOMA-IR= 3.38 in men, and 3.66 in women; ASF = 221.7 cm3 in men and 335.5 cm3 in women; AVF = 151.5 cm3 in men and 122.0 cm3 in women.
Statistical analysisContinuous variables with normal distribution were expressed as mean ± SD, and categorical variables were expressed as percentage. Variables with normal distribution were compared using the Student’s t-test and variables with asymmetrical distribution with the Mann-Whitney U test. Chi-square test was applied to compare the prevalence of coronary risk factors between the groups. The independence of the associations was assessed with a multivariate linear or logistic regression analysis [standardized β coefficient or odds ratio (OR)] and a 95% confidence interval (95% CI). Two-tailed p values < 0.05 were considered statistically significant. All analyses were performed using the statistical package SPSS version 15.0 (SPSS Chicago, II).
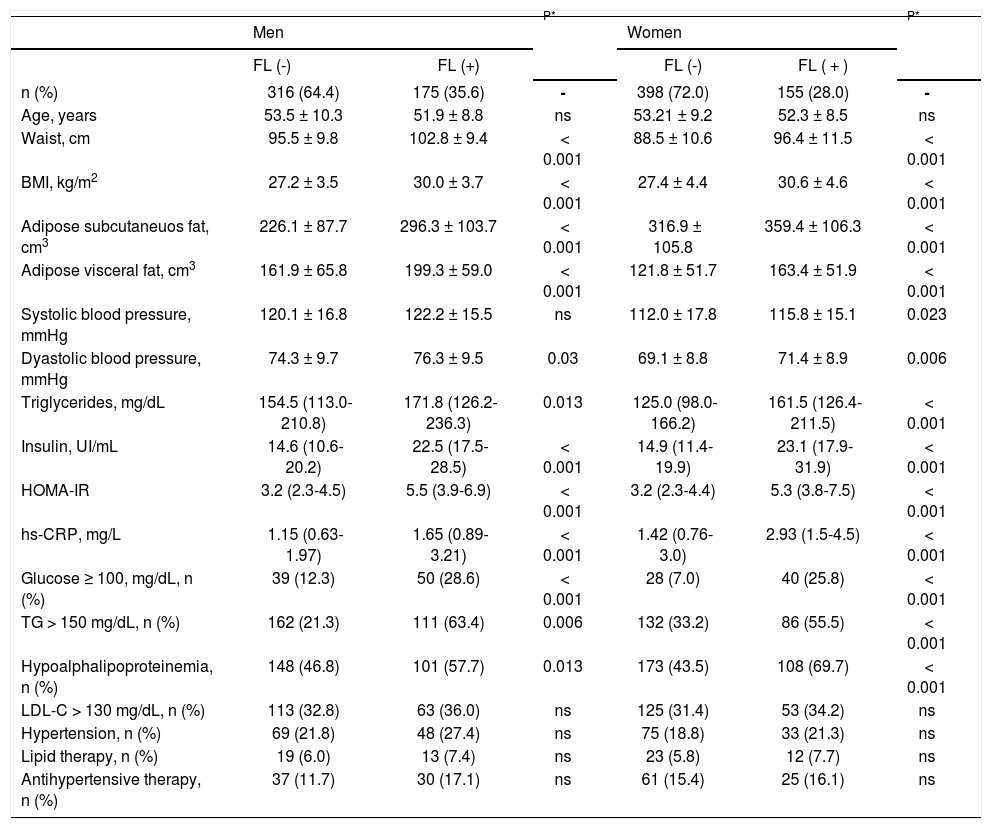
ResultsThe prevalence of FL in the study population was 31.6% (35.6% in men and 28.0% in women, p < 0.01). In subjects with FL compared to participants without FL, the prevalence of abdominal obesity, defined by waist circumference (93 vs. 70%, p < 0.001 in men and 94 vs. 80%, p < 0.001 in women) or AVF ≥ P75 (86 vs. 59%, p < 0.001 in men and 81 vs. 43%, p < 0.001 in women), was markedly increased. The high prevalence of abdominal obesity was associated with high rates of metabolic syndrome (55.3 vs. 38.1%, p < 0.001 in men and 48.4 vs. 23.2%, p < 0.001 in women) and insulin resistance (86.8 vs. 53.1%, p < 0.001 in men and 86.7 vs. 45.2%, p < 0.001 in women), in participants with and without FL, although significantly higher in subjects of both genders with intra-hepatic fat excess. Compared to subjects with non FL, all adiposity measurements were significantly higher in the subjects with FL (Table 1).
Clinical and biochemical characteristics of study subjects by gender and fatty liver.
| Men | P* | Women | P* | |||
|---|---|---|---|---|---|---|
| FL (-) | FL (+) | FL (-) | FL ( + ) | |||
| n (%) | 316 (64.4) | 175 (35.6) | - | 398 (72.0) | 155 (28.0) | - |
| Age, years | 53.5 ± 10.3 | 51.9 ± 8.8 | ns | 53.21 ± 9.2 | 52.3 ± 8.5 | ns |
| Waist, cm | 95.5 ± 9.8 | 102.8 ± 9.4 | < 0.001 | 88.5 ± 10.6 | 96.4 ± 11.5 | < 0.001 |
| BMI, kg/m2 | 27.2 ± 3.5 | 30.0 ± 3.7 | < 0.001 | 27.4 ± 4.4 | 30.6 ± 4.6 | < 0.001 |
| Adipose subcutaneuos fat, cm3 | 226.1 ± 87.7 | 296.3 ± 103.7 | < 0.001 | 316.9 ± 105.8 | 359.4 ± 106.3 | < 0.001 |
| Adipose visceral fat, cm3 | 161.9 ± 65.8 | 199.3 ± 59.0 | < 0.001 | 121.8 ± 51.7 | 163.4 ± 51.9 | < 0.001 |
| Systolic blood pressure, mmHg | 120.1 ± 16.8 | 122.2 ± 15.5 | ns | 112.0 ± 17.8 | 115.8 ± 15.1 | 0.023 |
| Dyastolic blood pressure, mmHg | 74.3 ± 9.7 | 76.3 ± 9.5 | 0.03 | 69.1 ± 8.8 | 71.4 ± 8.9 | 0.006 |
| Triglycerides, mg/dL | 154.5 (113.0-210.8) | 171.8 (126.2-236.3) | 0.013 | 125.0 (98.0-166.2) | 161.5 (126.4-211.5) | < 0.001 |
| Insulin, UI/mL | 14.6 (10.6-20.2) | 22.5 (17.5-28.5) | < 0.001 | 14.9 (11.4-19.9) | 23.1 (17.9-31.9) | < 0.001 |
| HOMA-IR | 3.2 (2.3-4.5) | 5.5 (3.9-6.9) | < 0.001 | 3.2 (2.3-4.4) | 5.3 (3.8-7.5) | < 0.001 |
| hs-CRP, mg/L | 1.15 (0.63-1.97) | 1.65 (0.89-3.21) | < 0.001 | 1.42 (0.76-3.0) | 2.93 (1.5-4.5) | < 0.001 |
| Glucose ≥ 100, mg/dL, n (%) | 39 (12.3) | 50 (28.6) | < 0.001 | 28 (7.0) | 40 (25.8) | < 0.001 |
| TG > 150 mg/dL, n (%) | 162 (21.3) | 111 (63.4) | 0.006 | 132 (33.2) | 86 (55.5) | < 0.001 |
| Hypoalphalipoproteinemia, n (%) | 148 (46.8) | 101 (57.7) | 0.013 | 173 (43.5) | 108 (69.7) | < 0.001 |
| LDL-C > 130 mg/dL, n (%) | 113 (32.8) | 63 (36.0) | ns | 125 (31.4) | 53 (34.2) | ns |
| Hypertension, n (%) | 69 (21.8) | 48 (27.4) | ns | 75 (18.8) | 33 (21.3) | ns |
| Lipid therapy, n (%) | 19 (6.0) | 13 (7.4) | ns | 23 (5.8) | 12 (7.7) | ns |
| Antihypertensive therapy, n (%) | 37 (11.7) | 30 (17.1) | ns | 61 (15.4) | 25 (16.1) | ns |
Values are expressed as mean ± SD, median (interquartile range), or n(%). FL: fatty liver. BMI: body mass index. HOMA-IR: homeostasis model assessment of insulin resistance. hs-CRP: high-sensitive C-reactive protein. TG: tyiglicerides. Hypoalphalipoproteinemia: < 40 mg/dL in men and < 50 mg/dL in women. LDL-C: low density lipoprotein cholesterol; hypertension: systolic ≥ 140 or diastolic ≥ 90.
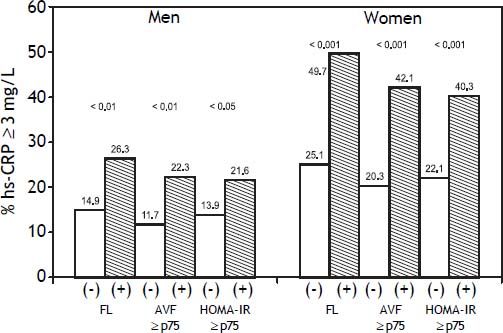
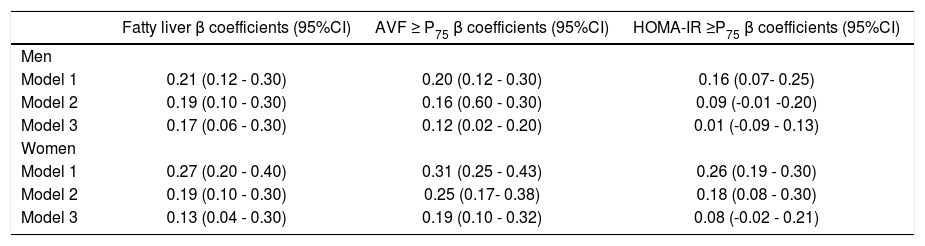
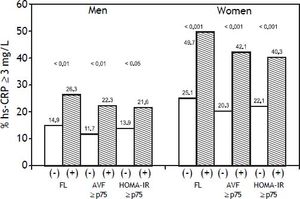
Overall, FL was associated with a more adverse metabolic profile (Table 1), characterized in both genders by higher blood pressure, triglycerides, insulin, HOMA-IR and hs-CRP values, as well as by significantly higher prevalence of impaired fasting glucose, increased triglycerides and low HDL-C. No significant differences were observed in age or the prevalence of increased LDL-C, hypertension, and the percentage of subjects under pharmacological treatment for these conditions in both groups. Figure 1 shows that compared to subjects without risk factors, subjects with FL, AVF ≥ P75 or HOMA-IR ≥ P75, particularly those of female gender, had a higher prevalence of hs-CRP ≥ 3 mg/L. The independence of the associations of FL, AVF ≥ P75 and HOMA-IR ≥ P75 with the natural logaritm of hs-CRP (Ln hsCRP) as a continuous variable, was determined by a multivariate linear regression analysis in each genever, ln hs-CRP was more strongly associated in women than in men (p < 0.01) (Table 2).
Association between fatty liver, adipose visceral fat ≥ P75 and HOMA-IR ≥ P75 with the natural logaritm of hs-CRP in multivariate linear regression analyses.
| Fatty liver β coefficients (95%CI) | AVF ≥ P75 β coefficients (95%CI) | HOMA-IR ≥P75 β coefficients (95%CI) | |
|---|---|---|---|
| Men | |||
| Model 1 | 0.21 (0.12 - 0.30) | 0.20 (0.12 - 0.30) | 0.16 (0.07- 0.25) |
| Model 2 | 0.19 (0.10 - 0.30) | 0.16 (0.60 - 0.30) | 0.09 (-0.01 -0.20) |
| Model 3 | 0.17 (0.06 - 0.30) | 0.12 (0.02 - 0.20) | 0.01 (-0.09 - 0.13) |
| Women | |||
| Model 1 | 0.27 (0.20 - 0.40) | 0.31 (0.25 - 0.43) | 0.26 (0.19 - 0.30) |
| Model 2 | 0.19 (0.10 - 0.30) | 0.25 (0.17- 0.38) | 0.18 (0.08 - 0.30) |
| Model 3 | 0.13 (0.04 - 0.30) | 0.19 (0.10 - 0.32) | 0.08 (-0.02 - 0.21) |
AVF: adipose visceral fat. HOMA-IR: homeostasis model assessment of insulin resistance. Model 1: unadjusted. Model 2: ajusted by age, hypertension, glucose. HDL-C: high density lipoprotein cholesterol, triglycerides, total kilocalories and physical activity. Model 3: model 2 plus AVF and HOMA-IR.
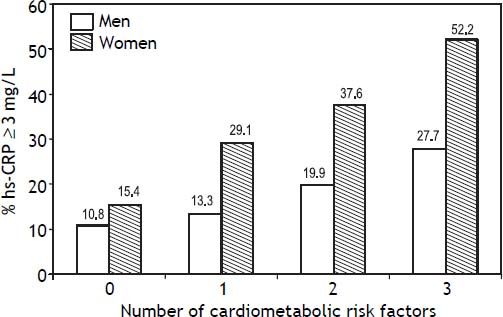
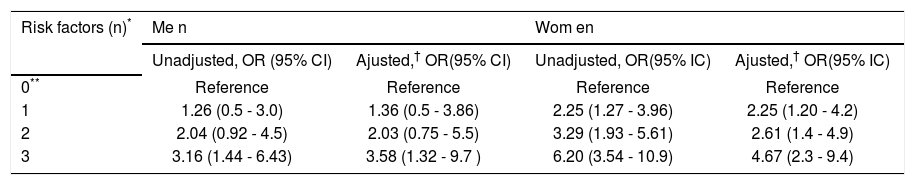
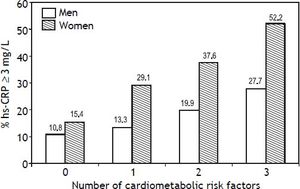
Other analyses were conducted to individually and jointly examine the association of FL, AVF ≥ P75 and HOMA-IR ≥ P75 with hs-CRP as a categorical variable (hs-CRP ≥ 3 mg/L). Figure 2 shows that increasing the number of cardiometabolic risk factors from zero to three raises the prevalence of elevated hs-CRP from 10.8 to 27.7% (p < 0.01) in men, and from 15.4 to 52.2% in women (p < 0.001). In addition the association of FL, AVF and HOMAIR with hs-CRP ≥ 3 mg/L in men, was statistically significant after adjust for traditional risk factors, only when combining all three cardiometabolic factors (OR [95% CI]:3.58 [1.32-9.7], p < 0.01) (Table 3). In women, the presence of a single factor was enough to raise the prevalence of hs-CRP ≥ 3 mg/L, which showed gradual increments (OR [95% CI]: 2.25 [1.20-4.2]) to 4.67 (2.3-9.4) as the number of risk factors increased.
Prevalence of hs-CRP (≥ 3 mg/L) associated with the presence of fatty liver, adipose visceral fat ≥ P75 (AVF: men = 151.5 cm3, women = 122 cm3) or HOMA-IR ≥P75 (men = 3.38, women = 3.66) and combination of two or three cardiometabolic risk factors. Trend in men p = 0.005, in women p < 0.001.
Association of fatty liver, adipose visceral fat ≥ P75 and HOMA-IR ≥ P75, with high hs-CRP (≥ 3 mg/L) in multivariate logistic regression analyses.
| Risk factors (n)* | Me n | Wom en | ||
|---|---|---|---|---|
| Unadjusted, OR (95% CI) | Ajusted,† OR(95% CI) | Unadjusted, OR(95% IC) | Ajusted,† OR(95% IC) | |
| 0** | Reference | Reference | Reference | Reference |
| 1 | 1.26 (0.5 - 3.0) | 1.36 (0.5 - 3.86) | 2.25 (1.27 - 3.96) | 2.25 (1.20 - 4.2) |
| 2 | 2.04 (0.92 - 4.5) | 2.03 (0.75 - 5.5) | 3.29 (1.93 - 5.61) | 2.61 (1.4 - 4.9) |
| 3 | 3.16 (1.44 - 6.43) | 3.58 (1.32 - 9.7 ) | 6.20 (3.54 - 10.9) | 4.67 (2.3 - 9.4) |
This study analyzes for the first time the influence of AVF, FL and HOMA-IR on hs-CRP in Mexican mestizo population. Both adipose stores and HOMA-IR had an independent and additive effect on the inflammation degree. FL and increased AVF were associated with hs-CRP concentration in both men and women independently of traditional risk factors and insulin resistance. The effect of FL was more evident in men while AVF was in women. These results suggest that, independent of insulin resistance, both adipose stores can predict an increased systemic inflammation. The combination of FL, AVF ≥ P75 and HOMA-IR ≥ P75 was associated with a gradual increase in the risk of hs-CRP ≥ 3 mg/L, which was 3.58 and 4.67 times higher in men and women in which all three factors were present, respectively.
It has been previously reported that compared to obese subjects with no liver impairment, obese subjects with steatosis or steatohepatitis had increased hs-CRP and other markers of inflammation.5 Also, serum C reactive protein was found associated with liver steatosis and visceral fat accumulation in type 2 diabetes mellitus patients but not in nondiabetic subjects.26 Another study in 2,388 Brazilian subjects, showed that hepatic steatosis, obesity, central obesity (with a crude estimation of waist circumference) and metabolic syndrome are independently and additionally associated with the likelihood of having hs-CRP ≥ 3 mg/L.10 In a study of Mexican subjects in which AVF was not determined, those with FL also showed a significant increase in ultrasensitivity CRP, after adjustment for traditional cardiovascular risk factors.14 Our data confirm and extend the findings from those studies by demonstrating that FL is associated with hsCRP even after controlling for visceral abdominal adiposity and insulin resistance. Although increases visceral abdominal adiposity and insulin resistance are closely related to hepatic steatosis,27,28 the observation that AVF and HOMA-IR are associated, independently from one another and together with systemic inflammation, and that they do not affect the association of FL with hs-CRP, could be interpreted as evidence that insulin resistance and AVF contribute to the risk of systemic inflammation by different but closely related pathophysiological mechanisms. Indeed previous evidence has shown that insulin-resistant adipose tissue plays a key role in the development of the metabolic and histological abnormalities present in obese subjects with FL.29,30 In physiological conditions, the adipose tissue is the main source of free fatty acids for the liver synthesis of triglycerides. However, when the adipose tissue is insulin-resistant, releases excessive amounts of fatty acids that accumulate in the liver along with other compounds such as diacylglycerols and ceramides, which trigger proinflammatory cytokine production.31,32 Oxidative stress, produced by the lipotoxic effect related to the accumulation of saturated fatty acids in the liver, is another inductive factor of inflammation.33,34 Together, these abnormalities could explain the direct and independent link of FL with hs-CRP, and HOMA-IR observed in our study.
Women of Latin American descent have one of the highest average hs-CRP concentrations.35–37 Epidemiologic and clinical trials have consistently shown that compared to men, women have higher hs-CRP concentrations, which are unrelated to age, BMI, ethnicity and hormone replacement therapy. The present study confirms that plasma hs-CRP concentration is higher in women than in men.38,39 The reason for this gender-based difference is unknown but some plausible explanations have been suggested:
- •
Because hormone replacement therapy increases CRP concentrations,40 it is possible that endogenous estrogen stimulates CRP synthesis.
- •
The larger total body fat reservoir in women could raise the synthesis of proinflammatory cytokines such as IL-6, that leads to increased production of CRP by the liver.41
The higher levels of subcutaneous abdominal fat and hs-CRP we observed in women support the latter hypothesis.
Study strengthsIn most previous trials, FL and CRP association was studied jointly in men and women and the effect of gender has been controlled with statistical models. In the present study, the results from a population consisting of 47.4% male and 52.6% female subjects without personal or family history of coronary artery disease or diabetes mellitus, selected from all socioeconomic strata, we used analyses adjusted for potential confounders to demonstrate independent associations of cardiometabolic factors with hs-CRP by gender. The majority of reports have assessed abdominal obesity by the crude measure of waist circumference, while in this study we used CAT scans, the best method for differential quantification of the subcutaneous and visceral central adiposity stores.18,19
Study limitationsDue to the cross sectional nature of the study, it is not possible to establish causal or temporal relationships between cardiometabolic risk factors and increased hs-CRP. Nevertheless, the findings suggest that FL is not only a cardiovascular risk marker, but also a factor with atherogenic effect mediated by systemic inflammation. It has been suggested that at least two hs-CRP concentration measurements in metabolically stable subjects are needed to appropriately determine inflammatory status.42 In this study hs-CRP was determined only once; however a great care was taken to avoid sampling of subjects with acute or chronic inflammatory conditions. The diagnosis of FL was established by CAT scans, but was not confirmed by liver biopsies. However, a significant correlation has been demonstrated between the liver attenuation images on CAT and the histological grade of steatosis.43 Although insulin resistance was not assessed by more sophisticated approaches, the HOMA-IR index has proven to be a reliable measure of insulin sensitivity in non-diabetics subjects.17
In conclusion, both steatosis and hs-CRP ≥ 3 mg/dL are present in 1 of 3 non-diabetic subjects free of clinical cardiovascular disease. FL, AVF ≥ P75 and HOMA-IR ≥ P75 are independently and additively associated in both genders with approximately twice the risk of increased hs-CRP values. The gender-based differences in the association of FL and the cardiometabolic risk factors with the systemic inflammatory status, suggest that hs-CRP related cardiovascular risk is greater in women than men.
Abbreviations- •
AVF: abdominal visceral fat.
- •
BMI: body mass index.
- •
CAD: coronary artery disease.
- •
CAT: computed axial tomography.
- •
FL: fatty liver.
- •
GEA: Genetics of Atherosclerotic Disease Study.
- •
HDL-C: high density lipoprotein cholesterol.
- •
HOMA-IR: homeostasis model assessment of insulin resistance.
- •
hs-CRP: high sensitivity C reactive protein.
- •
LDL-C: low density lipoprotein cholesterol.
- •
SAF: subcutaneous abdominal fat.
- •
TG: triglycerides.
This study was supported by CONACYT grant No. SALUD 2010-2 150537.
AcknowledgementsWe acknowledge to healthy volunteers and patients from the Instituto Nacional de Cardiología Ignacio Chávez, as well as the staff of the Endocrinology Department.